Abstract
The blood-brain barrier (BBB) and blood-cerebrospinal fluid (BCSF) barriers are critical determinants of CNS homeostasis. Additionally, the BBB and BCSF barriers are formidable obstacles to effective CNS drug delivery. These brain barrier sites express putative influx and efflux transporters that precisely control permeation of circulating solutes including drugs. The study of transporters has enabled a shift away from “brute force” approaches to delivering drugs by physically circumventing brain barriers towards chemical approaches that can target specific compounds of the BBB and/or BCSF barrier. However, our understanding of transporters at the BBB and BCSF barriers has primarily focused on understanding efflux transporters that efficiently prevent drugs from attaining therapeutic concentrations in the CNS. Recently, through the characterization of multiple endogenously expressed uptake transporters, this paradigm has shifted to the study of brain transporter targets that can facilitate drug delivery (i.e., influx transporters). Additionally, signaling pathways and trafficking mechanisms have been identified for several endogenous BBB/BCSF transporters, thereby offering even more opportunities to understand how transporters can be exploited for optimization of CNS drug delivery. This review presents an overview of the BBB and BCSF barrier as well as the many families of transporters functionally expressed at these barrier sites. Furthermore, we present an overview of various strategies that have been designed and utilized to deliver therapeutic agents to the brain with a particular emphasis on those approaches that directly target endogenous BBB/BCSF barrier transporters.
Keywords: ATP-binding cassette transporters, Blood-brain barrier, Blood-cerebrospinal fluid barrier, Drug Delivery, Neurovascular Unit, Solute Carriers, Transporters
Introduction
The blood-brain barrier (BBB) is a formidable physical and biochemical barrier to effective drug delivery to the brain, thus limiting the ability to effectively treat central nervous system (CNS) disorders. Within the past decade, intense research efforts have focused on directly targeting the BBB for optimization of drug delivery. Such BBB targets include influx and efflux transporters that are expressed at the level of the brain microvascular endothelium. Instead of physically circumventing the BBB by using mechanical (i.e., “brute-force”) techniques, identification and characterization of transporters enabled development of novel chemical approaches to utilize endogenous barrier components to deliver drugs to the brain. This approach provides a unique opportunity to improve efficacy of existing therapies while promoting development of new ones. In this review we provide an overview of BBB biology and the plethora of transporters expressed at the BBB endothelium. Furthermore, we highlight various techniques that have been developed to circumvent the BBB for CNS drug delivery, with a particular emphasis on opportunities provided by targeting endogenous BBB transporter systems.
The Blood-Brain Barrier/Neurovascular Unit
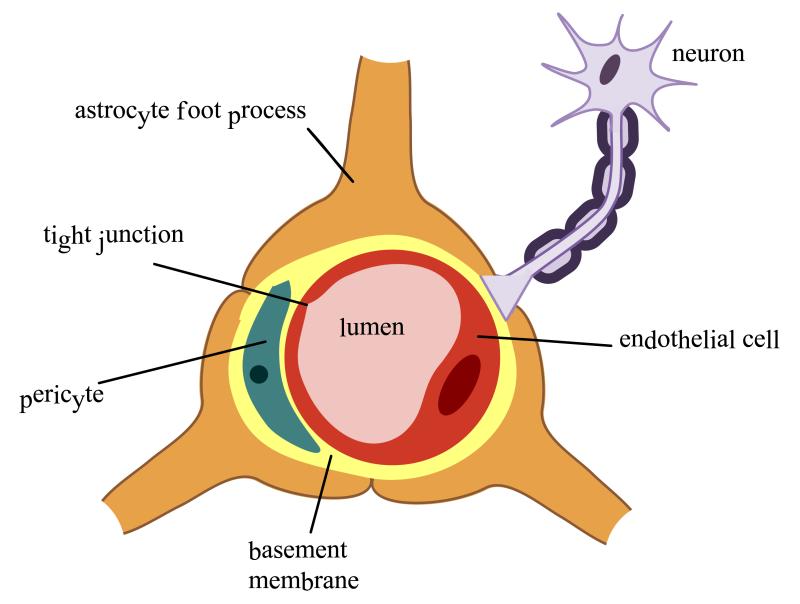
The neurovascular unit (NVU) is comprised of cellular constituents (i.e., endothelial cells, astrocytes, microglia, pericytes, neurons) and extracellular matrix (1). The concept of the NVU emphasizes that both brain function and dysfunction requires coordinated interaction between various NVU components. Disruption of any NVU component, either as a result of a physiological or pharmacological stressor, can alter BBB integrity, subsequently modifying brain microvascular permeability (2,3).
Components of the NVU
a) Endothelial Cells and the Blood-Brain Barrier
The CNS is the most sensitive and critical organ system in the human body. Therefore, proper function requires precise regulation of the brain extracellular milieu. Additionally, CNS metabolic demands are considerable, with the CNS accounting for approximately 20% of oxygen consumption in humans (2). The interface between the brain and the systemic circulation must possess highly selective and efficient mechanisms that are capable of facilitating nutrient transport, regulating ion balance, and providing a barrier to potentially toxic substances. To this end, brain entry of some substances must be permitted while permeation of others must be limited. This homeostatic function of the cerebral microvasculature occurs primarily at the level of brain microvascular endothelial cells, the principal cell type of the BBB.
Compared to peripheral vasculature, BBB endothelial cells are characterized by increased mitochondrial content, exhibit minimal pinocytotic activity, and lack fenestrations (3-5). Increased mitochondrial content is essential for these cells to maintain various active transport mechanisms such as those utilized to transport ions, nutrients, and waste products into and out of brain parenchyma, thus contributing to precise regulation of the CNS microenvironment and ensuring proper neuronal function. Cell polarity of endothelial cells is ascribed to differing functional expression of transporter proteins and metabolic enzymes that are differentially expressed on the luminal and abluminal membranes, which further contribute to the high selectivity of the BBB (6-8).Of the many transporters expressed at the BBB endothelium, several have been implicated in influx and/or efflux of drugs into the CNS. Examples of efflux transporters include P-glycoprotein (P-gp) (9), Breast Cancer Resistance Protein (BCRP in humans; Bcrp in rodents), Multidrug Resistance Proteins (MRPs in humans; Mrps in rodents). Transporters that facilitate drug entry into the brain include organic anion transporting polypeptides (OATPs in humans; Oatps in rodents), organic anion transporters (OATs in humans; Oats in rodents), organic cation transporters (OCTs in humans; Octs in rodents), nucleoside transporters, monocarboxylate transporters (MCTs in humans; Mcts in rodents), and putative transport systems for peptide transport.
BBB function is regulated, both in health and disease, by a variety of circulating mediators that are found in blood. Studies investigating effects of peripheral inflammatory pain (PIP) on BBB function found that PIP increased mRNA and protein levels of ICAM-1, a protein that plays an important role in immune-mediated cell-cell adhesive interactions (10). Increased levels of ICAM-1 were associated with changes in levels of several cytokines in the systemic circulation including IL-10 and INF-γ (10). Additionally, BBB function has been found to be modulated via the TGF-β1/ALK-5 pathway. Under conditions of PIP, altered expression of tight junction (TJ) proteins and transporters were associated with decreased levels of TGF-β1 and decreased expression of ALK-5, a critical TGF-β1 receptor. Changes in TGF- β /ALK5 signaling was also associated with increased BBB paracellular permeability to radiolabeled sucrose and increased expression of Oatp1a4, which resulted in increased brain delivery of taurocholate, an established Oatp substrate (11, 12). Additionally, cytokines have also been found to modulate activity of efflux transporters expressed at the BBB/NVU. Studies investigating endothelin-1 (ET-1) mediated signaling on blood-brain barrier P-glycoprotein-mediated transport revealed that exposure of rat brain capillaries to ET-1 produced a rapid and reversible reduction in P-gp transport activity (13). ET-1 regulation of P-gp was found to act through the endothelin-B receptor and protein kinase C signaling (13).
i.a) Molecular Characteristics of the BBB
1) Adherens Junctions (AJs)
Adherens junctions (AJs) are found throughout the CNS microvasculature and are responsible for intercellular adherence between adjacent endothelial cells (14). AJs are composed of multiple protein components including vascular endothelium (VE) cadherin, actinin, and catenin (8). Cell-cell adhesion is mediated by homophilic interactions of VE-cadherin expressed on adjacent endothelial cells. Such interactions mediate calcium-dependent cell adhesion by binding to the actin cytoskeleton. Cytoskeletal binding occurs via catenin accessory proteins. Specifically, β-catenin links VE-cadherin to α-catenin, an interaction that induces the direct binding to actin (3, 7).
Disruption of protein-protein interactions within AJs can result in decreased BBB functional integrity. For example, VE-cadherin protein expression was decreased in cultured bovine brain endothelial cells subjected to hypoxia/aglycemia conditions (11, 12). Hypoxia/aglycemic conditions also increased transendothelial permeability of the vascular marker 14C-sucrose in this same in vitro model system (15). Competitive inhibition of the catenin family member p120 using an epitope-tagged fragment corresponding to the juxtamembrane domain of VE-cadherin led to decreased interaction with VE-cadherin and a subsequent increase in permeability of albumin across confluent monolayers of bovine pulmonary artery endothelial cells. (16). The p120 catenin protein is a critical mediator of cell-cell adhesion via its direct interaction with VE-cadherin and emphasize the key role of AJs in restricting paracellular permeability across the BBB.
2) Tight Junctions (TJs)
Although disruption of AJs can result in increased BBB permeability, TJs are primarily responsible for restricting paracellular permeability at the BBB (14, 17). TJs form the primary physical barrier component of the BBB and function to greatly restrict paracellular entry of various endogenous and exogenous substances that can potentially be neurotoxic. Such TJs impart a high trans-endothelial electrical resistance (TEER) across the BBB (1500 - 2000 Ω cm2) that restricts free flow of ions and solutes (18). TJs are dynamic complexes of multiple protein constituents including junctional adhesion molecules (JAMs), occludin, claudins (i.e. claudin-1, -3, and -5), and membrane-associated guanylate kinase (MAGUK)-like proteins (i.e. ZO-1, -2 and -3) (14).
