Abstract
Background
Individuals with body dysmorphic disorder may have perceptual distortions for their appearance. Previous studies suggest imbalances in detailed relative to configural/holistic visual processing when viewing faces. No study has investigated the neural correlates of processing non-symptom-related stimuli. The objective of this study was to determine whether individuals with body dysmorphic disorder have abnormal patterns of brain activation when viewing non-face/non-body object stimuli.
Methods
Fourteen medication-free participants with DSM-IV body dysmorphic disorder and 14 healthy controls participated. We performed functional magnetic resonance imaging while participants matched photographs of houses that were unaltered, contained only high spatial frequency (high detail) information, or only low spatial frequency (low detail) information. The primary outcome was group differences in blood oxygen level-dependent signal changes.
Results
The body dysmorphic disorder group showed lesser activity in the parahippocampal gyrus, lingual gyrus, and precuneus for low spatial frequency images. There were greater activations in medial prefrontal regions for high spatial frequency images, although no significant differences when compared to a low-level baseline. Greater symptom severity was associated with lesser activity in dorsal occipital cortex and ventrolateral prefrontal cortex for normal and high spatial frequency images.
Conclusions
Individuals with body dysmorphic disorder have abnormal brain activation patterns when viewing objects. Hypoactivity in visual association areas for configural and holistic (low detail) elements and abnormal allocation of prefrontal systems for details is consistent with a model of imbalances in global vs. local processing. This may occur not only for appearance but also for general stimuli unrelated to their symptoms.
Keywords: body dysmorphic disorder, visual processing, perception, houses
Background
Body dysmorphic disorder (BDD) is a psychiatric disorder in which individuals are preoccupied with perceived defects of their appearance or are excessively concerned about a slight physical abnormality, which causes distress and/or functional impairment(American Psychiatric Association., 2000). Perceived defects can involve any body area but most often involve the face or head(Phillips et al., 1993). Individuals experience obsessive thoughts about their appearance and tend to engage in repetitive, time-consuming behaviors such as checking their appearance in the mirror and scrutinizing details of others' appearances to compare to their own(Phillips, 2005). BDD is estimated to affect approximately 0.7-2.4% of the population(Buhlmann et al., 2010, Faravelli et al., 1997, Koran et al., 2008, Otto et al., 2001, Rief et al., 2006), and is associated with high lifetime rates of psychiatric hospitalization (48%)(Phillips and Diaz, 1997) and suicide attempts (22-27.5%)(Phillips et al., 2005a, Phillips and Diaz, 1997, Veale et al., 1996). Very few have good insight and 36-60% are delusional(Gunstad and Phillips, 2003, Mancuso et al., 2010, Phillips et al., 2005b). Despite the prevalence and severity of the disorder, relatively little is known about the pathophysiology underlying various symptom domains.
One potentially important clinical symptom domain in BDD is distorted perception of appearance; they perceive certain features to be defective(American Psychiatric Association., 2000), e.g. their nose is crooked or their skin is pockmarked, contrary to what others perceive of them. In most cases, they seem to excessively focus on details of their appearance at the expense of global or configural aspects(Phillips, 2005). A possible explanation of these misperceptions might lie in visual perceptual abnormalities.
Neuropsychological and brain imaging studies suggest that abnormal information processing may contribute to perceptual abnormalities in BDD(Deckersbach et al., 2000, Feusner et al., 2010, Feusner et al., 2007b, Yaryura-Tobias et al., 2002). Early evidence of abnormalities in own-face processing comes from a study in which individuals with BDD perceived distortions of a digital photograph of their face, which were not actually present(Yaryura-Tobias et al., 2002). Additional evidence of abnormal visual processing of own faces comes from a functional magnetic resonance imaging (fMRI) study, which demonstrated hyperactivity in fronto-striatal systems for unaltered images and hypoactivity in visual cortical systems for low spatial frequency information that conveys holistic and configural elements(Feusner et al., 2010). Another fMRI study using others' faces demonstrated left hemisphere hyperactivity relative to healthy controls in higher-order visual processing regions, suggesting predominant detailed and analytic processing(Feusner et al., 2007b). These studies suggest abnormalities in visual processing in BDD, at least for symptom-relevant stimuli of own and others' faces.
Aside from abnormalities of face processing, whether individuals with BDD experience general abnormalities in visual processing is not yet clear. Early evidence that this may be the case comes from a study using the Rey-Osterrieth Complex Figure Test (RCFT) to compare visuospatial performance of individuals with BDD relative to healthy controls(Deckersbach et al., 2000). The BDD cohort demonstrated selective recall of details rather than larger organizational design features. This search strategy may implicate a deficit in executive functioning mediated by frontal-striatal circuits or other higher-order perceptual processing systems. Alternatively, deficits may have resulted from encoding an initially distorted lower-order visual perception, or from aberrant retrieval and reconstruction. In an earlier study Hanes et al. (1998) did not find abnormal performance on the RCFT in individuals with BDD relative to healthy controls(Hanes, 1998). Thus, it remains unclear if individuals with BDD demonstrate abnormal visual processing of objects or figures and if so, what systems may mediate this. No study to date has investigated the neural correlates of visual processing of non-face/non-body objects in individuals with BDD.
The objective of the current fMRI study was to test if individuals with BDD have abnormal visual processing for stimuli unrelated to their symptoms, i.e. non-face/non-body objects. We used houses as the object stimuli because they contain a combination of holistic (entire house as one precept), configural (e.g. spatial relationships between windows and roof) and detailed (e.g. shingles) information from which to understand different elements of visual processing. In addition, multiple previous studies have examined object processing in healthy controls using houses as stimuli, providing a framework of expected networks that includes the parahippocampal place area, fusiform gyrus, lateral occipital cortex, cuneus, and lingual gyrus(Epstein et al., 1999, Ewbank et al., 2005, Pourtois et al., 2005).
We used digital photographs of houses that were either unaltered or altered to contain only high spatial frequency (HSF) or only low spatial frequency (LSF) information. Detailed visual analysis relies on fine visual resolution, which is conveyed by HSF information(Norman and Ehrlich, 1987, Schyns and Oliva, 1999). Configural and holistic elements are primarily conveyed by LSF information(Costen et al., 1996, Sergent, 1985). We therefore used HSF, LSF, and normal spatial frequency (NSF) houses to functionally dissect visual processing systems. Our hypothesis was that individuals with BDD would demonstrate abnormalities for processing houses, similar in nature to that observed in the previous other-face study and therefore possibly representing a general underlying abnormal visual processing phenotype. Specifically, we hypothesized that the BDD group would demonstrate greater left-hemisphere activity in secondary and higher-order occipital and temporal object- and house-processing regions, such as the cuneus, lingual gyrus, fusiform gyrus, and parahippocampal place area. Based on what was observed for others' faces, we predicted the greatest degree of abnormalities for LSF images, followed by NSF images, and no difference from controls for HSF images. Based on what was observed for others' and own faces, we predicted that greater symptom severity would be associated with lesser activity in the dorsal visual stream for LSF images.
