Abstract
We demonstrate that therapeutically useful amounts of insulin are absorbed by the nasal mucosa of human beings when administered as a nasal spray with the common bile salts. By employing a series of bile salts with subtle differences in the number, position, and orientation of their nuclear hydroxyl functions and alterations in side chain conjugation, we show that adjuvant potency for nasal insulin absorption correlates positively with increasing hydrophobicity of the bile salts' steroid nucleus. As inferred from studies employing various concentrations of unconjugated deoxycholate and a constant dose of insulin, insulin absorption begins at the aqueous critical micellar concentration of the bile salt and becomes maximal when micelle formation is well established. These and other data are consistent with the complementary hypotheses that bile salts act as absorption adjuvants by producing high juxtamembrane concentrations of insulin monomers via solubilization in mixed bile salt micelles and forming reverse micelles within nasal membranes, through which insulin monomers can diffuse through polar channels from the nares into the blood stream.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altszuler N., Hampshire J. Intranasal instillation of oxytocin increases insulin and glucagon secretion. Proc Soc Exp Biol Med. 1981 Oct;168(1):123–124. doi: 10.3181/00379727-168-41245. [DOI] [PubMed] [Google Scholar]
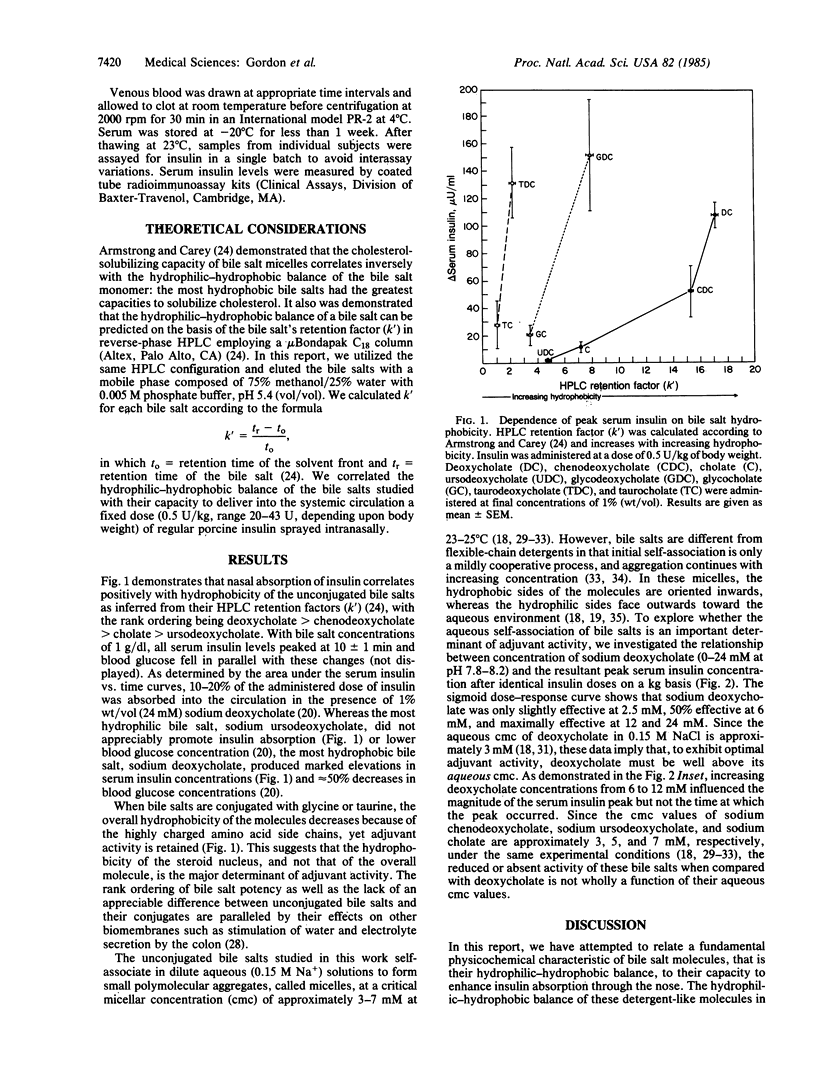
- Armstrong M. J., Carey M. C. The hydrophobic-hydrophilic balance of bile salts. Inverse correlation between reverse-phase high performance liquid chromatographic mobilities and micellar cholesterol-solubilizing capacities. J Lipid Res. 1982 Jan;23(1):70–80. [PubMed] [Google Scholar]
- Baumann G., Walser A., Desaulles P. A., Paesi F. J., Geller L. Corticotropic action of an intra-nasally applied synthetic ACTH derivative. J Clin Endocrinol Metab. 1976 Jan;42(1):60–63. doi: 10.1210/jcem-42-1-60. [DOI] [PubMed] [Google Scholar]