Several JAMs have been identified at the BBB including JAM-1, JAM-2, and JAM-3 (14). JAM-1 is believed to mediate early attachment of adjacent endothelial cells during BBB development through homophilic interactions and loss of JAMs is associated with BBB breakdown (19-22). For example, studies in an immortalized human brain endothelial cell line (hCMEC/d3) showed that inflammatory stimuli triggered movement of JAM away from the TJ, an observation that directly correlated with increased dextran leak across the BBB (23). Of particular note, JAMs are also implicated in the regulation of transendothelial migration of leukocytes (20, 24).
Monomeric occludin is a 60-65 kDa protein consisting of four transmembrane domains with two extracellular loops that span the intracellular cleft between the capillary endothelial cells (25). Occludin is highly expressed at the BBB and stains in a continuous pattern along cellular margins of the brain microvasculature (26). Expression of occludin at the TJ is associated with increased TEER, a marker for TJ “tightness” (27). For example, Madin-Darby canine kidney (MDCK) cells expressing a COOH-terminally truncated chicken occludin exhibited an increase in paracellular leak of various sizes of FITC-dextran (i.e., 4 kDa, 40 kDa, and 400 kDa). This increase in paracellular permeability was associated with discontinuous distribution of occludin at the TJ caused by deletion of the COOH-terminal domain of the protein (21, 28). Functional TJ-associated occludin assembles into dimers and oligomers via disulfide bond formation (29). Changes in relative amounts of oligomeric, dimeric, and monomeric occludin have been observed under pathological conditions such as PIP, primarily as an increase in monomeric occludin and a decrease in oligomeric isoforms (30). Such modulation of occludin oligomeric assemblies have been associated with loss of BBB integrity and increased paracellular permeability to vascular markers (i.e., sucrose) and drugs (i.e., codeine) (31). Similar results were observed under conditions of hypoxia and reoxygenation, a component of several pathophysiological conditions such as ischemic stroke (32). Taken together, these studies clearly demonstrate that occludin is a critical regulator of BBB functional integrity, particularly as a restrictor of paracellular solute permeability (25, 26, 27).
At least 24 claudins have been identified in mammalian tissues amongst which claudin-3 and -5 have been detected at the BBB endothelium (33). Claudins are 20 – 24 kDa proteins that have similar membrane topology to occludin, but do not share sequence homology (34). The extracellular loops of the claudins interact through heterophilic and homophilic interactions between adjacent endothelial cells (35). Overexpression of claudin isoforms results in formation of TJ strands in fibroblasts. Interestingly, expression of occludin does not promote formation of TJs. Rather, studies have shown that occludin does not localize to the TJs unless claudins are already localized at the TJ. Thus, it is believed that claudins form the primary “seal” of the tight junctions (19, 36).
BBB functional integrity requires association of transmembrane TJ proteins with accessory proteins localized within the endothelial cell cytoplasm. These include members of the MAGUK family, which includes TJ associated intracellular proteins ZO-1, ZO-2, and ZO-3. Such MAGUK proteins are necessary for clustering of TJ proteins to the cell membrane (37). ZO-1, the first protein identified to be directly associated with TJ complexes (38), is a 222 kDa phosphoprotein that is expressed in both endothelial and epithelial cells (39). ZO-1 links TJ proteins, such as occludin, to the actin cytoskeleton, thus maintaining both stability and function of the TJs (40). This is evidenced by the observation that nicotine-induced reduction of ZO-1 expression at TJs is associated with increased permeability (41). ZO-2, a 160 kDa, phosphoprotein, is localized at the TJs and has also been detected in non-TJ containing tissues (14, 42). ZO-2 may share many functions with ZO-1 and may act as a “stand in” for ZO-1 under conditions in which ZO-1 and TJ protein interactions are disrupted. In a study using mouse epithelial cell clones that lacked ZO-1, there was upregulation in recruitment of ZO-2 to the TJs allowing for formation of morphologically normal TJs (43). ZO-3 is expressed in some TJ-containing tissues; however, its role at the BBB has not been elucidated (44).
b) Astrocytes
Astrocytes are the most abundant cell type in the brain and display a fibroblast-like morphology within grey matter (45); however, this morphology can be influenced by their CNS location and associations with other cell types that are in close proximity (46). Astrocyte end-feet ensheathe over 99% of cerebral capillaries (47), leading to critical cell-cell interactions that directly modulate and regulate BBB characteristics (46). Several studies have demonstrated that astrocytes play a vital role in maintenance, and perhaps induction, of BBB characteristics. For example, Janzer and Raff (1987) injected purified astrocytes into the anterior eye chamber of adult rats and observed formation of capillaries and venules that demonstrated functional “tightness.” “Tightness” was determined by intravenous injection of Evans’ blue, a dye that conjugates with albumin thereby forming a large molecular weight complex that cannot cross the intact BBB. In this study, astrocyte aggregates did not stain with the dye indicating the presence of functionally tight capillaries and venules (48). Additionally, male Fisher rats treated with 3-chloropropanediol exhibited decreased barrier function as a result of loss of TJ proteins occludin and claudin-5 as well as cytoplasmic ZO-1. Loss of these TJ proteins resulted in BBB leak of 10 kDa dextran and fibrinogen (300 kDa), suggesting a dramatic reduction in BBB functional integrity (49). Several inducing factors secreted by astrocytes have been identified, including TGF-β, GDNF and BFGF, which are involved in induction and regulation of the BBB phenotype. Additionally, astrocytes can regulate brain microvascular permeability via Ca2+ signaling involving astrocyte-endothelial gap junctions and purigenic transmission (14, 19, 46). Astrocytes play a critical role in preventing excitotoxicity induced by acute elevations of glutamate in the brain. This is mediated via expression of astrocyte glutamate transporters EAAT1 and EAAT2 that are responsible for glutamate uptake into the astrocyte cell, thus reducing glutamate levels in the parenchyma (50).
Various transporters and enzymes are expressed on astrocytes including P-gp, BCRP/Bcrp, and MRP/Mrp isoforms (51-53). Expression of efflux transporters in astrocytes suggests that astrocytes may act a second barrier system to CNS drug penetration and distribution. Transporters expressed on astrocytes may work to sequester drugs within the astrocyte cell, thus limiting drug permeation into the brain parenchyma or they may concentrate drugs within the brain extracellular fluid. For more detailed information on functional expression of transporters in astrocytes, the reader is directed to recent reviews (19, 54).
c) Microglia
Microglia, the primary immune cells of the brain, are ubiquitously distributed in the CNS and are activated in response to systemic inflammation, trauma, and several CNS pathophysiologies (55, 56). Microglia present with a ramified morphology that is characterized by a small soma and fine cellular processes during their “resting state.” Microglial activation in response to pathophysiological stressors can trigger changes cell morphology, which include reduced complexity of cellular processes and transition from a ramified morphology to an amoeboid appearance (56). Activated microglia produce high levels of neurotoxic and proinflammatory mediators such as nitric oxide, peroxide, TNF-α, and proteases, all of which result in cell injury and neuronal death (19), As immune cells microglia scavenge apoptotic cells, tissue debris after trauma, or microbes (19). They can also act as scavengers of extracellular molecules such as amyloid-β (55, 56). Activation of microglia is associated with altered TJ protein expression and increased BBB permeability (10).
Several transporter proteins are expressed by microglia, including P-gp, Bcrp, Mrp-4, and Mrp-5. Inflammatory events may affect mRNA/protein expression of these transporters (57). For example, in vitro studies have shown that LPS-treated microglia express decreased mRNA/protein levels of several ABC transporters such as P-gp, Bcrp, Mrp2, Mrp4, and Mrp5 (58). Expression of these transporters indicates that microglia may play a role in CNS drug permeation and distribution; however, more studies are required to elucidate the role of microglia in drug uptake into the CNS.
d) Pericytes
Pericytes are attached at regular intervals to the abluminal side of brain capillary endothelial cells and on the luminal side of the astrocyte endfeet (14, 59). They have a round cell body, round nuclei (59, 60), and long processes that extend over the vessel walls of the brain capillaries (60). Pericytes are multi-functional cells that contribute not only to vascular contractility and immune responses but also to BBB functional integrity. The percentage of vasculature covered by pericytes correlates with “tightness” of the junctions between endothelial cells, suggesting that pericytes play a role in maintenance of BBB TJ protein complexes (59) .This “tightening” role for pericytes has been demonstrated by the observation that vascular tissues with fewer pericytes (i.e., spinal cord) are leakier than vessels localized within cerebral cortex tissue (54). This may relate to the production of pericyte-derived angiopoietin, which has been shown to induce occludin expression at the TJ in an in vitro endothelial-pericyte co-culture model (61). Similar findings have been reported at the blood-CSF barrier where pericyte-deficient mice displayed decreased expression of TJ proteins and increased solute leak at the choroid plexus (55). Furthermore, in vitro endothelial-pericyte co-culture studies have shown that pericytes are required to ensure proper localization of endogenous BBB proteins (i.e., P-gp, utrophin) in brain microvascular endothelial cells (62). Additionally, pericytes have been shown to induce BBB properties such as reducing paracellular permeability during in utero brain development which indicates that these cells are critical mediators of BBB development (63).
Studies using bovine brain tissue have shown that pericytes express several transporters including several Mrp isoforms (Mrp1, Mrp4, and Mrp5) (57). P-gp has also been shown to be localized to the pericyte plasma membrane in both human and rat brain tissue fixed in situ (64). Recently the cholesterol efflux regulatory protein (CERP) has been identified in a primary culture of brain pericytes, where it was reported to mediate cholesterol efflux (65).
e) Neurons
Modified BBB function has been observed in several CNS pathologies (i.e., inflammation, hypertension, ischemia) and is often accompanied by changes in cerebral blood flow. Such changes imply that the cerebral microcirculation must be highly responsive to the metabolic requirements of CNS tissue and suggests a need for direct innervation of the brain microvasculature. Indeed, there is considerable evidence for direct innervation of both brain microvessel endothelial cells and associated astrocyte processes via distinct connections with noradrenergic (66, 67), serotonergic (68), cholinergic (69, 70) and GABAergic (71) neurons. For example, studies have shown that loss of direct noradrenergic input from the locus coeruleus results in increased BBB susceptibility to effects of acute hypertension, resulting in significantly increased permeability to 125-I labeled albumin (57). Additionally, stimulation of the parasympathetic sphenopalatine ganglion has been found to induce BBB opening resulting in an increase in delivery of chemotherapeutic agents (i.e., anti-HER2 monoclonal antibody, etoposide) to the brain (72). In contrast, in a rodent model of traumatic brain injury (TBI), vagal stimulation following TBI resulted in a decrease in BBB permeability to FITC-dextran as compared to animals with TBI alone (73). It is noteworthy that many factors that modulate neuronal growth, development, and repair also regulate endothelial cell function. For example, within the CNS, VEGF supports neuronal growth and promotes neuronal migration in the developing CNS (74). VEGF is upregulated under hypoxia conditions and this upregulation is associated with increased BBB permeability (75). Thus, communication between neurons and endothelial cells may not simply regulate blood flow but BBB permeability as well.
f) Extracellular Matrix
The extracellular matrix of the basal lamina interact serves as an anchor for the cerebral microvascular endothelium. The anchoring function of the extracellular matrix is mediated via interactions between endothelial integrin receptors, lamin, and other matrix proteins. Disruption of extracellular matrix is associated with loss of barrier function, resulting in increased permeability. Additionally, matrix proteins have been shown to influence the expression of TJ proteins, such as occludin, suggesting that the extracellular matrix plays a role in maintaining TJ protein integrity (14, 19).