Methods
Participants
The UCLA Institutional Review Board approved the protocol for the study. Fourteen participants with BDD and 14 healthy controls, ages 20 to 48, provided informed consent. (All participants had participated in the previous own-face BDD fMRI study(Feusner et al., 2010)). BDD participants and controls were recruited from the community and were of equivalent gender, age, and level of education. All were right-handed, as determined by the Edinburgh Handedness Inventory(Oldfield, 1971). BDD participants met DSM-IV criteria for Body Dysmorphic Disorder, diagnosed by the first author (JDF), who has clinical expertise with this population. Diagnoses were made using the Body Dysmorphic Disorder Module(Phillips et al., 1995), a reliable diagnostic module modeled after the Structured Clinical Interview for DSM. In addition, we performed a clinical psychiatric evaluation and screened all participants with the Mini International Neuropsychiatric Inventory (MINI)(Sheehan et al., 1998). All BDD participants were required to have a score of ≥20 on the BDD version of the Yale-Brown Obsessive-Compulsive Disorder Scale (BDD-YBOCS)(Phillips et al., 1997). The BDD-YBOCS is a validated scale that is a widely-used standard to evaluate symptom severity in BDD, with a range of scores from 0 to 48 (Phillips et al., 1997). It has excellent interrater and test-retest reliability (intra-class correlation coefficients for total score=0.99 and 0.88, respectively), internal consistency (Cronbach's alpha=0.80), and convergent validity (r=.55 with the CGI) (Phillips et al., 1997). In addition to the BDD-YBOCS, we also administered the Hamilton Depression Rating Scale-17 (range of scores from 0 to 54) (Hamilton, 1960) and the Hamilton Anxiety Rating Scale (range of scores from 0 to 56) (Hamilton, 1969), which have similar standards for validity and reliability. These were included to provide a broader understanding of clinical severity of participants. We allowed participants with delusional beliefs about their appearance (score of 4 on item 11 of the BDD-YBOCS: “Lacks insight, delusional”).
Exclusion criteria for all participants included: substance abuse or dependence within the past 12 months, lifetime neurological disorder, pregnancy, or any current medical disorder that may affect cerebral metabolism. We excluded BDD participants with other Axis I disorders (current or past bipolar disorder, current or past panic disorder, current social phobia, current obsessive-compulsive disorder, current posttraumatic stress disorder, current or past psychotic disorders, current anorexia nervosa, or current bulimia nervosa). We did not exclude those with comorbid dysthymia, major depressive disorder, or generalized anxiety disorder; as depression and anxiety are so frequently comorbid in this population, we believed it would not be a representative sample to exclude these. However, we required that BDD be the primary diagnosis as defined by the MINI (“Which problem troubles you the most or dominates the others or came first in the natural history?”). We excluded any participants whom the investigator judged were suicidal. Healthy controls could not have any current or past Axis I disorder, as determined by the MINI.
BDD participants were free from psychoactive medications for at least 8 weeks prior to the study and were not receiving cognitive-behavioral therapy. Healthy controls were excluded if they were taking any psychiatric medication or were in any psychotherapy treatment. We only included participants with normal or corrected vision, as verified by Snellen eye chart. Any participants with ferromagnetic metal implantations or devices or weight greater than 280 lbs were excluded due to safety and capacity requirements of the MRI scanner.
Stimuli and Task
We obtained digitized, frontal view photographs of houses from the Internet and used Adobe Photoshop® CS3 software to convert to grayscale. We created HSF and LSF images, as previously described(Feusner et al., 2007b). This included using Scion Imaging Software (http://www.scioncorp.com) to Fourier-transform digital photographs, alter these images in their frequency domains, and then reverse Fourier-transform them to create high-pass and low-pass filtered images(Iidaka et al., 2004). A “high-pass filter” creates images that contain only the HSF information. A “low-pass filter” creates images that contain only LSF information. We normalized luminosity across stimuli (Fig. 1).
Fig. 1. Example houses stimuli.
Three different categories of houses comprised the tasks: A) NSF, B) HSF, or C) LSF. The control condition consisted of gray rectangle and square shapes, approximately the same size as the houses. We used MacStim 3.0 (White Ant Occasional Publishing, West Melbourne, Australia) to program the stimuli presentation and to record accuracy and reaction time. The matching task is a forced-choice, two alternative task. Each set of stimuli consisted of three houses or shapes: a target house or shape on top and two selection houses or shapes on the bottom. We instructed participants to: “Choose the house or shape on the bottom that is the same house or shape as the one on the top, by pressing the right or left button. Make your selection as rapidly and as accurately as possible.” Each set of houses or shapes appeared on the screen for 4 seconds (sufficient time to allow for visual inspection and for the task to be easy, yet short enough to likely minimize habituation of BOLD response), with a 1-second interstimulus interval.
Each block consisted of 4 sets of unique houses of the same spatial frequency that were A) NSF, B) HSF, or C) LSF; or 4 sets of the control task. Each set of 4 blocks (A-B-C-Control) was repeated four times in each run. The total time for each run was 6 minutes, 8 seconds. There were two runs, the second presented in a different order. Between participants, differently ordered runs were counter-balanced using a Latin Square design, to avoid possible order effects. Participants wore fMRI-compatible goggles to view the stimuli. If participants wore eyeglasses, appropriate corrective lenses for the goggles were used. After each run, participants rated their anxiety level during the tasks on a Likert-scale of 0-10.
fMRI
We used a 3-Tesla Allegra (Siemens) MRI scanner to evaluate BOLD contrast, using T2*-weighted echo planar imaging (EPI) gradient-echo pulse sequence (TR = 2.5 seconds, TE = 35 milliseconds, Flip-Angle = 90°, Matrix = 64×64, field-of-view = 24×24 cm, in-plane voxel size 3.1 mm × 3.1 mm, slice thickness 3mm, 1mm intervening spaces, and 28 total slices). We obtained matched-bandwidth T2 as well as MP-RAGE T1-weighted images to provide detailed anatomy during structural image acquisition.
Image processing included motion correction, skull stripping, spatial smoothing of 5mm full-width/half-maximum Gaussian kernel, mean-based intensity normalization of all volumes by the same factor, and high-pass temporal filtering. We co-registered functional images of each participant to corresponding matched-bandwidth structural images in native space, then performed a second-stage registration to their higher-resolution MP-RAGE scans, and finally registered these to structural standard images, defined by the Montreal Neurological Institute averaged 152 standard brain.
Data Analysis
Behavioral data
We performed a two-way repeated-measures analysis of variance to compare response times (RT) and accuracy rates between groups, using PROC GLM in SAS®. Group (BDD and healthy controls) was the between-subject factor and stimulus type (NSF, HSF, LSF, and control stimuli) was the within- factor.
