Abstract
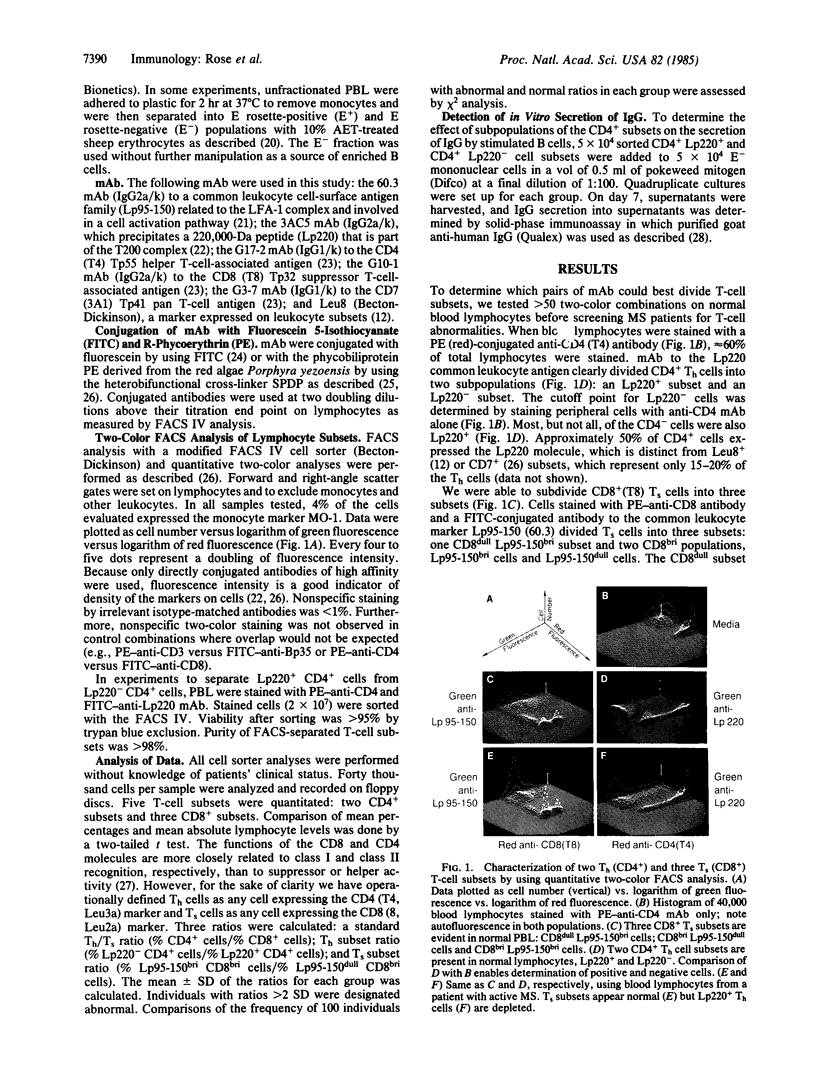
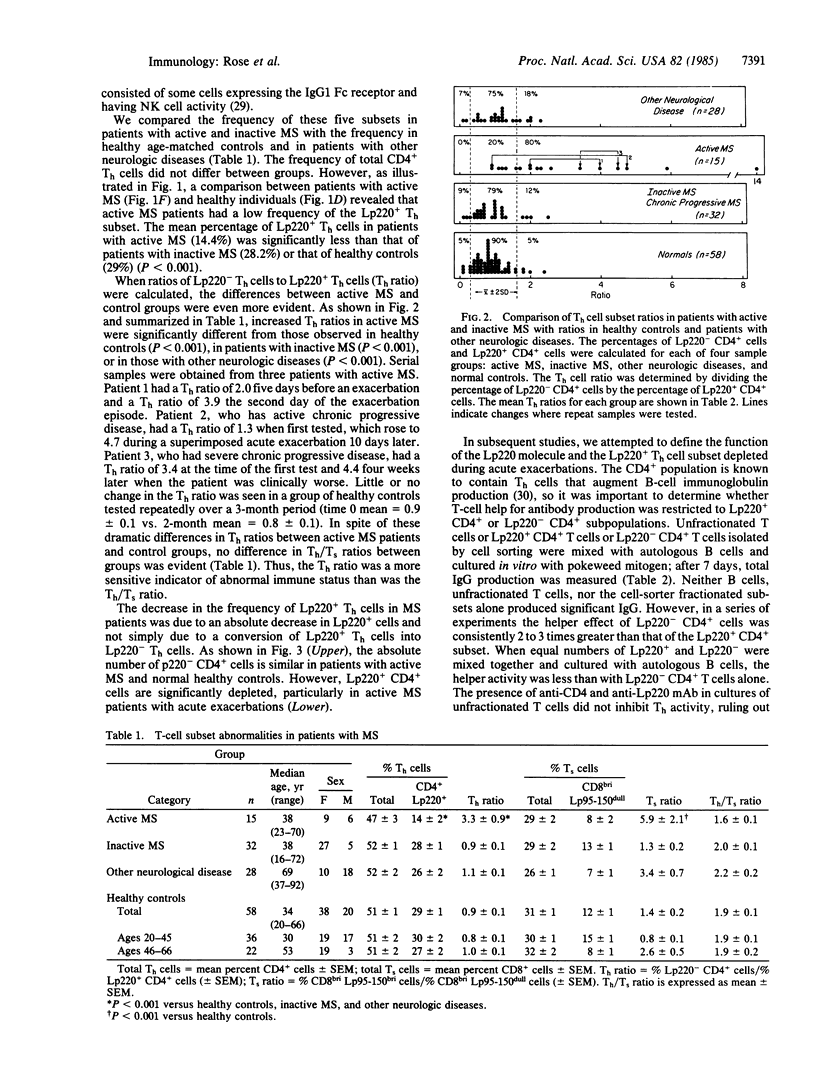
Patients with active multiple sclerosis (MS) have a selective loss of a subset of T helper cells (Th), detectable by two-color fluorescence-activated cell sorter analysis of peripheral blood lymphocytes. By using pairs of monoclonal antibodies to the T-cell subset markers CD4 (Th) and CD8 [T suppressor/cytotoxic cell (Ts)] and the common leukocyte markers Lp220 and Lp95-150, five phenotypically distinct T-cell subsets have been identified in peripheral blood: two CD4+ Th cell subsets and three CD8+ Ts cell subsets. The frequencies and absolute numbers of these five populations were measured in patients with active and inactive MS and were compared with those in healthy age-matched controls and in patients with other neurologic diseases. A high frequency of patients with active MS (80%) had a selective reduction of one Th subset (CD4+ Lp220+) compared with normal controls (P less than 0.001) or patients with inactive MS (P less than 0.001). Three patients examined sequentially had a further loss of the Lp220+ Th subset as disease activity progressed. The proportion of two Ts subsets was also abnormal in patients with active MS, but this defect was not restricted to that group. Total Th and Ts cell frequencies and Th/Ts ratios were not significantly different between patient and normal control groups. Thus, two-color analysis of T-cell subsets may be a more sensitive indicator than conventional single-marker assays of abnormal immune status in MS patients.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beatty P. G., Ledbetter J. A., Martin P. J., Price T. H., Hansen J. A. Definition of a common leukocyte cell-surface antigen (Lp95-150) associated with diverse cell-mediated immune functions. J Immunol. 1983 Dec;131(6):2913–2918. [PubMed] [Google Scholar]
- Clark E. A., Shu G., Ledbetter J. A. Role of the Bp35 cell surface polypeptide in human B-cell activation. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1766–1770. doi: 10.1073/pnas.82.6.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey J. L., Detels R., Gottlieb M. Immune-cell augmentation (with altered T-subset ratio) is common in healthy homosexual men. N Engl J Med. 1983 Apr 7;308(14):842–843. doi: 10.1056/NEJM198304073081414. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Conjugation of antibodies with fluorochromes: modifications to the standard methods. J Immunol Methods. 1976;13(3-4):215–226. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- Hauser S. L., Bahn A. K., Che M., Gilles F., Weiner H. L. Redistribution of Lyt-bearing T cells in acute murine experimental allergic encephalomyelitis: selective migration of Lyt-1 cells to the central nervous system is associated with a transient depletion of Lyt-1 cells in peripheral blood. J Immunol. 1984 Dec;133(6):3037–3042. [PubMed] [Google Scholar]
- Hauser S. L., Reinherz E. L., Hoban C. J., Schlossman S. F., Weiner H. L. Immunoregulatory T-cells and lymphocytotoxic antibodies in active multiple sclerosis: weekly analysis over a six-month period. Ann Neurol. 1983 Apr;13(4):418–425. doi: 10.1002/ana.410130408. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Honda M., Herzenberg L. A., Steinberg A. D., Herzenberg L. A. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2494–2498. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Parks D. R., Herzenberg L. A. The "Ly-1 B" cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983 Jan 1;157(1):202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURTZKE J. F. FURTHER NOTES ON DISABILITY EVALUATION IN MULTIPLE SCLEROSIS, WITH SCALE MODIFICATIONS. Neurology. 1965 Jul;15:654–661. doi: 10.1212/wnl.15.7.654. [DOI] [PubMed] [Google Scholar]
- Kastrukoff L. F., Paty D. W. A serial study of peripheral blood T lymphocyte subsets in relapsing-remitting multiple sclerosis. Ann Neurol. 1984 Mar;15(3):250–256. doi: 10.1002/ana.410150308. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Loken M. R. Human lymphocyte subpopulations identified by using three-color immunofluorescence and flow cytometry analysis: correlation of Leu-2, Leu-3, Leu-7, Leu-8, and Leu-11 cell surface antigen expression. J Immunol. 1984 Jan;132(1):151–156. [PubMed] [Google Scholar]
- Ledbetter J. A., Rose L. M., Spooner C. E., Beatty P. G., Martin P. J., Clark E. A. Antibodies to common leukocyte antigen p220 influence human T cell proliferation by modifying IL 2 receptor expression. J Immunol. 1985 Sep;135(3):1819–1825. [PubMed] [Google Scholar]