- Berson S. A., Yalow R. S. Insulin in blood and insulin antibodies. Am J Med. 1966 May;40(5):676–690. doi: 10.1016/0002-9343(66)90148-3. [DOI] [PubMed] [Google Scholar]
- Blundell T. L., Cutfield J. F., Cutfield S. M., Dodson E. J., Dodson G. G., Hodgkin D. C., Mercola D. A. Three-dimensional atomic structure of insulin and its relationship to activity. Diabetes. 1972;21(2 Suppl):492–505. doi: 10.2337/diab.21.2.s492. [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr The Banting Memorial Lecture 1971. Physiology of insulin in man. Diabetes. 1971 Dec;20(12):785–799. doi: 10.2337/diab.20.12.785. [DOI] [PubMed] [Google Scholar]
- Carey M. C., Montet J. C., Phillips M. C., Armstrong M. J., Mazer N. A. Thermodynamic and molecular basis for dissimilar cholesterol-solubilizing capacities by micellar solutions of bile salts: cases of sodium chenodeoxycholate and sodium ursodeoxycholate and their glycine and taurine conjugates. Biochemistry. 1981 Jun 9;20(12):3637–3648. doi: 10.1021/bi00515a052. [DOI] [PubMed] [Google Scholar]
- Carey M. C., Montet J. C., Small D. M. Surface and solution properties of steroid antibiotics: 3-acetoxylfusidic acid, cephalosporin P1 and helvolic acid. Biochemistry. 1975 Nov 4;14(22):4896–4905. doi: 10.1021/bi00693a018. [DOI] [PubMed] [Google Scholar]
- Castellino F. J., Violand B. N. 31P-nuclear magnetic resonance and 31P(1H) nuclear Overhauser effect analysis of mixed egg phosphatidylcholine-sodium taurocholate vesicles and micelles. Arch Biochem Biophys. 1979 Apr 1;193(2):543–550. doi: 10.1016/0003-9861(79)90061-4. [DOI] [PubMed] [Google Scholar]
- Dobbins J. W., Binder H. J. Effect of bile salts and fatty acids on the colonic absorption of oxalate. Gastroenterology. 1976 Jun;70(6):1096–1100. [PubMed] [Google Scholar]