Anxiety levels
A two-sample t-test was used to compare mean subjective anxiety scores between groups.
Functional neuroimaging data
We used FEAT software (FMRI Expert Analysis Tool) Version 5.4, part of the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain Software Library (FSL), www.fmrib.ox.ac.uk/fsl.
For within-group analyses, we performed a random-effects analysis with subject as the random factor to identify typical patterns of brain activity in BDD participants and healthy controls. We modeled the hemodynamic response function by performing a simple convolution of the blocked experimental paradigms of each condition vs. control task with the canonical hemodynamic response function and its temporal derivative(Aguirre et al., 1998). To determine the patterns of activations in the BDD group and in the control group, we analyzed the normalized data with multiple regression by using three regressors to model hemodynamic changes associated with the HSF, LSF, and NSF tasks, each contrasted to the control task.
Model fitting generated whole brain images in native space of parameter estimates and corresponding variance, representing average signal change during each particular contrast. We used FMRIB's Improved Linear Model (FILM) for time-series statistical analysis, using local autocorrelation correction(Woolrich et al., 2001). We thresholded Z statistic images using clusters determined by Z>2.0 and a (corrected) cluster significance threshold of P=0.05(Worsley et al., 1992).
For between-group analyses, we directly compared BDD participants and controls using a voxel-wise, mixed-effects analysis in FSL. After we performed within-groups analyses, we used FLAME (FMRIB's Local Analysis of Mixed Effects) stages 1 and 2(Beckmann et al., 2003, Woolrich et al., 2004). We thresholded Z statistic images using clusters determined by Z>2.0, and a (corrected) cluster significance threshold of P=0.05(Worsley et al., 1992). A two-sample t-test identified group mean differences in activity at each voxel.
To graphically represent BOLD signal changes in regions identified from the voxelwise analysis as differentially active between groups, we performed post hoc percent signal change analyses. For this we created a set of spherical ROIs (6 mm radii) at local maxima for significant clusters from the between-group analyses. Parameter estimate data were then extracted from each ROI for each participant using FSL command line tools(Poldrack, 2007).
To investigate the relationship between symptom severity in BDD participants and regional brain activation, we entered results from the within-group analysis into a higher-level analysis with de-meaned BDD-YBOCS scores as a covariate of interest. This produced a voxelwise map of regions whose activity correlated with BDD symptom severity. We also extracted post hoc percent signal change from local maxima (as above) to create scatterplots depicting the relationship between symptom severity and brain activity across BDD participants.
Results
Demographics and psychometrics
Table 1 summarizes demographic and psychometric data. One BDD participant had comorbid major depressive disorder, one had generalized anxiety disorder, four had major depressive disorder and generalized anxiety disorder, and one had both dysthymic disorder and generalized anxiety disorder. All participants had preoccupations with perceived facial defects.
Table 1. Demographics and psychometric scores.
| Characteristic | BDD group (N=14) | Control group (N=14) | P valuea |
|---|---|---|---|
| Age, mean (SD), y | 28.1 (7.3) | 26.9 (5.1) | 0.616 |
| Female/male, No. | 7/7 | 7/7 | >0.99 |
| Right-handedness, No. | 14 | 14 | >0.99 |
| Education, mean (SD), y | 15.1 (2.6) | 16.9 (2.4) | 0.0627 |
| BDD-YBOCS score, mean (SD) | 29.2 (5.1) | N/A | N/A |
| HDRS-17 score, mean (SD) | 11.6 (7.7) | 1.3 (1.2) | <0.001 |
| HARS score, mean (SD) | 13.9 (8.0) | 1.5 (1.5) | <0.001 |
Abbreviations: BDD: body dysmorphic disorder; BDD-YBOCS: BDD version of the Yale-Brown Obsessive-Compulsive Scale; HDRS-17: 17-item Hamilton Depression Rating Scale; HARS: Hamilton Anxiety Rating Scale
t-test for all comparisons except gender and handedness (X2 test)
Behavioral Data
Matching task
The BDD group had slower mean RT across all tasks including the control task (1.28±0.22 sec. vs. 1.03±0.35 sec. for NSF, 1.26±0.22 sec. vs. 0.99±0.37 sec. for LSF, 1.65±0.33 sec. vs. 1.39±0.47 sec. for HSF, and 1.22±0.23 sec. vs. 0.96±0.30 sec. for control stimuli, for BDD and healthy control groups, respectively). There were significant main effects of group (F1,26 = 5.14, P=0.0320) (slower RT for the BDD group) and stimulus (F3,78 = 79.41, P<0.0001) (slower RT for HSF images) but no group by stimulus interaction effect (F3,78 = 0.02, P=0.996).
Mean accuracy rates were similar between groups across all stimuli (99.33±1.81% vs. 99.09±1.50% for NSF, 99.33±1.33% vs.98.42±2.07% for LSF, 98.73±1.55% vs. 98.88±1.56% for HSF, and 96.91±2.88% vs. 96.36±3.93% for control stimuli, for BDD and healthy control groups, respectively). There was a significant main effect of stimulus (F3,78 = 8.02, P=<0.0001), (lower accuracy for control stimuli) but no significant main effects of group (F1,26 = 0.77, P=0.389) or group by stimulus interaction (F3,78 = 0.29, P=0.829). In the BDD group, correlations were nonsignificant between RT (collapsed across all stimuli because of absence of a group by stimulus interaction effect) and BDD-YBOCS scores (r=0.34, P=0.24), HDRS scores (r=0.29, P=0.31), HARS scores (r=0.16, P=0.60), and task anxiety (r=-0.14, P=0.62).
Anxiety levels
Mean anxiety during the experiment was slightly higher for the BDD than the healthy control group, but not significantly different (3.32±2.45 vs. 2.04±1.88, t=1.55, d.f.=26, P=0.13). We explored using task anxiety as a covariate in the two-way repeated measures ANOVA. When controlling for anxiety, there were still significant main effects of group (F1,25 = 5.88, P=0.0229) (slower RT for the BDD group) and stimulus (F3,75 = 29.86, P<0.0001) (slower RT for HSF images) but no group by stimulus interaction effect (F3,75 = 0.05, P=0.977) for RT. For accuracy rate, when controlling for anxiety there was a significant main effect of stimulus (F3,75 = 4.83, P=<0.0119) (lower accuracy for control stimuli), but no significant main effects of group (F1,25 = 0.59, P=0.451) or group by stimulus interaction (F3,75 = 0.62, P=0.543).
fMRI
Within-groups analyses
For all stimulus types, the BDD and healthy control groups activated extensive bilateral visual cortex, as well as fusiform cortex and parahippocampal gyri. There was also activation for both groups for NSF and HSF (but not LSF) images evident in subcortical (thalamic) and prefrontal (paracingulate, supplementary motor area, and inferior frontal gyrus) regions. For HSF images, activation in bilateral frontal poles was evident for the BDD but not healthy control group.