- McFarlin D. E., McFarland H. F. Multiple sclerosis (first of two parts). N Engl J Med. 1982 Nov 4;307(19):1183–1188. doi: 10.1056/NEJM198211043071905. [DOI] [PubMed] [Google Scholar]
- Merrill J. E., Mohlstrom C., Uittenbogaart C., Kermaniarab V., Ellison G. W., Myers L. W. Response to and production of interleukin 2 by peripheral blood and cerebrospinal fluid lymphocytes of patients with multiple sclerosis. J Immunol. 1984 Oct;133(4):1931–1937. [PubMed] [Google Scholar]
- Meuer S. C., Schlossman S. F., Reinherz E. L. Clonal analysis of human cytotoxic T lymphocytes: T4+ and T8+ effector T cells recognize products of different major histocompatibility complex regions. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4395–4399. doi: 10.1073/pnas.79.14.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingioli E. S., McFarlin D. E. Leukocyte surface antigens in patients with multiple sclerosis. J Neuroimmunol. 1984 Apr;6(2):131–139. doi: 10.1016/0165-5728(84)90034-1. [DOI] [PubMed] [Google Scholar]
- Nicholson J. K., McDougal J. S., Spira T. J., Cross G. D., Jones B. M., Reinherz E. L. Immunoregulatory subsets of the T helper and T suppressor cell populations in homosexual men with chronic unexplained lymphadenopathy. J Clin Invest. 1984 Jan;73(1):191–201. doi: 10.1172/JCI111190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oger J., Roos R., Antel J. P. Immunology of multiple sclerosis. Neurol Clin. 1983 Aug;1(3):655–679. [PubMed] [Google Scholar]
- Oi V. T., Glazer A. N., Stryer L. Fluorescent phycobiliprotein conjugates for analyses of cells and molecules. J Cell Biol. 1982 Jun;93(3):981–986. doi: 10.1083/jcb.93.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino M. A., Ferrone S., Dierich M. P., Reisfeld R. A. Enhancement of sheep red blood cell human lymphocyte rosette formation by the sulfhydryl compound 2-amino ethylisothiouronium bromide. Clin Immunol Immunopathol. 1975 Jan;3(3):324–333. doi: 10.1016/0090-1229(75)90019-7. [DOI] [PubMed] [Google Scholar]
- Perussia B., Fanning V., Trinchieri G. A human NK and K cell subset shares with cytotoxic T cells expression of the antigen recognized by antibody OKT8. J Immunol. 1983 Jul;131(1):223–231. [PubMed] [Google Scholar]