- Ericsson B., Larsson K., Fontell K. A cubic protein-monoolein-water phase. Biochim Biophys Acta. 1983 Mar 23;729(1):23–27. doi: 10.1016/0005-2736(83)90451-0. [DOI] [PubMed] [Google Scholar]
- Evans W. S., Borges J. L., Kaiser D. L., Vance M. L., Sellers R. P., MacLeod R. M., Vale W., Rivier J., Thorner M. O. Intranasal administration of human pancreatic tumor GH-releasing factor-40 stimulates GH release in normal men. J Clin Endocrinol Metab. 1983 Nov;57(5):1081–1083. doi: 10.1210/jcem-57-5-1081. [DOI] [PubMed] [Google Scholar]
- Gibaldi M. Role of surface-active agents in drug absorption. Fed Proc. 1970 Jul-Aug;29(4):1343–1349. [PubMed] [Google Scholar]
- Harai S., Ikenaga T., Matsuzawa T. Nasal absorption of insulin in dogs. Diabetes. 1978 Mar;27(3):296–299. doi: 10.2337/diab.27.3.296. [DOI] [PubMed] [Google Scholar]
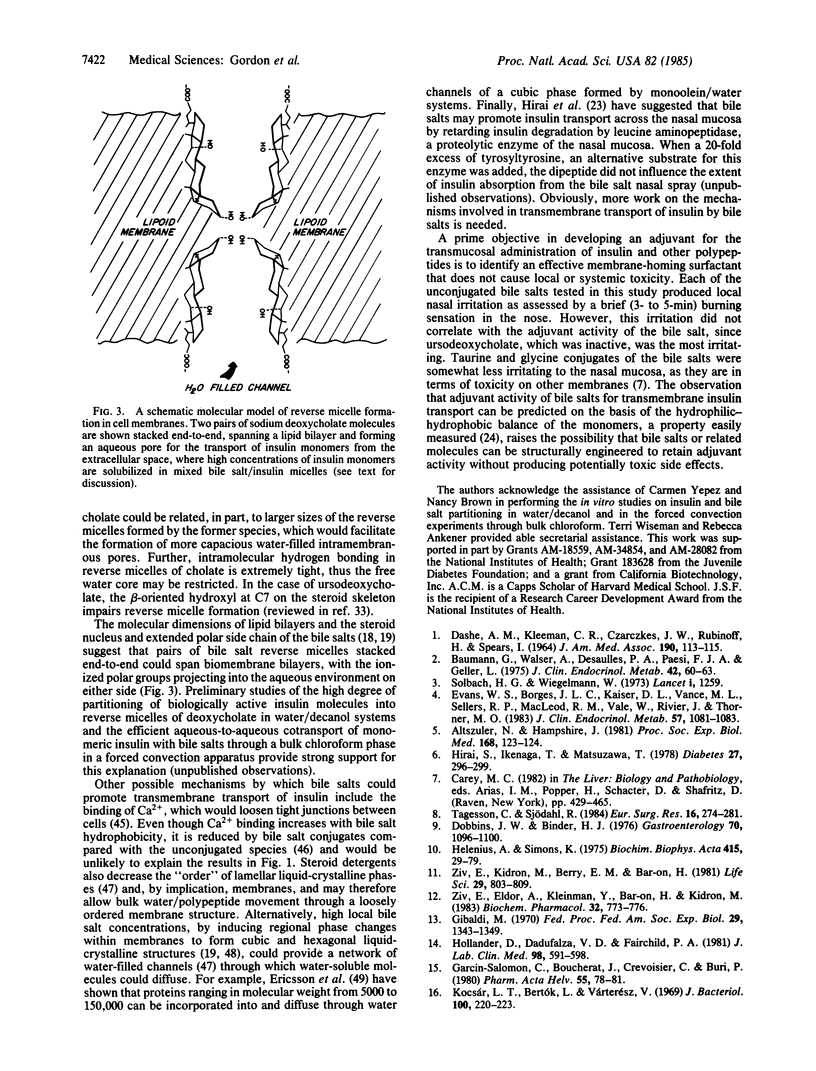
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Hisadome T., Nakama T., Itoh H., Furusawa T. Physical-chemical properties of chenodeoxycholic acid and ursodeoxycholic acid. Gastroenterol Jpn. 1980;15(3):257–263. doi: 10.1007/BF02774276. [DOI] [PubMed] [Google Scholar]
- Hollander D., Dadufalza V. D., Fairchild P. A. Intestinal absorption of aspirin. Influence of pH, taurocholate, ascorbate, and ethanol. J Lab Clin Med. 1981 Oct;98(4):591–598. [PubMed] [Google Scholar]
- Hunt G. R. A comparison of triton X-100 and the bile salt taurocholate as micellar ionophores or fusogens in phospholipid vesicular membranes. A 1H NMR method using the lanthanide probe ion Pr3+. FEBS Lett. 1980 Sep 22;119(1):132–136. doi: 10.1016/0014-5793(80)81014-3. [DOI] [PubMed] [Google Scholar]
- Hyslop P. A., York D. A., Sauerheber R. D. Effects of insulin on the lipid structure of liver plasma membrane measured with fluorescence and ESR spectroscopic methods. Biochim Biophys Acta. 1984 Oct 3;776(2):267–278. doi: 10.1016/0005-2736(84)90216-5. [DOI] [PubMed] [Google Scholar]