Between-groups analyses
There were no significant differences between groups for NSF stimuli.
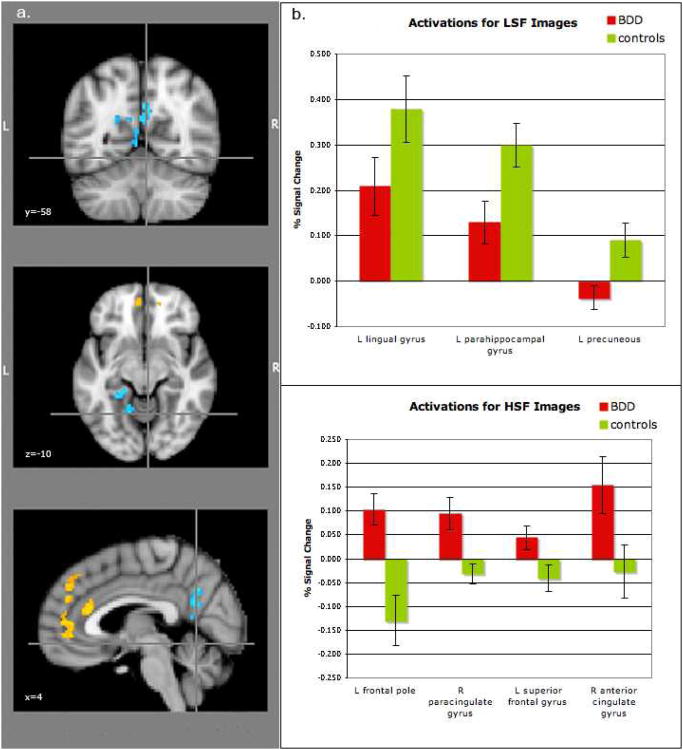
For the LSF images, the BDD group relative to the healthy control group showed lesser activity in the left parahippocampal cortex, the left hippocampus, left lingual gyrus, left posterior cingulate, and bilateral precuneus (Fig. 2a, Table 2). This was accounted for primarily by lesser activation in the BDD group relative to healthy controls in these regions (see percent signal changes – Fig. 2b).
Fig. 2. Significant differences in regional brain activity between BDD and healthy control groups.
a) Lesser activation for BDD relative to control participants (blue) for low spatial frequency (LSF) stimuli in the left lingual gyrus and right precuneus (top image), and left parahippocampal gyrus and left hippocampal gyrus (middle image). There was greater activation for BDD than control participants (orange) for high spatial frequency (HSF) stimuli in left frontal pole (middle image), left superior frontal gyrus (not shown), and right anterior cingulate gyrus and right paracingulate gyrus (bottom image). (See Table 2 for list of all unique local maxima).
b) Percent signal changes for several regions found to be significant between groups in the voxelwise analysis for LSF (top) and HSF (bottom) stimuli.
Table 2. Local maxima for significant between-groups differences.
| healthy controls > BDD | ||
|---|---|---|
| LSF House vs. control stimuli | Z score | x, y, z |
| Left lingual gyrus | 3.56 | -20, -48, -4 |
| Left precuneus | 3.43 | -18, -52, 18 |
| Left parahippocampal gyrus | 3.05 | -20, -36, -10 |
| Left hippocampus | 2.98 | -22, -38, 0 |
| BDD > healthy controls | ||
| HSF House vs. control stimuli | Z score | x, y, z |
|
| ||
| Left frontal pole | 4.28 | -22, 50, 22 |
| Right frontal pole | 3.56 | 14, 44, 36 |
| Left superior frontal gyrus | 3.98 | -12, 26, 52 |
| Right paracingulate gyrus | 3.53 | 16, 38, 18 |
| Right anterior cingulate gyrus | 2.98 | 2, 28, 22 |
BDD = body dysmorphic disorder. LSF = low spatial frequency. HSF = high-spatial frequency. Only local maxima from unique regions are represented. There were no significant differences for normal spatial frequency (NSF) images.
For HSF images, BDD participants relative to controls showed greater activity in bilateral frontal pole, left superior frontal gyrus, right anterior cingulate gyrus, and right paracingulate gyrus (Fig. 2a, Table 2). This was accounted for by activation in the BDD group relative to the control task and deactivation in healthy controls relative to the control task in these regions (see percent signal changes – Fig. 2b). To verify if this pattern represented deactivation in healthy controls and activation for the BDD group for the HSF task vs. “true” baseline (as opposed to HSF vs. control task) we performed a post hoc analysis contrasting the HSF task as well as the control task to the rest periods of the scan (during which participants viewed a small crosshair in the middle of the screen). Within-groups results revealed deactivation in the healthy controls for both HSF and control tasks in bilateral dorsal and ventral medial prefrontal cortex, precuneus/posterior cingulate, and angular gyrus. The BDD group demonstrated similar deactivations in these regions for both HSF vs. baseline and control task vs. baseline, except for dorsal medial prefrontal cortex (see Fig. 5 in supplemental material online). There were no significant differences between groups for HSF vs. baseline or for the control task vs. baseline.
Response time covariate analysis
Due to the fact that there were behavioral differences between groups in response times on all tasks, we performed a between-groups analysis using response time on the tasks as a covariate of non-interest. For the LSF analysis, there were no significant differences between groups at a threshold of Z>2.0, but at Z>1.9 there was relative hypoactivation in the BDD group in the left lingual gyrus, the left parahippocampal gyrus, the left hippocampus, and the left precuneus (similar to the regional activation without RT as a covariate). For the HSF analysis, the regional activation was essentially unchanged in the prefrontal regions compared to results without RT as a covariate. For the NSF analysis, there remained no significant differences between groups.
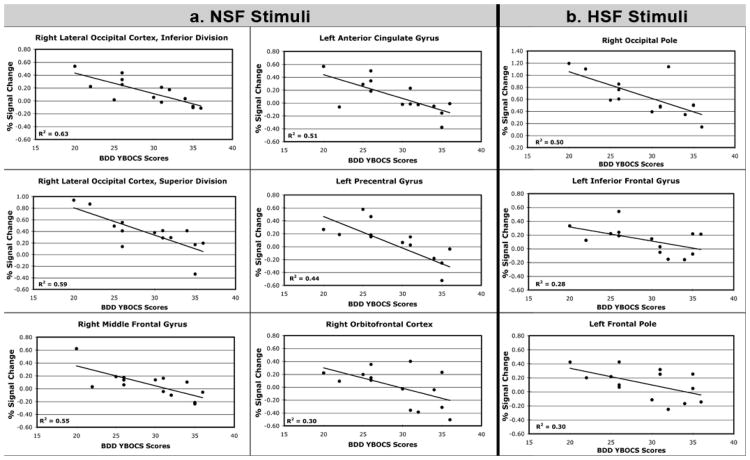
Correlation with BDD-YBOCS
For NSF images, there were inverse associations between BDD-YBOCS scores and activity in bilateral middle frontal gyrus, right lateral dorsal occipital cortex, bilateral orbitofrontal cortex, left anterior cingulate gyrus, left precentral gyrus, and left inferior frontal gyrus (See Fig. 3a; see also Fig. 4 and Table 3 in supplemental online material).