The Blood-CSF (BCSF) Barrier
The blood-CSF (BCSF) barrier is formed by the choroid plexus, the primary interface between the systemic circulation and the CSF. It is comprised of fenestrated capillaries, which are joined together by TJs that link adjacent choroid plexus epithelial cells and limit paracellular diffusion of hydrophilic substances (76). The structure of the choroid plexus consists of an external cuboidal epithelium that surrounds a vascular bed embedded in loose connective tissue. The cuboidal epithelium is continuous with the ependyma, a thin epithelial membrane that lines the ventricular system of both the brain and the spinal cord (77). TEER values for the BCSF barrier are significantly lower compared to TEER values across the BBB, which suggests that many solutes can permeate the BCSF barrier to a greater degree than at the BBB. For example, large substances such as peptides can cross the BCSF more efficiently (albeit still minimally) via limited paracellular diffusion due to incomplete TJs as well as reduced pinocytosis/exocytosis (78).
In addition to its barrier function, the choroid plexus produces CSF, which is secreted into the lateral, third and fourth ventricles, thus the choroid plexus plays a role in regulating fluid pressure within the CSF (46). CSF secretion is regulated by ion exchange across the epithelium and is driven by activity of Na+-K+ ATPase and of carbonic anhydrase (77). CSF is continually secreted and reabsorbed into the circulation resulting in the total volume of CSF being replaced four to five times per day. This results in a “sink effect” that reduces the steady state concentration of substances entering the CSF and brain (79, 80). The “sink effect” is much more pronounced for large molecular weight and hydrophilic drugs. The CSF also contains approximately 0.3% plasma protein that totals between 15-40 mg/mL (81). This is in direct contrast to the extracellular space of the brain, which does not contain detectable concentrations of plasma proteins (82).
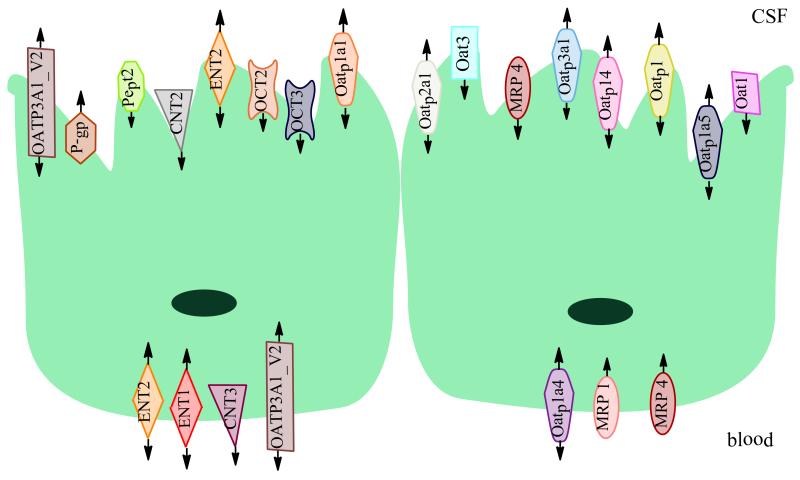
The choroid plexus barrier and secretory functions are aided by expression of a variety of transporters, allowing for precise regulation of ion and nutrient content of the CSF, as well the removal of waste products and limited entry of potentially neurotoxic compounds (76). Several transporters have been identified at the choroid plexus through use of quantitative gene analysis in vivo biotinylation, immunohistochemistry, and Western blot analysis. These transporters include organic anion transporters (Oat 3, Oat 2) (83, 84), peptide transporters (PEPT2) (85), organic cation transporters (Oct 3), organic anion polypeptide transporters (Oatp 1a1, Oatp 1c4, Oatp1a6, Oatp2a1, Oatp4a1) (78), amino acid transporters (Lat1) (86), monocarboxylate transporters (MCT 3) (87), and multidrug resistance proteins (Mrp 1, Mrp 4) (86). Additionally, P-gp (88), BCRP (89), and nucleoside transporters (90) are expressed at the BCSF barrier.
Transport Across Brain Barriers
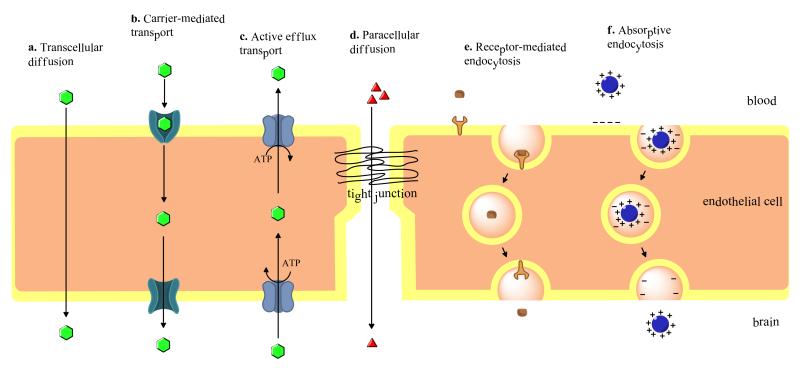
Several disorders of the CNS remain difficult to treat pharmacologically due to an inability of many drugs to attain efficacious concentrations in the brain. In part, this is due to active efflux transport processes that restrict blood-to-brain drug uptake. However, drugs may still cross brain barriers (i.e. BBB, BCSF barrier) and accumulate in the CNS by various mechanisms that favor uptake including passive diffusion, carrier-mediated transport, and endocytosis. A brief description of each process is provided in this section (Fig 1).
Figure 1.
Cross section of brain capillary endothelial cell. Factors secreted by astrocytes and pericytes, as well as neuronal input help maintain tight junction integrity and function, allowing for limited paracellular permeability of substances into the CNS.
a) Passive Diffusion
Passive diffusion involves movement of solutes across biological membranes along their concentration gradient without expenditure of biological energy or involvement of a carrier protein. Several factors influence a substance’s ability to passively diffuse: lipid solubility, polarity, molecular size, concentration in blood, and surface area available for diffusion. In general, polar, hydrophilic substances cannot diffuse easily across membranes. There is a strong correlation between a substance’s lipid solubility and membrane permeability, with more lipid soluble substances being able to easily move across cell membranes. Although molecular size influences permeability, with smaller substances passing more easily, its influence on permeability is not as great as that of lipid solubility. Size limitations of a compound can be overcome by lipid solubility, particularly in compounds that are highly hydrophobic (91). Hydrogen bonding capability of a compound also influences passive diffusion across biological membranes. Generally, the fewer the number of hydrogen bonds formed, the greater the ability of a compound to passively diffuse across a membrane (92). Despite limitations imposed by their physicochemical properties, small, polar, or charged molecules (i.e., ions, water) can traverse biological membranes via aqueous channels traversing the lipid bilayer (93). Examples of drugs that can passively diffuse across biological membranes include opioids (i.e. morphine, heroin), diphenhydramine, and steroids (93-95).
b) Carrier-Mediated Transport
Carrier-mediated transport involves interactions of a substrate with a transport/carrier protein, providing a route for diffusion of substances across a membrane with the direction of transport dictated by the solute concentration gradient. Such transport systems are utilized for transport of essential nutrients (i.e., glucose) into the brain as well as elimination of metabolic waste. A classic example of carrier-mediated transport is the Glut-1 transporter, which is localized to both the luminal and abluminal membrane of the BBB and whose transport is concentration-dependent (86, 96). Transport by such carriers is often categorized according to the requirement for a co-transport substrate (i.e., monovalent ions) along with the substance being primarily transported (92). CNS drugs may also be transported via carrier-mediated transport. For example, the sodium-independent large neutral amino acid transporter (LAT-1) mediates transport of L-Dopa, the “gold-standard” therapeutic used for treatment of Parkinson’s disease (97).
c) Endocytosis
Vesicular transport across the BBB occurs via receptor-mediated, adsorptive, or bulk-phase endocytosis (46, 92, 98). Receptor-mediated endocytosis involves interaction of a substrate with a receptor expressed on the membrane surface. Binding of a substrate triggers internalization of the substrate-receptor complex into the intracellular compartment where dissociation of the substrate from the receptor occurs (99). Internalization of the substrate-receptor complex involves invagination of the luminal membrane, which encapsulates the complex in vesicles. Vesicles then pinch off from the membrane and are internalized. Once internalized, the vesicles release their contents within the intracellular space or the vesicles fuse with the abluminal membrane after which their contents can be released directly into brain parenchyma (99). Both the transport of the iron transport protein transferrin and insulin occur via receptor-mediated endocytosis (100, 101). In adsorptive endocytosis, cationic proteins bind to the luminal membrane of capillary endothelial cells via electrostatic interactions with anionic sites on the membrane. These anionic sites are due to expression of acidic glycoproteins on the luminal membrane (i.e., glycocalyx) (102, 103). Bulk-phase endocytosis does not require a receptor and involves uptake of substances that are solubilized in extracellular fluid. The attachment of clathrin to the membrane creates clathrin cages on the cytoplasmic surface of the cell membrane. Cage formation is followed by invagination of the membrane and formation of a clathrin-coated pit and subsequent generation of a closed vesicle. The vesicle detaches from the membrane via membrane fission. Similar to receptor-mediated endocytosis, the internalized vesicle may release its contents in the intracellular space or fuse to with the abluminal membrane (104).
d) Active transport
Active transport of substrates across the BBB is energy dependent and usually coupled to ATP-hydrolysis (46, 105). Such processes enable movement of substances against their concentration gradient. There are a multitude of energy dependent transporters expressed at the BBB endothelium that work to transport essential nutrients, ions, and other endogenous compounds into the CNS. Additionally, other active transport mechanisms are responsible for restricting/regulating entry of potentially toxic substances (46). Many therapeutic drugs are transported by active transport processes in the CNS, including opioid analgesic drugs, opioid analgesic peptides, HMG CoA reductase inhibitors, HIV-1 protease inhibitors, cardiac glycosides, antineoplastic agents, calcium channel blockers and antibiotics.