- Igimi H., Carey M. C. pH-Solubility relations of chenodeoxycholic and ursodeoxycholic acids: physical-chemical basis for dissimilar solution and membrane phenomena. J Lipid Res. 1980 Jan;21(1):72–90. [PubMed] [Google Scholar]
- Martindale H., Marsh J., Hallett F. R., Albisser A. M. Examination of insulin formulations using quasi-elastic light scattering. Diabetes. 1982 Apr;31(4 Pt 1):364–366. doi: 10.2337/diab.31.4.364. [DOI] [PubMed] [Google Scholar]
- Mazer N. A., Carey M. C., Kwasnick R. F., Benedek G. B. Quasielastic light scattering studies of aqueous biliary lipid systems. Size, shape, and thermodynamics of bile salt micelles. Biochemistry. 1979 Jul 10;18(14):3064–3075. doi: 10.1021/bi00581a024. [DOI] [PubMed] [Google Scholar]
- Mekjian H. S., Phillips S. F., Hofmann A. F. Colonic secretion of water and electrolytes induced by bile acids: perfusion studies in man. J Clin Invest. 1971 Aug;50(8):1569–1577. doi: 10.1172/JCI106644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses A. C., Gordon G. S., Carey M. C., Flier J. S. Insulin administered intranasally as an insulin-bile salt aerosol. Effectiveness and reproducibility in normal and diabetic subjects. Diabetes. 1983 Nov;32(11):1040–1047. doi: 10.2337/diab.32.11.1040. [DOI] [PubMed] [Google Scholar]
- Mukerjee P., Moroi Y., Murata M., Yang A. Y. Bile salts as atypical surfactants and solubilizers. Hepatology. 1984 Sep-Oct;4(5 Suppl):61S–65S. doi: 10.1002/hep.1840040811. [DOI] [PubMed] [Google Scholar]
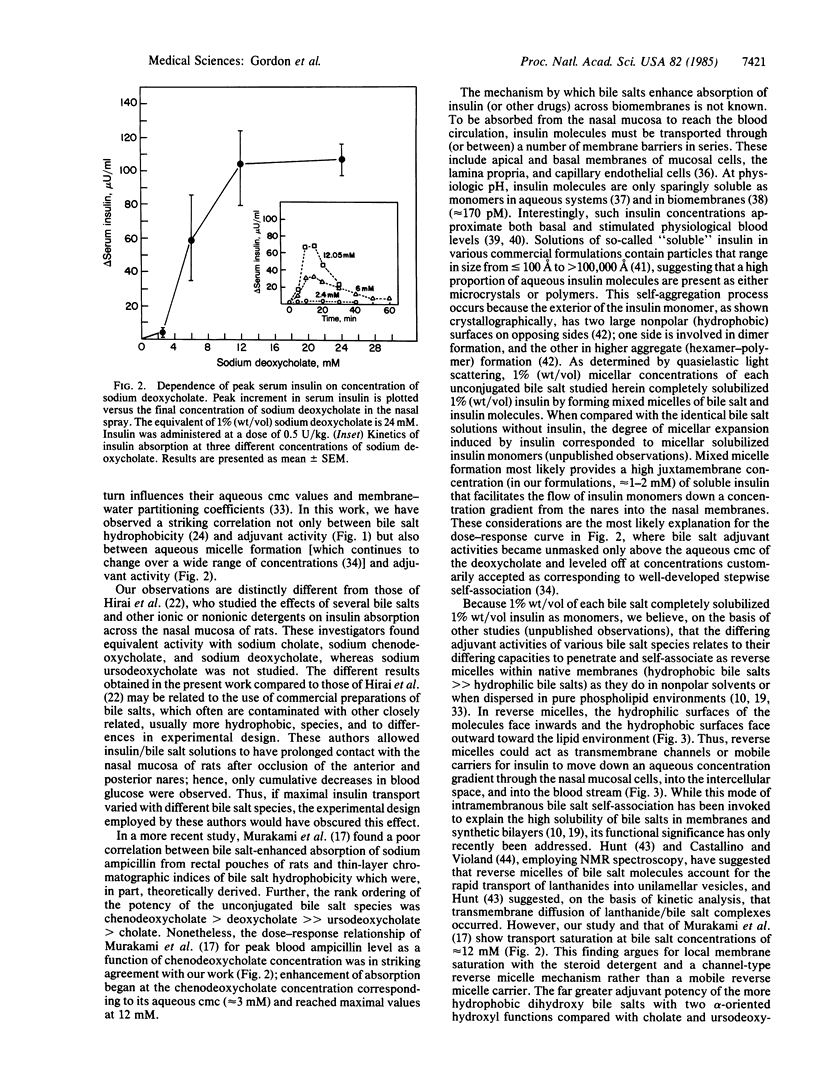
- Murakami T., Sasaki Y., Yamajo R., Yata N. Effect of bile salts on the rectal absorption of sodium ampicillin in rats. Chem Pharm Bull (Tokyo) 1984 May;32(5):1948–1955. doi: 10.1248/cpb.32.1948. [DOI] [PubMed] [Google Scholar]