Fig. 3. Percent signal change as a function of symptom severity.
Percent signal change (y axis) in regions found to be significantly associated with symptom scores on the body dysmorphic disorder version of the Yale-Brown Obsessive Compulsive Scale (BDD YBOCS) (x axis) for a) NSF – normal spatial frequency; and b) HSF – high spatial frequency images. No regions were significantly associated with LSF – low spatial frequency – images. (Note that in Fig. 3b the left lateral occipital cortex, right cuneus, and left occipital pole are not depicted because the local max were on cortical surface regions such that spherical ROI extraction for percent signal change would not be valid).
For LSF images, there were no significant associations between BDD-YBOCS scores and brain activity.
For HSF images, there were inverse associations between BDD-YBOCS scores and activity in left lateral occipital cortex, bilateral occipital pole, right dorsal cuneus, left inferior frontal gyrus, and left frontal pole. (See Fig. 3b; see also Fig. 4 and Table 3 in supplemental online material).
There were no significant positive associations between BDD-YBOCS scores and brain activity for any image type.
Discussion
This study demonstrates that unmedicated individuals with BDD have abnormal brain activation patterns when viewing non-face/non-body objects. The primary finding is less activation than healthy controls in secondary visual processing systems for configural and holistic elements. Moreover, greater BDD symptom severity is associated with lesser activity in the dorsal visual stream and in ventrolateral prefrontal regions. Because images of houses are not symptom-related stimuli, the current study therefore suggests the possibility of general, aberrant visual processing in BDD.
These results confirmed our hypothesis of abnormal activity for LSF information in occipital and temporal visual processing systems in individuals with BDD. Contrary to our predictions, however, a pattern of predominant left hemisphere hyperactivity was not evident; instead, there was abnormal hypoactivity in left lingual gyrus and parahippocampal cortex. The parahippocampal cortex (in addition to a distributed network in bilateral ventral temporal and occipital cortex(Ishai et al., 2000, Ishai et al., 1999)) has been identified as an important region for recognition and encoding of places, scenes, and houses, and has been termed the “parahippocampal place area”(Epstein et al., 1999, Ewbank et al., 2005, Pourtois et al., 2009). The BDD group also demonstrated relative hypoactivation for LSF information in systems involved in directed and sustained attention including the posterior cingulate and precuneus(Cabeza and Nyberg, 2000, Cavanna and Trimble, 2006). Together, these findings provide evidence that individuals with BDD may under-activate systems involved in secondary visual processing and attention, specifically for configural and holistic visual elements.
The nature of these findings fit with results from a previous neuropsychological study of complex figure processing using the RCFT, in which individuals with BDD appeared to underutilize configural and holistic elements while selectively recalling and reproducing visual details(Deckersbach et al., 2000). It also fits with clinical observation that they often focus on details of their appearance, for example small skin blemishes or small asymmetries of their nose, at the expense of global or configural aspects.
For HSF information, there were differences in patterns of brain activation between groups. However, the post hoc between-groups analysis of the HSF task relative to a low-level baseline (as opposed to the matching task of circles and ovals) revealed that there were no significant differences. The within-groups post hoc analysis demonstrates that the overall pattern of deactivation in both groups appears to represent task-related deactivation in the default mode network (DMN); this includes medial prefrontal cortex, precuneus/posterior cingulate and inferior parietal lobule. Deactivation of the DMN is commonly observed in a wide variety of cognitive tasks, and activation is evident during rest (see (Buckner et al., 2008) for review). The within-groups analysis in this study suggests that there may have been lesser deactivation in dorsal medial prefrontal regions in the BDD group. Thus, it is possible that the BDD group may be somewhat less able to deactivate specifically the dorsal medial prefrontal portions of the DMN when engaging in the task. Since a subsystem of the DMN involving the dorsal medial prefrontal cortex is thought to be involved in self-referential thinking(Castelli et al., 2000, Gusnard et al., 2001) one speculation about these findings is that the BDD group may have difficulty in switching out of self-referential thinking during the task, perhaps related to intrusive thoughts about themselves or other spontaneous stimulus-independent thoughts(Andrews-Hanna et al., 2010, McGuire et al., 1996).
Greater BDD symptom severity was associated with lesser activity in the dorsal occipital cortex for HSF and NSF images, although not for LSF images, as hypothesized. Dorsal occipital cortex and parieto-occipital regions, part of the dorsal visual stream(Ungerleider and Mishkin, 1982), are involved in motion perception and extracting shapes and three-dimensional cues from objects(Durand et al., 2007, Peuskens et al., 2004, Sereno et al., 2002). Such information extracted from objects is then integrated with information related to object identification and recognition extracted via the ventral visual stream (see Farivar, 2009 for review(Farivar, 2009)). Therefore, greater BDD symptom severity may be associated with abnormalities in extracting configural visual information within unaltered and HSF images related to shape.
Greater BDD symptom severity was also associated with lesser activity in ventrolateral prefrontal regions. Previous studies have found that the ventrolateral prefrontal cortex (and to a lesser extent dorsolateral prefrontal cortex) is important for selection, comparison, and judgment, as evidenced by involvement in simultaneous matching tasks(Corbetta et al., 1991, Grady et al., 1996, Haxby et al., 1994, Kosslyn et al., 1994, McIntosh et al., 1996, Rushworth et al., 1997), and on tasks involving working memory and short and long-term memory(Haxby et al., 1995, Petrides, 1996, Rushworth et al., 1997). These are regions that are involved in face, form, color, and spatial matching(Corbetta et al., 1991, Grady et al., 1996, Haxby et al., 1994, Kosslyn et al., 1994) and have connections to visual processing regions in the temporal and occipital lobes (Catani et al., 2002, Webster et al., 1994). A possible interpretation of the results, then, is that greater severity of BDD symptoms may be associated with impaired integration of visual and prefrontal systems.
As this is the first functional neuroimaging study to investigate object processing in BDD, other similar studies are not available for comparison. However, other fMRI studies of other-face processing(Feusner et al., 2007b) and own-face processing(Feusner et al., 2010) have demonstrated both similar and different patterns. For example, in the own-face processing study, the BDD group had similar relative visual cortical hypoactivity in left primary and secondary visual processing regions for LSF images as in this study, although in posterior and inferior regions of the occipital cortex(Feusner et al., 2010). In contrast to the findings in this study, there were no significant differences from controls for HSF stimuli. In that study subjective aversiveness of faces was inversely associated with activity in bilateral dorsal lateral occipital cortex and precuneous for LSF images. In addition, greater BDD symptom severity was inversely associated with activity in the left dorsal occipital cortex and the right lateral occipital cortex for the LSF images, while in this study there were similar inverse correlations although with HSF and NSF images.