Functional Expression of Transporters at Brain Barrier Sites
For many therapeutic compounds, uptake into the brain and extrusion from the brain is mediated by transport proteins. A variety of efflux and influx transporter systems have been identified at brain barriers including ATP-binding cassette (ABC) transporters, organic anion and organic cation transporters, peptide transporters, nucleoside transporters, and monocarboxylate transporters. Below, we provide an overview of each of these transport systems and their relevance to CNS drug delivery.
a) ATP-binding Cassette (ABC) Transporters
The ATP-binding cassette (ABC) transporter superfamily is among the largest and most ubiquitously expressed protein families known to date. The ABC transporter superfamily consists of 48 genes, which are subdivided into 7 distinct subfamilies (ABCA -ABCG) (106). ABC transporters are involved in various physiological functions, such as maintenance of lipid bilayers, peptide transport, and sterol transport. Perhaps the most clinically relevant role of ABC transporters is their direct contribution to development of the multidrug resistance (MDR) phenotype (106). The MDR phenotype is defined as the simultaneous resistance to several structurally unrelated compounds that does not result from independent genetic mutations that confer resistance to a single xenobiotic (107).
ABC genes are categorized as either full transporters or half transporters, with full transporters exhibiting the prototypical two transmembrane domains and two nucleotide binding domains. Half transporters contain only one transmembrane domain and one nucleotide binding domain and are believed to homo- or heterodimerize in order to achieve functionality (108). All ABC superfamily members possess three highly conserved motifs known as the Walker A and Walker B motifs and the ABC signature C motif (i.e., ALSGGQ) (109, 110). The exact role of the ABC signature sequence is unknown, although it has been suggested that this domain may be involved in substrate recognition or ATP hydrolysis (111). ATP hydrolysis is required by ABC transporters as a source of biological energy for transport of substances in a single direction across membranes against a concentration gradient (106, 112).
Members of subfamily ABCA play a role in phospholipid (i.e., cholesterol) trafficking across plasma membranes. Loss of function of these transporters can result in development of dyslipidemia in affected individuals. For example, loss of function mutations in ABCA1, which plays a critical role in reverse cholesterol transport, leads to development of Tangier disease (113, 114). Tangier disease is an autosomal recessive disorder characterized by a severe deficiency of high-density lipoprotein and apolipoprotein A-I as well as the accumulation of cholesterol esters throughout the body (113, 115). Mutant variants of the ABCA4 gene, which is localized to rod photoreceptors, have been detected in multiple ophthalmic disorders including Stargardt disease, recessive retinitis pigmentosum, and recessive rod-cone dystrophy (113). The ABCB subfamily consists of 11 members that are responsible for transport of various solutes such as drugs, peptides, phosphatidylcholine, and iron (113). Perhaps the most well studied member of the ABCB family is P-gp, a major contributor to the MDR phenotype that is involved in cellular efflux of therapeutic agents. Other members of the ABCB subfamily include bile salt export protein (ABCB11) and transporter associated with antigen processing 1 and 2 (TAP1 and TAP2; also known as ABCB2 and ABCB3 respectively). Mutations in ABCB genes have been observed in various diseases including progressive familial intrahepatic cholestasis, ankylosing spondylitis, insulin-dependent diabetes mellitus, and celiac disease (113).
There are 13 members of the ABCC subfamily whose functions include ion transport, signal transduction, and toxin secretion (113). Disruption and/or loss of function of these transporters results in an array of pathophysiological conditions including hyperinsulinemic hypoglycemia (ABCC8) (116, 117) and Dubin-Johnson syndrome (ABCC2) (118). Additionally, cystic fibrosis results from a loss of function mutation in the CFTR transporter (ABCC7), a chloride ion channel (119). MRP/Mrp isoforms are also members of the ABCC subfamily and are associated with development of the MDR phenotype (113). The ABCC family also includes sulfonylurea receptors (SUR) 1 and 2 and a truncated protein that does not mediate transport (ABCC13) (113).
The ABCD subfamily consists of 4 genes (ABCD1-4) that encode half transporters found exclusively in peroxisomes. ABCD family members are believed to be involved in transport of coenzyme A esters of very-long-chain fatty acids (120). Mutations in the ABCD1 gene results in the X-linked disease adrenoleukodystrophy, which is characterized by progressive demyelination and impaired cognition, vision, hearing, and motor function (121). Unlike the other ABC subfamilies, the ABCE and ABCF subfamilies contain genes that contain nucleotide-binding domains, but do not encode transmembrane domains. The OABP protein is the only known member of the ABCE subfamily and is responsible for recognizing oligoadenylate produced during viral infections (122). The functions of ABCF1-3 have not been fully characterized; however, the hABCF1 has been found to be part of the ribosome complex (113). Members of the human ABCG subfamily are comprised of six transporters, which include ABCG2, also known as BCRP/Bcrp. BCRP/Bcrp plays a critical role in conferring the MDR phenotype and is known to be involved in efflux transport of several drugs. Other members of the ABCG subfamily include ABCG1, ABCG5, and ABCG8 that are involved in transport of sterols such as cholesterol (103).
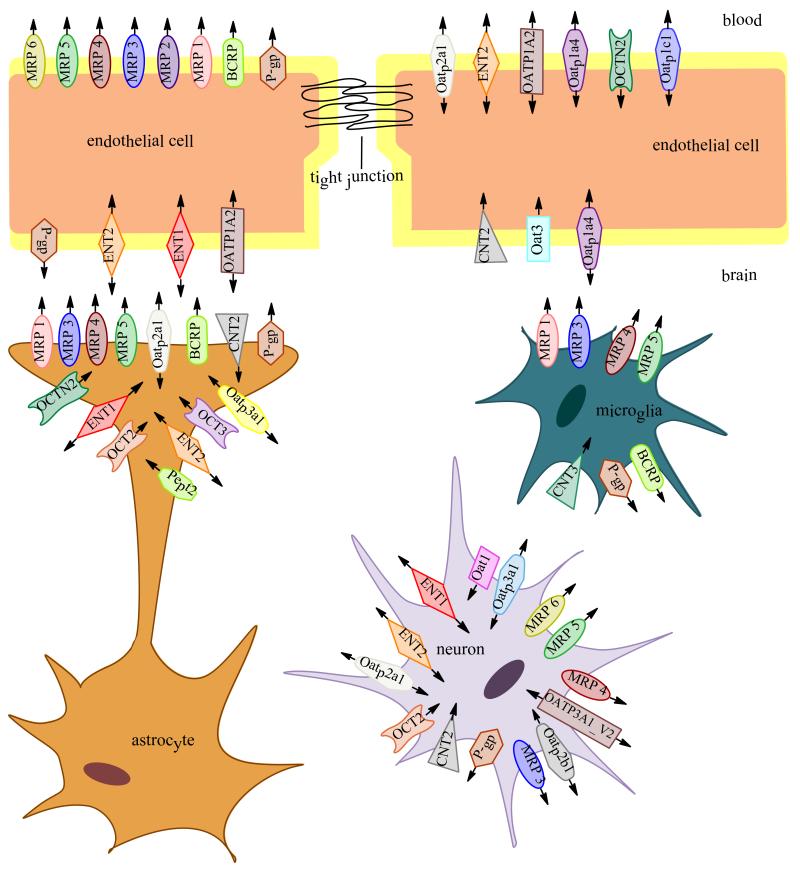
In the CNS, the most studied members of the ABC superfamily are P-gp, MRP/Mrp isoforms, and BCRP/Bcrp as they are known to play a critical role in limiting therapeutic drug entry into the brain thereby limiting the effectiveness of pharmacotherapy for treatment of neurological disease (112, 123).
P-glycoprotein (P-gp)
P-gp is a 170-kDa efflux transporter encoded by the MDR gene (107). Two MDR isoforms have been identified in human tissues, MDR-1 and MDR-2 (124, 125); however, P-gp expression in rodent tissues is encoded by three distinct mdr isoforms designated mdr-1a, mdr-1b, and mdr-2. While over expression of MDR-1/mdr-1a/mdr1b confers the MDR phenotype (107, 126), MDR-2/mdr-2 is primarily expressed in the liver and is involved in biliary transport of phosphatidylcholine (107, 127). In humans, the MDR1 gene product is 1280 amino acids in length and consists of two homologous halves, each containing six transmembrane domains. Each homologous half also contains one ATP-binding site. Two to four glycosylation sites have been located on the first extracellular loop. Studies using glycosylation-deficient P-gp found lower levels of this transporter at the cell surface but transport function remained unaffected (128). Mature P-gp is phosphorylated on the linker region between the two homologous halves (TM6-TM7) (107). Phosphorylation may protect non-glycosylated P-gp from breakdown by endoplasmic reticulum proteases or from proteasomal degradation prior to glycosylation and trafficking to the plasma membrane. For example, in vitro studies have demonstrated that activation of Pim-1 kinase, a serine/threonine kinase, decreased P-gp degradation and increased cell surface expression (129), which suggests that phosphorylation may be a critical step in processing of a mature and functional P-gp transporter.
Since its initial discovery in Chinese hamster ovary cells that were resistant to colchicine (130), P-gp expression has been observed in multiple tissues, including kidney, liver, gastrointestinal tract, placenta, and testes (131). In the brain, P-gp is localized to both the luminal and abluminal membranes of the BBB endothelium (64) and to the apical plasma membrane of choroid plexus epithelial cells (88). Expression of P-gp at the BBB likely evolved to protect the CNS from exposure to potentially neurotoxic xenobiotics and to maintain the precise homeostatic environment required for proper neuronal function (132). The importance of P-gp’s role in CNS protection is highlighted by studies using mdr1a/mdr1b knockout mice. Mdr-1a/mdr1b null mice showed a 100-fold increase in brain uptake of ivermectin, a neurotoxic pesticide, when compared to their wild-type counterparts (133). Furthermore, mdr1a/mdr1b null mice displayed multiple symptoms of ivermectin toxicity (i.e., tremors, paralysis, coma, and death) that are directly attributed to increased brain penetration (133). Similar observations were reported in collies where increased sensitivity to ivermectin was directly correlated to a complete absence of the mdr1 gene (134). Additionally, P-gp expression has been detected in brain parenchyma cellular compartments such as astrocytes, microglia, and neurons (135-139).