- Pontiroli A. E., Alberetto M., Secchi A., Dossi G., Bosi I., Pozza G. Insulin given intranasally induces hypoglycaemia in normal and diabetic subjects. Br Med J (Clin Res Ed) 1982 Jan 30;284(6312):303–306. doi: 10.1136/bmj.284.6312.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope J. L. Crystallization of sodium taurocholate. J Lipid Res. 1967 Mar;8(2):146–147. [PubMed] [Google Scholar]
- Roda A., Hofmann A. F., Mysels K. J. The influence of bile salt structure on self-association in aqueous solutions. J Biol Chem. 1983 May 25;258(10):6362–6370. [PubMed] [Google Scholar]
- Small D. M., Bourgès M., Dervichian D. G. Ternary and quaternary aqueous systems containing bile salt, lecithin, and cholesterol. Nature. 1966 Aug 20;211(5051):816–818. doi: 10.1038/211816a0. [DOI] [PubMed] [Google Scholar]
- Solbach H. G., Wiegelman W. Intranasal application of luteinising-hormone releasing hormone. Lancet. 1973 Jun 2;1(7814):1259–1259. doi: 10.1016/s0140-6736(73)90581-3. [DOI] [PubMed] [Google Scholar]
- Tagesson C., Sjödahl R. Passage of molecules through the wall of the gastrointestinal tract. Intestinal permeability to polyethylene glycols in the 414- to 1,074-dalton range. Eur Surg Res. 1984;16(5):274–281. doi: 10.1159/000128419. [DOI] [PubMed] [Google Scholar]
- Ulmius J., Lindblom G., Wennerström H., Johansson L. B., Fontell K., Söderman O., Arvidson G. Molecular organization in the liquid--crystalline phases of lecithin--sodium cholate-water systems studied by nuclear magnetic resonance. Biochemistry. 1982 Mar 30;21(7):1553–1560. doi: 10.1021/bi00536a014. [DOI] [PubMed] [Google Scholar]
- Ziv E., Eldor A., Kleinman Y., Bar-On H., Kidron M. Bile salts facilitate the absorption of heparin from the intestine. Biochem Pharmacol. 1983 Mar 1;32(5):773–776. doi: 10.1016/0006-2952(83)90575-0. [DOI] [PubMed] [Google Scholar]
- Ziv E., Kidron M., Berry E. M., Bar-On H. Bile salts promote the absorption of insulin from the rat colon. Life Sci. 1981 Aug 24;29(8):803–809. doi: 10.1016/0024-3205(81)90036-9. [DOI] [PubMed] [Google Scholar]