In the other-face processing study, the BDD group, compared to healthy controls, had greater left hemisphere activity in temporal, parietal, and prefrontal regions compared to healthy controls; group differences were most prominent for LSF stimuli but were also evident for NSF and to a lesser extent HSF stimuli(Feusner et al., 2007b). In contrast, in the current study there was lesser left hemisphere activity for LSF images in temporal regions. In the other-face study there was relative hypoactivity in the left and right occipital cortex for NSF stimuli, as in the current study, although not in the dorsal visual stream. Greater BDD symptom severity was found to be associated with lesser activity in left occipital and parieto-occipital (dorsal) regions for LSF other-face images(Feusner et al., 2007a), although not for HSF and NSF images as in the current study.
In total, these own- and other-face studies and the current houses study share evidence of abnormal visual cortical hypoactivity, in addition to lesser dorsal visual stream activity associated with greater symptom severity. However, the observation that specific aspects of abnormal visual processing in BDD are not identical across house, own-face, and other-face stimuli may be due to the differential relevance in the context of their symptoms. For example own-face stimuli are directly relevant to core appearance concerns regarding the face, and therefore may evoke emotional arousal and/or urges to engage in compulsive behaviors. Other-face stimuli may also be relevant to their symptoms, albeit indirectly, as individuals with BDD typically engage in scrutiny of others' faces to compare to their own. These symptom-relevant stimuli may engage prefrontal and/or frontostriatal systems and may lead to secondary top-down modulation of visual processing systems. House stimuli, which are not related to appearance preoccupations and are less likely to elicit emotional arousal or urges to engage behavioral sequences, therefore may be less likely to result in top-down modulation (however, this remains to be tested directly). Visual cortical abnormalities in the current study of house processing suggest the possibility of general, lower-level visual processing abnormalities in BDD, although it is still possible that top-down attentional modulation may affect these secondary visual systems.
Similar experiments using non-face/non-body objects have not yet been conducted in other disorders that share overlapping phenotypes with BDD, such as OCD, other anxiety disorders, and eating disorders. However, neuropsychological studies of visuospatial functioning have found similar detail-focused, piecemeal processing strategies on the Rey-Osterrieth Complex Figure Task in BDD(Deckersbach et al., 2000), OCD(Mataix-Cols et al., 2003, Savage et al., 2000), and anorexia nervosa(Sherman et al., 2006). These implicate abnormalities in executive functioning and/or visual memory encoding(Deckersbach et al., 2000). In addition, several neuroimaging studies in OCD have found possible abnormalities in visual processing systems including below normal glucose metabolic activity in parieto-occipital regions(Kwon et al., 2003, Nordahl et al., 1989), and abnormal hyperactivity when viewing biological motion in the ventral visual stream (left fusiform and left inferior temporal gyrus)(Jung et al., 2009). Future studies in these related disorders using similar stimuli as in the current study may be useful to explore possible shared phenotypes or endophenotypes of abnormal visual processing.
Behaviorally, the BDD group was slower on all tasks including the control task. It is unlikely that the results obtained for the BOLD signal were affected by offset of timing behaviorally because it was a blocked design and because we used temporal derivatives. Moreover, using response times as a covariate of non-interest, the findings in prefrontal regions for HSF images were essentially unchanged. Findings in secondary visual processing systems for LSF images were also essentially unchanged, although the power was reduced. This suggests that the between-groups differences are not just a function of slower behavioral responses for configural/holistic information.
This study has several limitations. The cross-sectional design precludes causal understandings of whether these abnormalities represent an endophenotype that predisposes individuals to develop BDD, or are secondary results of having BDD symptoms. Another limitation is that the temporal resolution of fMRI does not allow a determination of whether observed abnormalities are the result of top-down modulation of temporal and occipital systems. The sample size may have limited our power to detect small differences between groups, (however, this may not have been the case as a different study of 16 individuals with autism spectrum and 16 healthy controls using similar house stimuli found statistically significant differences of 0.02% signal change)(Bird et al., 2006). Due to the exploratory nature of this study as the first to investigate non-face/non-body object processing in BDD, and the fact that our hypotheses involved multiple regions that may be involved in distributed networks, we did not utilize a priori regions of interest to make use of small-volume corrections. It is possible that group differences in brain activity may have been influenced by different behavioral eye-tracking patterns; the use of an eye-tracking camera in future studies would help clarify this.
In conclusion, this study suggests that unmedicated individuals with BDD may have general abnormalities in higher- and lower-order visual processing, beyond that for their own appearance or for faces in general. These findings are consistent with a model of imbalances in global vs. local processing, which may exist for symptom-related and non-related visual stimuli. Future studies using techniques with higher temporal resolution such as EEG may help elucidate whether visual cortical abnormalities are the result of top-down modulation. Future studies will also be useful to characterize the inherited vs. acquired nature of these findings, for example, by studying unaffected first degree relatives or twins, or by studying individuals very early in their development of the disorder.
Supplementary Material
Acknowledgments
Funding for this study was provided by the National Institute for Mental Health (5K23MH079212 to Dr. Feusner).
We would like to thank Dr. Susan Bookheimer, Ph.D and Courtney Sheen, M.A. for their comments on the manuscript.
Footnotes
Results presented in part at the 18th European Congress of Psychiatry; March, 2010: Munich, Germany
The authors have no conflicts of interest to report.