P-glycoprotein has an immense substrate profile that renders it a formidable obstacle to CNS drug delivery. In fact, the number of compounds known to be P-gp substrates is continuously expanding as more and more research is done. P-gp substrates are generally non-polar, weakly amphipathic compounds that vary considerably in molecular size. For example P-gp is known to transport small molecule drugs such as daunorubicin (563.99 Da) as well as larger molecules such as actinomycin D (1255.42 Da) (132). The list of known substrate categories includes, but is not limited to, antibiotics, calcium channel blockers, cardiac glycosides, chemotherapeutics, immunosupressants, anti-epileptics, anti-depressants, and HIV-1 protease inhibitors (140, 141). Recent studies have demonstrated that many HMG CoA reductase inhibitors (i.e., pitavastatin, pravastatin) are transported across biological membranes by P-gp (142, 143). Studies have also shown that opioid analgesic drugs such as morphine and the opioid peptide DPDPE are directly extruded from brain tissue by P-gp (12, 144-146). Endogenous substrates of P-glycoprotein may include cytokines, lipids, steroid hormones, and peptides (132). However, caution must be exercised with regards to use of pharmacological inhibitors of P-gp for enhanced tissue delivery. Specifically, the ubiquitous expression of P-gp throughout the body coupled with the large inhibitor doses required to effectively block P-gp has often resulted in significant systemic toxicity (147).
Additionally, several substrates of P-gp have been found to be competitive transport inhibitors. Examples of such drugs include calcium channel blockers (i.e., verapamil), antipsychotics (i.e., chlorpromazine), immunosuppressive agents (i.e., cyclosporine A) and the cyclosporine A analog PSC 833 (i.e., valspodar) (132). HMG CoA reductase inhibitors have also been found to block P-gp transport function and several studies are exploring the possibility of using these drugs to reverse P-gp induced drug resistance in tumor cells (148).
Breast Cancer Resistance Protein (BCRP/Bcrp)
BCRP/Bcrp was originally identified in the MCF-7/AdrVp breast cancer cell line that was developed to study methods to overcome the MDR phenotype (149). Despite the absence of P-gp or MRP-1, these cells exhibited ATP-dependent efflux transport of both adriamycin and rhodamine 123, suggesting the presence of a novel transporter protein (150, 151). This novel transporter was later cloned from the MCF-7/AdrVp cell line and subsequently named “breast cancer resistance protein” (152). BCRP/Bcrp is comprised of 655 amino acids and has a molecular weight of approximately 72 kDa. It is commonly referred to as a “half-transporter,” composed of six transmembrane domains (prototypical ABC transporters have 12 transmembrane domains), with the C- and N-termini located on the intracellular side of the plasma membrane (152). In addition, 2-3 N-glycosylation sites are located on the extracellular loops of the protein. These glycosylation sites do not appear to have any direct influence on functional capabilities of the transporter or on its cellular localization (153, 154). It is believed that BCRP/Bcrp forms functional homo- or heterodimers, which is required for efflux activity (155, 156). BCRP/Bcrp has been identified in several tissues types including liver, gastrointestinal tract, placenta, and testes (157). Within the CNS, BCRP/Bcrp is expressed at the luminal side of BBB capillary endothelial cells as well as in astrocytes and in microglia (158-160). BCRP/Bcrp has also been detected at the rat choroid plexus but only at the mRNA level (161).
Despite studies demonstrating expression of BCRP/Bcrp within the CNS, there is still debate as to its functionality. In vitro studies using cultured rodent and human capillary endothelial cells have demonstrated BCRP/Bcrp-mediated transport activity (159, 162), but the BCRP/Bcrp functional expression might result from overexpression of BCRP/Bcrp in this cell culture system. Therefore, the transport activity reported might not be an accurate reflection of in vivo BCRP/Bcrp function (163, 164). In vivo studies have also shown conflicting data regarding functional expression of Bcrp. Studies investigating efflux transport of dehydroepiandrosterone sulfate (DHEAS) and mitoxantrone across the mouse BBB concluded that Bcrp played only a minor role in the active efflux of these transport substrates (160, 165). This conclusion was based on in situ perfusion data acquired from both wild-type and P-gp knockout mice. Both studies demonstrated increased uptake of radiolabeled DHEAS and mitoxantrone when treated with the dual P-gp/Bcrp inhibitor GF120918 (i.e., elacridar), indicating the presence of a P-gp-independent efflux transporter (160, 165). However, transport studies using ABCG2(−/−) (i.e., Bcrp) knockout mice showed that brain uptake of radiolabeled DHEAS and mitoxantrone was comparable to levels of uptake observed in wild-type mice (160, 165). Addition of GF120918 had a similar effect on substrate uptake into the brain in both Bcrp and wild-type mice (160, 165). While these data may point to only a minor role for Bcrp in drug efflux transport at brain barrier sites, the conclusions of the above studies may have been a function of the substrates used. For example, an earlier study by Breedveld and colleagues showed that clearance of intravenous imantinib, an established Bcrp substrate, was significantly decreased (~1.6 fold) in Bcrp knockout mice compared to wild-type controls. Furthermore, brain penetration of imantinib, measured at two hours post-administration, was significantly higher in the Bcrp knockout mice (2.5 fold) than levels observed in the wild-type mice (166). Additionally, administration of elacridar increased brain penetration of imantinib in wild-type mice, suggesting that efflux transport of this antineoplastic drug is mediated by Bcrp (166).
There is significant overlap between the substrate profiles of BCRP/Bcrp and P-gp (Table 1) (167). In addition to physiological substrates such as steroid hormones, glutathione, and folic acid (167, 168), BCRP/Bcrp also transports many structurally diverse therapeutic compounds. Among substrates transported by BCRP/Bcrp are chemotherapeutic agents (i.e., mitoxantrone), anthracyclines (i.e., etoposide, teniposide), and campothecin derivatives (i.e., topotecan, irinotecan) (149, 151, 169-171).
Table l.
OATP Localization in the CNS and Known Substrates
| OATP Isoform | CNS Barrier Localization |
Substrates |
|---|---|---|
| Rodent | ||
|
Oatplal (slcolal) |
Choroid plexus (ap) |
BQ123, BSP-DNP-SG, cholate, cortisol, CRC220, deltorphin II, DPDPE, enalapril, Estradiol-17 β- glucuronide, estrone-3-sulfate, fexofenadine, gadoxetate, glutathione, glycocholate, hyodeoxycholic acid, ochratoxin A, ouabain, pravastatin, S-dinitrophenyl, sulfotaurolithocholate, taurochenodeoxycholate (TCDCA), taurolithocholic acid sulfate, tauroursodeoxycholate (TUDCA), taurocholate, temocaprilat |
|
Oatp1a4 (slco1a4) |
BBB (ap, bl); choroid plexus (bl) |
BQ123, cholate, dehydroepiandrosterone sulfate (DHEAS), digoxin, DPDPE, estradiol-17 β- glucuronide, estrone-3-sulfate, fexofenadine, glycocholate, ouabain, pravastatin, taurocholate, T3, T4, TCDCA, TUDCA |
|
Oatp1a5 (slco1a5) |
Choroid plexus (ap) |
Bromosulfophthalein, BQ123, cholate, cortisol, dexamethasone, DHEAS, estradiol-17 β- glucuronide, estrone-3-sulfate, glycochenodeoxycholate, glycocholate, glycodeoxycholate, GW4064, oleic acid, prostaglandinE2, T3, T4, taurocholate, taurodeoxycholate, TCDCA, TUDCA, ursodeoxycholic acid |
|
Oatplcl (slcolcl) |
BBB (ap); choroid plexus (bl) |
Estradiol-17 β-glucuronide, cerivastatin, reverse T3, T4, troglitazone sulfate |
|
Oatp2a1 (sclo2a1) |
BBB | ProstaglandinE2 |
| Human | ||
|
OATP1A2 (SLCO1A2) |
BBB (ap, bl) | Acebutolol, atenolol, atrasentan, bilirubin, bromosulfophthalein, BSP, BQ-123, celiprolol, ciprofloxacin, cholate, enoxacin, erythromycin, ethotrexate, darunavir, deltorphin II, DHEAS, DPDPE, estrone-3-sulfate, fexofenadine, gatifloxacin, glycocholate, hydroxyurea, imatinib, labetalol, levofloxacin, lomefloxacin, lopinavir, mesylate, microcystin - LR, N - methylquinine, N, methylquinidine, norfloxacin, ouabain, pitavastatin, prostaglandinE2, sotalol, rocuronium, rosuvastatin, reverse T3, saquinavir, T4, T3, talinolol, taurocholate, taurochenodeoxycholate, tauroursodeoxycholate, TCDCA, TUDCA, a metabolite of unoprostone |
|
OATP3A_v1 (SLCO3A1_v1) |
Choroid plexus (bl) |
Benzylpenicillin, BQ-123, deltorphin II, estrone-3- sulfate, prostaglandinE1, prostaglandinE2, T4, vasopressin |
|
OATP3A_v2 (SLCO3A_v2) |
Choroid plexus (ap) |
Arachidonic acid, BQ-123, prostaglandinE1, prostaglandinE2, T4, vasopressin |
|
OATP1C1 (SLCO1C1) |
Detected in brain but localization not confirmed |
Bromosulfophthalein, estradiol-17 β-glucuronide, estrone-3-sulfate, thyroid hormones, thyroxine sulfate |
|
OATP2B1 (SCLO2Bl) |
Detected in brain but localization not confirmed |
Atorvastatin, bosentan, fexofenadine, fluvastatin, montelukast, pravastatin, pitavastatin, rosuvastatin, talinolol, aliskiren, benzylpenicillin, bromosulfophthalein, dehydroepiandrosterone-3- sulfate, estrone-3-sulfate, ezetimibe glucuronide, glibenclamide, mesalazine, pregnenolone sulfate, taurocholate, tebipenem pivoxil, a metabolite of unoprostone |
Multidrug Resistance Proteins (MRPs/Mrps)
The primary role of MRPs/Mrps is to extrude xenobiotics from cells, thereby contributing to development of the MDR phenotype. MRPs/Mrps differ from P-gp in that their substrate profile is more restrictive. Specifically, MRP/Mrp isoforms generally transport organic anions and their glucuronidated, sulfated, and glutathione-conjugated metabolites (172). Specific properties and functional significance of individual MRP/Mrp isoforms is difficult to determine due to existence of 9 homologues with overlapping substrate profiles, designated MRP1-MRP9. MRP1/Mrp1-MRP6/Mrp6 have been detected at both the BBB and BCSF barrier, though with some controversy regarding localization and/or functional expression (172). MRP1/Mrp1, MRP2/Mrp2, MRP3/Mrp3, and MRP6/Mrp6 are structurally similar in that each has 3 transmembrane domains (TMD) designated TMD0, TMD1, and TMD2 respectively. TMD0 contains 5 alpha helices, while both TMD1 and TMD2 contain 6 alpha helices. MRP/Mrp TMDs are believed to assemble into a plasma membrane pore through which substrates can be transported (173). In contrast, MRP4/Mrp4 and MRP5/Mrp5 are more similar in structure to P-gp in that they lack TMD0 and are therefore smaller, having lower molecular weights than other MRP/Mrp homologues (173, 174). The cytoplasmic linker (L0) portion of the protein is conserved throughout all MRP/Mrp homologues and is essential for transport function. L0 is located between TMD0 and TMD1 on those MRP homologues with 3 transmembrane domains, while in those with 2 TMDs, L0 is a cytoplasmic segment of the protein between TMD1 and the N-terminus (175). Nucleotide-binding domains have been identified in the cytoplasmic region of the protein between TMD1 and TMD2 as well as between TMD2 and the C-terminus (173).