- Perussia B., Trinchieri G. Antibody 3G8, specific for the human neutrophil Fc receptor, reacts with natural killer cells. J Immunol. 1984 Mar;132(3):1410–1415. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4061–4065. doi: 10.1073/pnas.76.8.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Morimoto C., Penta A. C., Schlossman S. F. Regulation of B cell immunoglobulin secretion by functional subsets of T lymphocytes in man. Eur J Immunol. 1980 Jul;10(7):570–572. doi: 10.1002/eji.1830100715. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Weiner H. L., Hauser S. L., Cohen J. A., Distaso J. A., Schlossman S. F. Loss of suppressor T cells in active multiple sclerosis. Analysis with monoclonal antibodies. N Engl J Med. 1980 Jul 17;303(3):125–129. doi: 10.1056/NEJM198007173030303. [DOI] [PubMed] [Google Scholar]
- SCHUMACHER G. A., BEEBE G., KIBLER R. F., KURLAND L. T., KURTZKE J. F., MCDOWELL F., NAGLER B., SIBLEY W. A., TOURTELLOTTE W. W., WILLMON T. L. PROBLEMS OF EXPERIMENTAL TRIALS OF THERAPY IN MULTIPLE SCLEROSIS: REPORT BY THE PANEL ON THE EVALUATION OF EXPERIMENTAL TRIALS OF THERAPY IN MULTIPLE SCLEROSIS. Ann N Y Acad Sci. 1965 Mar 31;122:552–568. doi: 10.1111/j.1749-6632.1965.tb20235.x. [DOI] [PubMed] [Google Scholar]
- Santoli D., Moretta L., Lisak R., Gilden D., Koprowski H. Imbalances in T cell subpopulations in multiple sclerosis patients. J Immunol. 1978 Apr;120(4):1369–1371. [PubMed] [Google Scholar]
- Traugott U., Reinherz E. L., Raine C. S. Multiple sclerosis: distribution of T cell subsets within active chronic lesions. Science. 1983 Jan 21;219(4582):308–310. doi: 10.1126/science.6217550. [DOI] [PubMed] [Google Scholar]
- Trotter J., Sriram S., Rassenti L., Chou C. H., Fritz R. B., Steinman L. Characterization of T cell lines and clones from SJL/J and (BALB/c x SJL/J)F1 mice specific for myelin basic protein. J Immunol. 1985 Apr;134(4):2322–2327. [PubMed] [Google Scholar]
- Waldor M. K., Sriram S., Hardy R., Herzenberg L. A., Herzenberg L. A., Lanier L., Lim M., Steinman L. Reversal of experimental allergic encephalomyelitis with monoclonal antibody to a T-cell subset marker. Science. 1985 Jan 25;227(4685):415–417. doi: 10.1126/science.3155574. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Hauser S. L. Neuroimmunology I: Immunoregulation in neurological disease. Ann Neurol. 1982 May;11(5):437–449. doi: 10.1002/ana.410110502. [DOI] [PubMed] [Google Scholar]