References
- Aguirre GK, Zarahn E, D'Esposito M. The variability of human, BOLD hemodynamic responses. Neuroimage. 1998;8:360–9. doi: 10.1006/nimg.1998.0369. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-IV-TR. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL. Evidence for the default network's role in spontaneous cognition. Journal of Neurophysiology. 2010;104:322–35. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann M, Jenkinson M, Smith S. General multi-level linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Bird G, Catmur C, Silani G, Frith C, Frith U. Attention does not modulate neural responses to social stimuli in autism spectrum disorders. Neuroimage. 2006;31:1614–24. doi: 10.1016/j.neuroimage.2006.02.037. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The Brain's Default Network: Anatomy, Function, and Relevance to Disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buhlmann U, Glaesmer H, Mewes R, Fama JM, Wilhelm S, Brahler E, Rief W. Updates on the prevalence of body dysmorphic disorder: a population-based survey. Psychiatry Research. 2010;178:171–5. doi: 10.1016/j.psychres.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Castelli F, Happe F, Frith U, Frith C. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage. 2000;12:314–25. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE. Selective and divided attention during visual discriminations of shape, color, and speed: functional anatomy by positron emission tomography. Journal of Neuroscience. 1991;11:2383–402. doi: 10.1523/JNEUROSCI.11-08-02383.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costen NP, Parker DM, Craw I. Effects of high-pass and low-pass spatial filtering on face identification. Perception & Psychophysics. 1996;58:602–12. doi: 10.3758/bf03213093. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Savage C, Phillips K, Wilhelm S, Buhlmann U, Rauch S, Baer L, Jenike M. Characteristics of memory dysfunction in body dysmorphic disorder. Journal of the International Neuropsychological Society. 2000;6:673–81. doi: 10.1017/s1355617700666055. [DOI] [PubMed] [Google Scholar]
- Durand JB, Nelissen K, Joly O, Wardak C, Todd JT, Norman JF, Janssen P, Vanduffel W, Orban GA. Anterior regions of monkey parietal cortex process visual 3D shape. Neuron. 2007;55:493–505. doi: 10.1016/j.neuron.2007.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N. The parahippocampal place area: recognition, navigation, or encoding? Neuron. 1999;23:115–25. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- Ewbank MP, Schluppeck D, Andrews TJ. fMR-adaptation reveals a distributed representation of inanimate objects and places in human visual cortex. Neuroimage. 2005;28:268–79. doi: 10.1016/j.neuroimage.2005.06.036. [DOI] [PubMed] [Google Scholar]
- Faravelli C, Salvatori S, Galassi F, Aiazzi L, Drei C, Cabras P. Epidemiology of somatoform disorders: a community survey in Florence. Social Psychiatry and Psychiatric Epidemiology. 1997;32:24–9. doi: 10.1007/BF00800664. [DOI] [PubMed] [Google Scholar]
- Farivar R. Dorsal-ventral integration in object recognition. Brain Research and Reviews. 2009;61:144–53. doi: 10.1016/j.brainresrev.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Feusner JD, Moody T, Townsend J, McKinley M, Hembacher E, Moller H, Bookheimer S. Abnormalities of visual processing and fronto-striatal systems in body dysmorphic disorder. Archives of General Psychiatry. 2010;67:197–205. doi: 10.1001/archgenpsychiatry.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feusner JD, Townsend J, Bystritsky A, Bookheimer S. Correlation between symptoms of body dysmorphic disorder and brain activation patterns with visual processing: preliminary results. Organization for Human Brain Mapping 13th Annual Meeting; Chicago, IL. 2007a. [Google Scholar]
- Feusner JD, Townsend J, Bystritsky A, Bookheimer S. Visual information processing of faces in body dysmorphic disorder. Archives of General Psychiatry. 2007b;64:1417–25. doi: 10.1001/archpsyc.64.12.1417. [DOI] [PubMed] [Google Scholar]
- Grady CL, Horwitz B, Pietrini P, Mentis MJ, Ungerleider LG, Rapoport SI, Haxby JV. Effect of task difficulty on cerebral blood flow during perceptual matching of faces. Human Brain Mapping. 1996;4:227–39. doi: 10.1002/(SICI)1097-0193(1996)4:4<227::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Gunstad J, Phillips KA. Axis I comorbidity in body dysmorphic disorder. Comprehensive Psychiatry. 2003;44:270–6. doi: 10.1016/S0010-440X(03)00088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences U S A. 2001;98:4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, & Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. Diagnosis and rating of anxiety. British Journal of Psychiatry. 1969;3:76–79. [Google Scholar]
- Hanes K. Neuropsychological performance in body dysmorphic disorder. Journal of the International Neuropsychological Society. 1998;4:167–71. doi: 10.1017/s1355617798001672. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Horwitz B, Ungerleider LG, Maisog JM, Pietrini P, Grady CL. The functional organization of human extrastriate cortex: a PET-rCBF study of selective attention to faces and locations. Journal of Neuroscience. 1994;14:6336–53. doi: 10.1523/JNEUROSCI.14-11-06336.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Ungerleider LG, Horwitz B, Rapoport SI, Grady CL. Hemispheric differences in neural systems for face working memory: A PET-rCBF study. Human Brain Mapping. 1995;3:68–82. [Google Scholar]
- Iidaka T, Yamashita K, Kashikura K, Yonekura Y. Spatial frequency of visual image modulates neural responses in the temporo-occipital lobe. An investigation with event-related fMRI. Cognitive Brain Research. 2004;18:196–204. doi: 10.1016/j.cogbrainres.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Martin A, Haxby JV. The representation of objects in the human occipital and temporal cortex. Journal of Cognitive Neuroscience. 2000;12(2):35–51. doi: 10.1162/089892900564055. [DOI] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Martin A, Schouten JL, Haxby JV. Distributed representation of objects in the human ventral visual pathway. Proceedings of the National Academy of Sciences U S A. 1999;96:9379–84. doi: 10.1073/pnas.96.16.9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung WH, Gu BM, Kang DH, Park JY, Yoo SY, Choi CH, Lee JM, Kwon JS. BOLD response during visual perception of biological motion in obsessive-compulsive disorder : an fMRI study using the dynamic point-light animation paradigm. European Archives of Psychiatry and Clinical Neuroscience. 2009;259:46–54. doi: 10.1007/s00406-008-0833-8. [DOI] [PubMed] [Google Scholar]
- Koran LM, Abujaoude E, Large MD, Serpe RT. The prevalence of body dysmorphic disorder in the United States adult population. CNS Spectrums. 2008;13:316–22. doi: 10.1017/s1092852900016436. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Alpert NM, Thompson WL, Chabris CF, Rauch SL, Anderson AK. Identifying objects seen from different viewpoints. A PET investigation. Brain. 1994;117(Pt 5):1055–71. doi: 10.1093/brain/117.5.1055. [DOI] [PubMed] [Google Scholar]
- Kwon JS, Kim JJ, Lee DW, Lee JS, Lee DS, Kim MS, Lyoo IK, Cho MJ, Lee MC. Neural correlates of clinical symptoms and cognitive dysfunctions in obsessive-compulsive disorder. Psychiatry Research. 2003;122:37–47. doi: 10.1016/s0925-4927(02)00104-x. [DOI] [PubMed] [Google Scholar]