It is well established that multiple MRPs/Mrps are expressed at both the BBB and BSCF barrier. MRP1/Mrp1-MRP6/Mrp6 are localized to the luminal membrane of brain capillary endothelial cells (172, 175). This localization suggests that MRP/Mrp family members play a critical role in efflux transport of drugs from the brain into the blood. While Mrp1 and Mrp3-Mrp5 have been detected at the plasma membrane of astrocytes and microglia, expression of Mrp2 and Mrp6 in glial cells appears to be minimal (164). Neither mRNA nor protein has been found consistently for Mrp2 or Mrp6 in rat microglia or astrocytes though some controversy surrounding mRNA levels in rat fetuses has arisen (175). In neonatal Wistar rat astrocytes, Hirrlinger and colleagues identified Mrp1 and Mrp3-Mrp5 mRNA but were unable to detect Mrp2 mRNA (176). In another study by Ballerini and colleagues, discovery of Mrp1-Mrp6 mRNA was reported in rat fetal astrocytes (177). Taken together, these data imply that Mrp expression levels differ between the prenatal and postnatal state in rodents thereby accounting for these discrepancies.
MRP1/Mrp1 is a 1531 amino acid, 190 kDa protein that is ubiquitously expressed throughout the body with highest levels found in lung, testes, kidney, and peripheral blood mononuclear cells (173-175). Substrates transported by MRP1/Mrp1 are diverse, including both organic anions and some cationic compounds. Glucuronide conjugates such as estradiol-17β-glucuronide (E217βG) and sulfate conjugates such as estrone 3-sulfate are also preferred substrates (140). The chemotherapeutic agent methotrexate is an established substrate (173). The high affinity that MRP1 has for the cysteinyl leukotriene LTC4, suggests that MRP1 may contribute to immune responses though the details of this potential involvement remains unknown. MRP1 is also able to transport various metalloids in the form of oxoanions. The ability of MRP1 to efflux various toxins, carcinogens, and drugs attests to its role in xenobiotic detoxification (178). The oxidized form of glutathione (GSSG), along with reduced glutathione (GSH), and GSH conjugates such as 2,4-dinitrophenyl-S-glutathione (DNP-SG), are transported by MRP1 (140). It is highly probable that this physiological function of MRP1/Mrp1 is key to maintaining normal redox balance within the brain due to its localization at brain barrier sites and in brain parenchyma (i.e., astrocytic and microglial membranes). For example, inhibition of Mrp1 results in reduced efflux of GSSG from primary culture that is formed as a byproduct of oxidative stress (179). GSH transport by Mrp1 is increased in the presence of verapamil, a known P-gp inhibitor (180). Verapamil itself is not transported by Mrp1, implying that it allosterically enhances Mrp1 transport of GSH by increasing its affinity for this endogenous antioxidant (180). Therefore, endogenous compounds that exert the same effect as verapamil on Mrp1 (i.e., the bioflavonoid apigenin) (180), may need to accumulate intracellularly in order to enhance Mrp1-mediated GSH transport. Additionally, GSH stimulates transport of the anionic conjugate estrone-3 sulfate without directly transporting GSH itself. Based on these observations, it has been proposed that GSH can allosterically modulate Mrp1 transport (179). Mrp1 is capable of co-transporting certain cationic compounds with GSH such as the anti-cancer drugs etoposide and vincristine (140). This evidence supports the notion that free GSH is required for MRP1/Mrp1-mediated transport of some substances within the CNS.
Immunohistochemical analysis and drug transport studies established functional expression of Mrp1 in primary cell cultures established from neonatal rat choroid plexus, where Mrp1 was localized to the basolateral side of choroid plexus epithelial cells (88). Localization of Mrp1 at the rat choroid plexus was further confirmed by studies using a branched DNA signal amplification method to measure mRNA expression levels (84). MRP1’s basolateral localization allows for efflux of substances into blood circulation from CSF (175). However, following administration of established MRP/Mrp substrates E217βG and DNP-GS into cerebral ventricles, Mrp1 knockout mice showed no significant difference in E217βG and DNP-GS CSF concentrations as compared to wild type mice (174). This finding suggests the presence of another organic anion transporter that actively extrudes substances from the CSF at the BCSF barrier such as members of the OAT/Oat family.
Encoded by the ABCC2 gene, MRP2 is comprised of 1545 amino acids and has a molecular weight of 174 kDa (173). In the CNS, MRP2/Mrp2 is primarily expressed at the luminal membrane of brain capillary endothelial cells (175). The substrate profile for MRP2/Mrp2 is very similar to that of MRP1/Mrp1; however, many of these common substrates display lower affinity for MRP2/Mrp2 than for MRP1/Mrp1 (181). For example, MRP2 has been shown to transport the anti-cancer drug cisplatin while MRP1 does not (181). Originally discovered in the canalicular (apical) side of hepatocyte membranes, MRP2/Mrp2 is often referred to as the canalicular multispecific organic anion transporter (cMOAT). MRP2/Mrp2 was found to be a major transporter of bilirubin, organic anions, and glucuronides into bile from the hepatic circulation (173). This localization of MRP2/Mrp2 to the bile canaliculus of hepatocytes renders this transporter critical for biliary excretion of various metabolites. Absence of fully functional MRP2 in hepatocytes results in Dubin-Johnson syndrome, in which bilirubin conjugates accumulate appreciably in the blood.
In many regards, MRP3/Mrp3 is closely related to MRP2 /Mrp2. At 1527 amino acids and 169 kDa, MRP3/ Mrp3 is similar in size and substrate profile to both MRP1/Mrp1 and MRP2/Mrp2 (173, 174). MRP3/Mrp3 is localized to the luminal side of the BBB and is also expressed at the plasma membranes of astrocytes and microglia (182). MRP3/Mrp3 is known to transport antineoplastic drugs such as teniposide and methotrexate, but not cisplatin, vincristine, or doxorubicin (173, 175). MRP3/Mrp3 is the only MRP that transports univalent bile salts such as glycocholate (175). One of the key differences between MRP3/Mrp3 and MRP1/Mrp1 and/or MRP2/Mrp2 is the increased affinity of MRP3/Mrp3 has for glucuronide conjugates over conjugates of GSH. In fact, MRP3/Mrp3 is not known to transport GSH as demonstrated by the observation that cells overexpressing MRP3/Mrp3 do not show measurable transport of GSH (183). MRP3 transports etoposide without requiring GSH as a co-transport substrate (156). This is in direct contrast to MRP1/Mrp1 and MRP2/Mrp2, which are dependent on GSH to transport etoposide (175).
MRP4/Mrp4 transports a variety of compounds and is unique compared to other MRPs/Mrps. MRP4 has a molecular weight of 150 kDa, is composed of 1325 amino acids, and contains only two transmembrane domains (173). Like MRP1/Mrp1 and MRP2/Mrp2, MRP4/Mrp4 is able to transport E217βG and methotrexate; however, MRP 4/Mrp4 is not capable of transporting other substrates common to MRP1/Mrp1-MRP3/Mrp3 such as etoposide, DNP-SG, and LTC4. The substrate profile of MRP4 lately has broadened to exceed original predictions (175). Mrp4 confers resistance to cells to HIV-1 therapeutics such as azidothymidine monophosphate (AZT-MP) and 9-(2-phosphonylmethoxyethyladenine) (PMEA) (138). Cyclic nucleotides cAMP and cGMP, the acyclic nucleotide analog phosphonylmethoxyethyl guanine (PMEG), a compound with anti-proliferative activity, as well as purine analogs such as 6-thioguanine and 6-mercaptopurine have been shown to be MRP4/Mrp4 substrates (173, 175). The presence of GSH has been shown to promote Mrp4-mediated transport of the bile acids cholylglycine, choline, and cholyltaurine in hepatocytes (184). This finding suggests that GSH may be required as a co-transport substrate for specific MRP4/Mrp4 substrates. In both MRP4 and MRP5-transfected cells, intracellular GSH levels have been shown to decrease, signifying that GSH alone is a substrate of these transporters (179). MRP4/Mrp4 is highly expressed in the CNS at the BBB and BCSF barrier as well as in astrocytes and microglia (140). Localized to the luminal aspect of brain microvessel endothelial cells and to the basolateral side of choroid plexus epithelial cells, MRP4/Mrp4 is thought to restrict influx of a wide variety of compounds into brain parenchyma and CSF (185).
MRP5/Mrp5 is 1437 amino acids in length and has a molecular weight of 161 kDa (173). MRP5/Mrp5 is ubiquitously expressed with high levels found mainly in the brain, heart, and skeletal muscle. Mrp5 knockout mice show no discernible dysfunctions (186). Similar to MRP4/Mrp4, MRP5/Mrp5 appears to be an active pump of nucleotide analogues. Similar to MRP4/Mrp4, MRP5/Mrp5 has been identified in the plasma membranes of astrocytes and microglia, and at both the BBB and BCSF barrier (140). Mrp5 can transport PMEA and 6-thioinosine monophosphate as well as cAMP and cGMP (173). MRP5/Mrp5 lacks the ability to bind and transport common MRP1/Mrp1 substrates such as etoposide, LTC4, and vincristine. One substrate unique to MRP5/Mrp5 is the anti-HIV-1 drug stavudine monophosphate. Aside from substrates discussed here, no other endogenous CNS substrates have been identified for MRP5/Mrp5 (179). Another unique characteristic of MRP5/Mrp5 is the apparent intracellular expression of the protein. Nonpolarized HEK293 cells transfected with MRP5 have shown greater concentration of MRP within the cell than at the plasma membrane as where polarized MDCKII cells show MRP5 at the basolateral membrane (175, 186). There have also been reports of MRP5 transfected cells exhibiting resistance to heavy metals such as potassium antimonyl tartrate and cadmium chloride (173). Compared with MRP4/Mrp4, MRP5/Mrp5 has a much narrower substrate profile though more investigation into substrate specificity and localization is needed.
MRP6/Mrp6 is a 1503 amino acid and 165 kDa protein that is expressed in low levels in many tissues throughout the body but is principally expressed in the kidney and liver where it is localized to the basolateral membrane of proximal tubules and hepatocytes (162, 164). At the BBB, MRP6/Mrp6 has currently only been confirmed at the luminal side of brain capillaries—expression in astrocytes and microglia is thought to be negligible. MRP6/Mrp6 has also not yet been detected at the BCSF barrier (140). Transport of etoposide, cisplatin, and doxorubicin has been demonstrated though MRP6/Mrp6 appears to not interact with methotrexate, vincristine, or E217βG (175). The inability of MRP6/Mrp6 to transport glucuronide conjugates separates it from MRP1/Mrp1-MRP3/Mrp3 even though all these proteins are structurally similar with three transmembrane domains. MRP6/Mrp6 also has not been shown to transport cyclic nucleotides or methotrexate, common substrates of MRP4/Mrp4.
Solute Carrier (SLC) Superfamily
The solute carrier (SLC) superfamily mediates transport of anionic and cationic small molecules as well as peptides and nucleosides across biological membranes. Of the 43 known subfamilies of SLC transporters (SLC1-SLC43), proteins from SLC15A1, SLC21A, SLC22, SLC28, and SLC29 are expressed at the BBB and/or the BCSF barrier (187, 188). Members of SLC21 and SLC22 subfamilies include OATs/Oats, OCTs/Octs and OATPs/Oatps (189). Members of the SLC15A1 family include peptide transporters while nucleoside transporters are members of the SLC28 and SLC29 families (188). Although many of these transporters are capable of bidirectional transport, SLC transporters generally favor cellular uptake of drugs (187).
Unlike their ABC transporter counterparts, most SLC transporters do not require ATP to translocate substrates across biological membranes. Instead, transport is driven either by electrochemical gradients (i.e., Na+ or H+ gradient) or by concentration gradients established by the solutes that are being transported. Therefore, these transporters are categorized as either facilitated transporters or secondary active transporters (19, 112).
Organic Anion Transporters Polypeptides (OATPs/Oatps)
OATPs/Oatps are classified within the larger SLC superfamily (190). In 2004, to clarify nomenclature inconsistencies in the literature and to prevent future confusion, the OATP/Oatp naming system was revised. OATPs/Oatps were placed in an OATP/SLCO superfamily and subdivided into family, subfamily and individual gene product/gene based on phylogenetic relationships (190, 191). Family and subfamily members share 40% and 60% amino acid identity, respectively. Human OATP isoforms are identified by the root protein symbol “OATP” or gene symbol “SLCO” followed by a family number, subfamily letter and individual protein/gene number. Rodent orthologues are distinguished from human proteins and genes by lowercase root symbols (Oatp, Slco).
OATPs/Oatps are involved in transcellular transport of molecules across cellular barriers. This group of transporters has broad substrate specificity and is involved in absorption, distribution and excretion of xenobiotics. Directionality of transport is dependent on the transmembrane concentration gradient of an OATP/Oatp substrate. The OATP/Oatp transport mechanism is sodium-independent and does not require expenditure of ATP to move substrates across membranes. Similar to other SLC transporters, it is believed that OATP/Oatp mediated transport is governed by electrochemical gradients that utilize an inorganic or organic solute as a driving force. While intracellular bicarbonate, glutathione and glutathione conjugates have been identified as possible co-transport substrate candidates, the exact driving force of these transporters has not been conclusively shown (192). Additionally, functional activity of some OATP/Oatp family members is profoundly affected by extracellular pH. For example, it has been demonstrated that OATP2B1 transport function is significantly increased at low pH (193, 194). This increase in transport activity was shown to be substrate-specific, an effect that can be attributed to increased substrate affinity and/or increased substrate turnover rate (195, 196). The affinity for some OATP/Oatp substrates can also be increased at acidic pH via protonation of extracellular histidine residues (196). The pH dependence of OATP2B1 transport is clinically relevant; OATP2B1 is expressed in the small intestine, oral bioavailability of OATP2B1 substrates may be increased at low pH. Since OATP2B1 is also expressed in brain tissue, pH sensitivity of this transporter may imply altered CNS delivery of OATP/Oatp substrates in response to changes in blood pH (i.e., metabolic/ respiratory acidosis and alkalosis). Nonetheless, pH dependence for all OATP/Oatp family members remains controversial. While some studies have shown OATP1B1 and OATP1B3 to be electrogenic transporters with high sensitivity to extracellular pH changes (197), alteration of transport of the well established OATP/Oatp substrate estrone-3-sulfate in response to extracellular acidification was not observed (198).
OATPs/ Oatps are widely expressed in numerous tissues, including liver, kidney, retina, lung, testis, thyroid, spleen, placenta, leukocytes, heart, peripheral blood, and breast (123). In the brain, OATP/Oatp expression has been identified in BBB endothelial cells and choroid plexus epithelial cells (123). Of the 36 OATP/Oatp isoforms that have been identified, those that have been found to localize to CNS barriers include Oatp1a1, Oatp1a4, Oatp1a5, Oatp1c1, and Oatp2a1 in rodents and OATP1A2, OATP1C1 and OATP2B1 in humans. At the BBB and the BCSF barrier, OATPs/Oatps are responsible for CNS uptake of a vast array of amphipathic, organic compounds. OATP/Oatp family members have also been detected in brain parenchyma cellular compartments such as astrocytes and neurons (104). For more detailed information regarding OATP/Oatp localization and functional expression in the brain, the reader is directed to a recent review by Ronaldson and Davis (1).
Human OATPs expressed at the BBB and/or BCSF Barrier
While expression of Oatps at the rodent BBB has been well established, identification of OATPs at the human BBB has been controversial. For example, immunofluorescent staining of frontal brain cortex has demonstrated expression of OATP1A2 at the human BBB (199). In contrast, a recent study using a targeted absolute proteomics approach offers contradictory evidence (199, 200). In this proteomic study, all OATP family members including OATP1A2 were below the detection limit of the approach. It should be acknowledged that the brain tissue samples in this study came from subjects who died of peripheral diseases that have been previously shown to modulate expression of BBB transport proteins (200). As demonstrated by our group, presence of a pathological stressor in the periphery can have dramatic effects on BBB transporter expression and, subsequently, CNS drug delivery (12, 31, 144, 201, 202). Therefore, these proteomic data cannot be interpreted to suggest that OATP family members are absent from the human BBB or that these transporters do not represent viable targets for optimization of CNS drug delivery. Rather, mechanisms of OATP regulation in both health and disease need to be rigorously examined in order to fully comprehend OATP localization and expression at the human BBB. Additionally, the work of Uchida and colleagues underscores the need for in vivo studies to assess involvement of OATP isoforms in CNS drug delivery at the human BBB (200).
OATP1A2 was the first identified human OATP and the only human OATP/Oatp isoform whose expression is widely accepted at the BBB. This 670 amino acid protein shares 67% amino acid identity with rat Oatp1a1. OATP1A2 has been localized to both the luminal and abluminal membranes of human BBB endothelial cells (199). Known substrates of OATP1A2 include therapeutic agents such as antibiotics, antihistamines, antineoplastic drugs, beta-blockers, cardiac glycosides, endothelin-A receptor antagonists, HIV-1 protease inhibitors, HMG CoA reductase inhibitors, neuromuscular blocking agents, and opioid analgesic peptides (191). Endogenous OATP1A2 substrates include bilirubin, bromosulfophthalein, cholate, deltorphin II, estradiol-17β-glucuronide, estrone-3-sulfate, glycocholate, hydroxyurea, PGE2, reverse T3, taurocholate, taurochenodeoxycholate, tauroursodeoxycholate, T4, T3, and a metabolite of unoprostone (191).
In humans, two different OATP3A1 splice variants have been reported at the BCSF barrier with differing localization (203). While OATP3A1_v1 has been reported at the basolateral surface of choroid plexus epithelial cells, OATP3A1_v2 has been observed at the apical membrane of the BCSF barrier (203). Both variants (OATP3A1_v1 and OATP3A1_v2) are capable of transporting drugs (i.e., BQ-123) as well as physiological substrates (i.e., PGE1, PGE2, T4, vasopressin) (191). These splice variants do, however, have some differences in substrate profile. It has been demonstrated that deltorphin II is a substrate specific to OATP3A1_v1 while arachidonic acid is a substrate specific to OATP3A1_v2 (203).