- Mancuso SG, Knoesen NP, Castle DJ. Delusional versus nondelusional body dysmorphic disorder. Comprehensive Psychiatry. 2010;51:177–82. doi: 10.1016/j.comppsych.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Alonso P, Hernandez R, Deckersbach T, Savage CR, Manuel Menchon J, Vallejo J. Relation of neurological soft signs to nonverbal memory performance in obsessive-compulsive disorder. Journal of Clinical and Experimental Neuropsychology. 2003;25:842–51. doi: 10.1076/jcen.25.6.842.16470. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Paulesu E, Frackowiak RS, Frith CD. Brain activity during stimulus independent thought. Neuroreport. 1996;7:2095–9. [PubMed] [Google Scholar]
- McIntosh AR, Grady CL, Haxby JV, Ungerleider LG, Horwitz B. Changes in limbic and prefrontal functional interactions in a working memory task for faces. Cerebral Cortex. 1996;6:571–84. doi: 10.1093/cercor/6.4.571. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Benkelfat C, Semple WE, Gross M, King AC, Cohen RM. Cerebral glucose metabolic rates in obsessive compulsive disorder. Neuropsychopharmacology. 1989;2:23–8. doi: 10.1016/0893-133x(89)90003-1. [DOI] [PubMed] [Google Scholar]
- Norman J, Ehrlich S. Spatial frequency filtering and target identification. Vision Research. 1987;27:87–96. doi: 10.1016/0042-6989(87)90145-3. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Otto MW, Wilhelm S, Cohen LS, Harlow BL. Prevalence of body dysmorphic disorder in a community sample of women. American Journal of Psychiatry. 2001;158:2061–3. doi: 10.1176/appi.ajp.158.12.2061. [DOI] [PubMed] [Google Scholar]
- Petrides M. Specialized systems for the processing of mnemonic information within the primate frontal cortex. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 1996;351:1455–61. doi: 10.1098/rstb.1996.0130. discussion 1461-2. [DOI] [PubMed] [Google Scholar]
- Peuskens H, Claeys KG, Todd JT, Norman JF, Van Hecke P, Orban GA. Attention to 3-D shape, 3-D motion, and texture in 3-D structure from motion displays. Journal of Cognitive Neuroscience. 2004;16:665–82. doi: 10.1162/089892904323057371. [DOI] [PubMed] [Google Scholar]
- Phillips KA. The Broken Mirror. Oxford University Press; New York: 2005. [Google Scholar]
- Phillips KA, Atala KD, Pope HG., Jr . Diagnostic Instruments for body dysmorphic disorder. In: A. P. Association, editor. American Psychiatric Association 148th Annual Meeting. Miami, FL: 1995. p. 157. [Google Scholar]
- Phillips KA, Coles ME, Menard W, Yen S, Fay C, Weisberg RB. Suicidal ideation and suicide attempts in body dysmorphic disorder. Journal of Clinical Psychiatry. 2005a;66:717–25. doi: 10.4088/jcp.v66n0607. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Diaz SF. Gender differences in body dysmorphic disorder. Journal of Nervous and Mental Disorders. 1997;185:570–7. doi: 10.1097/00005053-199709000-00006. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Hollander E, Rasmussen SA, Aronowitz BR, DeCaria C, Goodman WK. A severity rating scale for body dysmorphic disorder: development, reliability, and validity of a modified version of the Yale-Brown Obsessive Compulsive Scale. Psychopharmacology Bulletin. 1997;33:17–22. [PubMed] [Google Scholar]
- Phillips KA, McElroy SL, Keck PE, Jr, Pope HG, Jr, Hudson JI. Body dysmorphic disorder: 30 cases of imagined ugliness. American Journal of Psychiatry. 1993;150:302–8. doi: 10.1176/ajp.150.2.302. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Menard W, Fay C, Weisberg R. Demographic characteristics, phenomenology, comorbidity, and family history in 200 individuals with body dysmorphic disorder. Psychosomatics. 2005b;46:317–25. doi: 10.1176/appi.psy.46.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R. Tools of the trade, region of interest analysis for fMRI. SCAN. 2007;2:67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourtois G, Schwartz S, Seghier ML, Lazeyras F, Vuilleumier P. View-independent coding of face identity in frontal and temporal cortices is modulated by familiarity: an event-related fMRI study. Neuroimage. 2005;24:1214–24. doi: 10.1016/j.neuroimage.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Schwartz S, Spiridon M, Martuzzi R, Vuilleumier P. Object representations for multiple visual categories overlap in lateral occipital and medial fusiform cortex. Cerebral Cortex. 2009;19:1806–19. doi: 10.1093/cercor/bhn210. [DOI] [PubMed] [Google Scholar]
- Rief W, Buhlmann U, Wilhelm S, Borkenhagen A, Brahler E. The prevalence of body dysmorphic disorder: a population-based survey. Psychological Medicine. 2006;36:877–85. doi: 10.1017/S0033291706007264. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Nixon PD, Eacott MJ, Passingham RE. Ventral prefrontal cortex is not essential for working memory. Journal of Neuroscience. 1997;17:4829–38. doi: 10.1523/JNEUROSCI.17-12-04829.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage C, Deckersbach T, Wilhelm S, Rauch S, Baer L, Reid T, Jenike M. Strategic processing and episodic memory impairment in obsessive-compulsive disorder. Neuropsychology. 2000;14:141–151. doi: 10.1037//0894-4105.14.1.141. [DOI] [PubMed] [Google Scholar]
- Schyns P, Oliva A. Dr. Angry and Mr. Smile: when categorization flexibly modifies the perception of faces in rapid visual presentations. Cognition. 1999;69:243–65. doi: 10.1016/s0010-0277(98)00069-9. [DOI] [PubMed] [Google Scholar]
- Sereno ME, Trinath T, Augath M, Logothetis NK. Three-dimensional shape representation in monkey cortex. Neuron. 2002;33:635–52. doi: 10.1016/s0896-6273(02)00598-6. [DOI] [PubMed] [Google Scholar]
- Sergent J. Influence of task and input factors on hemispheric involvement in face processing. Journal of Experimental Psychology Human Perception Performance. 1985;11:846–61. doi: 10.1037//0096-1523.11.6.846. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59(20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- Sherman B, Savage C, Eddy K, Blais M, Deckersbach T, Jackson S, Franko D, Rauch S, Herzog D. Strategic memory in adults with anorexia nervosa: are there similarities to obsessive compulsive spectrum disorders? International Journal of Eating Disorders. 2006;39:468–76. doi: 10.1002/eat.20300. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of Visual Behavior. MIT Press; Cambridge: 1982. pp. 549–586. [Google Scholar]
- Veale D, Boocock A, Gournay K, Dryden W, Shah F, Willson R, Walburn J. Body dysmorphic disorder. A survey of fifty cases. British Journal of Psychiatry. 1996;169:196–201. doi: 10.1192/bjp.169.2.196. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Bachevalier J, Ungerleider LG. Connections of inferior temporal areas TEO and TE with parietal and frontal cortex in macaque monkeys. Cerebral Cortex. 1994;4:470–83. doi: 10.1093/cercor/4.5.470. [DOI] [PubMed] [Google Scholar]
- Woolrich M, Behrens T, Beckmann M, Jenkinson M, Smith S. Multi-level linear modeling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich M, Brady M, Smith S. Hierarchical fully Bayesian spatio-temporal analysis of FMRI data; Seventh International Conference on Functional Mapping of the Human Brain; Brighton, UK. 2001. [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. Journal of Cerebral Blood Flow and Metabolism. 1992;12:900–18. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Yaryura-Tobias J, Neziroglu F, Chang R, Lee S, Pinto A, Donohue L. Computerized perceptual analysis of patients with body dysmorphic disorder. CNS Spectrums. 2002;7:444–446. doi: 10.1017/s1092852900017958. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.