Other OATP isoforms, such as OATP1C1 (204) and OATP2B1 (205), have been detected in human brain tissue. The exact localization and functional expression of these isoforms at CNS barriers has yet to be determined. However, there is evidence that these OATP isoforms are also expressed by glial cells (206, 207), suggesting a potential role for OATPs as determinants of CNS drug distribution. OATP1C1 has a more restrictive substrate profile compared to other OATP1 family members. Although OATP1C1 can transport prototypical OATP substrates (i.e., bromosulfophthalein, estradiol-17β-glucuronide, estrone-3-sulfate), it primarily functions in the blood-to-tissue delivery of thyroid hormones (1). In contrast, OATP2B1 is involved in transport of many therapeutic compounds, including atorvastatin, bosentan, fexofenadine, fluvastatin, montelukast, pravastatin, pitavastatin, rosuvastatin, and talinolol (208). Other known substrates for OATP2B1 include aliskiren, benzylpenicillin, bromosulfophthalein, dehydroepiandrosterone-3-sulfate, estrone-3-sulfate, ezetimibe glucuronide, glibenclamide, mesalazine, pregnenolone sulfate, taurocholate, tebipenem pivoxil, and a metabolite of unoprostone (208). Given the ability of OATPs to transport structurally and therapeutically diverse drugs, targeting OATP-mediated drug influx may represent an excellent opportunity for efficient and effective delivery of drugs to the brain.
Rodent Oatps expressed at the BBB and/or BCSF Barrier
Oatp1a1 is a 670 amino acid protein expressed in multiple tissues including the brain (192). Originally cloned from rat liver, RT-PCR approaches were used to demonstrate its mRNA expression at the choroid plexus (209). Protein expression of Oatp1a1 at the choroid plexus has yet to be confirmed, which is partly due to cross-reactivity of currently available Oatp1a1 antibodies with other Oatps such as Oatp1a5. Oatp1a1 substrates include bile salts, organic anions, organic cations and drugs (i.e., pravastatin, DPDPE, fexofenadine) (192). The functional relevance of Oatp1a1 at the choroid plexus remains unclear.
Oatp1a4 is a 661 amino acid protein that is expressed at the luminal and abluminal membranes of the rodent BBB endothelium and at the choroid plexus (192). Substrates include drugs (i.e., opioid analgesic peptides, HMG CoA reductase inhibitors), bile salts, hormones, peptides and endogenous organic cations (210). Along with Oatp1a5 at the BCSF barrier and Oatp1c1 at the BBB, Oatp1a4 is responsible for thyroid hormone uptake into the CNS (210). It has also been proposed that Oatp1a4 is the primary drug transporting Oatp isoform expressed at the rat BBB (190). For example, Oatp1a4 mediates blood-to-brain transport of DPDPE (12, 211) and pravastatin (212). Recently, increased Oatp1a4 functional expression was demonstrated following a pathological stressor, PIP (12). After eliminating P-gp-mediated efflux by pharmacological inhibition, the relative contribution of Oatp1a4 to brain uptake of DPDPE was shown to increase from 56% in saline controls to 71% in animals subjected to PIP (12). These data are critical because they demonstrate that OATP/Oatp isoforms may represent a viable target that can be exploited for delivery of drugs to the brain.
Oatp1a5, a 670 amino acid protein, is the most highly expressed Oatp family member at the rodent BCSF barrier (187, 192, 207). Specifically, Oatp1a5 is localized to the apical brush-border membrane of choroid plexus epithelial cells. Substrates of Oatp1a5 include bile salts, steroid conjugates and thyroid hormones (210). Its presence on the opposite membrane as Oatp1a4 and Oatp1c1 could facilitate movement of common substrates into or out of the CSF (192).
Oatp1c1 is a 716 amino acid protein that is expressed at both the BBB and the BCSF barrier. At the BBB, Oatp1c1 has been localized to the luminal and abluminal membranes of rodent BBB endothelial cells (192). In the choroid plexus, expression is primarily at the basolateral membrane of choroid plexus epithelial cells (192). Oatp1c1 has a more restrictive substrate profile than most other OATPs/Oatps, functioning primarily as a high-affinity thyroxine transporter (213). In addition to thyroxine, Oatp1c1 transports reverse T3 (3,3′, 2,5′-triiodothyronine), cerivastatin and estradiol E217βG (192).
Oatp2a1 is ubiquitously expressed in rodents and exclusively transports PGE2 (214). At the BBB, its expression pattern shifts from predominantly luminal to predominantly cytoplasmic in response to lipopolysaccharide, suggesting that this transporter may play a role in the physiology of fever (215). Although Oatp2b1 has been detected in rodent brain tissue, its localization at brain barrier sites has yet to be determined (192).
Organic Anion Transporters (OATs/Oats)
OATs/oats are members of family 22 of the SLC superfamily (SLC22A) (187). Current members of the OAT/oat family include OAT/oat 1-6 and the renal specific transporter (RST) (216-225). OATs are classified according to their energy requirements: Na+ dependent, Na+ independent, and ATP-dependent (138, 226). These transporters mediate movement of organic anions across biological membranes. This includes a variety of endogenous and exogenous molecules such as prostaglandins, hormones, and anionic neurotransmitter metabolites as well as drugs and their associated metabolites (123, 227). OAT/Oat substrates generally possess a negative charge at physiological pH and therefore require a specific transport system to cross biological membranes. The prototypical OAT/Oat substrate is p-aminohippurate (PAH), a low molecular weight organic anion. OATs/Oats have a broad substrate profile and can transport small amphiphilic organic anionic compounds with varied chemical structures, uncharged molecules, and some organic cations (123, 227).
The predicted membrane topology of OATs/Oats includes 12 membrane-spanning α-helices and several glycosylation and PKC sites, which are located on extracellular loops connecting helices 6 and 7 (226, 227). OATs/Oats are found primarily in epithelial tissues (kidney, liver), but are also expressed in the brain at the choroid plexus and in brain microvasculature (216-220, 228). OAT1/Oat1 is weakly expressed in the brain (199, 206-208). OAT2 protein is expressed in both the liver and the brain; however, Oat2 mRNA has only been detected in rat choroid plexus (84, 219, 229, 230). In contrast, OAT3/Oat3 is the most highly expressed OAT/Oat isoform in the brain. Oat3 has been shown to transport PAH, estrone sulfate, taurocholate, ochratoxin, benzylpenicillin, cimetidine, and ranitidine (187, 231, 232). Studies have shown that Oat3 plays a critical role in transport of anionic neurotransmitter metabolites of epinephrine, norepinephrine, dopamine, and serotonin (219). It is localized to the apical membrane of choroid plexus epithelial cells where it mediates entry of substrates into the choroid plexus and out of the CSF (231). Oat3 is known to be localized to the basolateral membrane, and may be present at the apical membrane, of rat brain capillary endothelial cells (233). Expression of Oat3 on both the apical and basolateral membranes would allow for both blood-to-brain and brain-to-blood transport of Oat3 substrate drugs. Thus, this family of transporters may be a potential target for increasing delivery of therapeutic compounds into the CNS.
OAT4/Oat4 is also known to be expressed at brain barriers but its exact protein localization has not been confirmed. Oat4 mRNA has been detected in epithelial cells of the choroid plexus (234). Expression of OAT4 has also been detected in human brain microvessel endothelial cells but only at the mRNA level (235). Oat4 transport has been characterized as bidirectional and sodium independent. Studies have shown that Oat4 is involved in transport of prostaglandins (236). Other substrates include estrone sulfate and ochratoxin A (220, 237). Currently there is no evidence to suggest the expression of OAT5/Oat5 and/or OAT6/Oat6 in the brain (225, 228).
Organic Cation Transporters (OCTs/Octs)
Organic cations have either a transient or permanent positive charge and, therefore, do not permeate biological membranes to any appreciable extent. Such substances require specific transport mechanisms to enable them to traverse biological membranes and accumulate within cells (225, 226). The identified organic cation transporters (OCTs) are members of family 22 of the solute carrier superfamily (SLC22A) (238) and are categorized into two groups according to their transport capabilities. Oligospecific organic cation transporters facilitate transport of one main substrate and/or its analogs. This category of OCTs include Na+ co-transporters for neurotransmitters, high affinity transporters of thiamine (vitamin B1) and vesicular and plasma membrane transporters for choline (i.e., the precursor of acetylcholine) (239). Polyspecific OCTs transport organic cations with a variety of chemical structures (240-242). OCT systems are further characterized as being either potential-sensitive organic cation transporters (OCTs) or H+ gradient-dependent novel organic transporters (OCTNs). OCTs include OCT/Oct1-3 while OCTN/Octn transport systems includes OCTN1-3 in human and Octn1-3 in rodents (243-245). In general, OCTs/Octs are involved in cellular influx of cationic substrates (244, 246) while OCTNs/Octns mediate cellular efflux of various cationic substances (246, 247).
OCT/Oct family members are predicted to have a structure that consists of 12 α-helical transmembrane domains with intracellular N and C-termini. Additionally, the predicted membrane includes a large extracellular loop between TM1 and TM2 that contains several N-glycosylation sites and a small intercellular loop that connects TM6 to TM7. The C-terminus contains consensus sequences to several kinase-dependent phosphorylation sites (240, 247, 248). OCT/Oct1-3 are primarily localized to the basolateral membrane of polarized cells including brain microvessel endothelial cells and choroid plexus epithelial cells. OCT1 mRNA and protein is expressed in human brain endothelial cells (hCMEC/D3) where it mediates transport of lamotrigine, an antiepileptic drug (249). Unlike OCT1/Oct1, OCT2/Oct2 is expressed in a variety of tissues within the CNS, such as neurons, choroid plexus, and cortical astrocytes. Within neurons, OCT2 mediates the transport of neurotransmitters such as serotonin, dopamine and norepinephrine (250-252). OCT3/Oct3 is expressed in a variety of tissues, including the brain (253-255). Within the CNS, expression of Oct3 mRNA is higher than that reported for Oct1 and Oct2 (256). Expression of Oct3 has been detected in neurons and the rodent choroid plexus (254, 257, 258) where it mediates transport of tetraethylammonium (TEA), dopamine, serotonin, and amphetamines (256). In addition to the transport of endogenous organic cations, OCTs/Octs may play a role in CNS drug penetration/distribution. For example, an in vitro study using conditionally immortalized rat brain capillary endothelial cells (TR-BBB13) revealed the role of a putative organic cation transporter in cellular uptake of oxycodone (259).
To date, only three isoforms of Octns have been identified in rodents, Octn 1-3. In contrast, two OCTN isoforms, designated OCTN1 and OCTN2, have been reported in humans (260). All OCTNs transport organic cations and carnitine, an amino acid required for transport of fatty acids across the inner mitochondrial membrane for ATP generation (261-263). While OCTN1 has not been reported in human CNS tissue (264)Octn1 has been detected in rodent spinal cord, choroid plexus, hippocampus, cortex, and cerebellum (84, 250, 265). In addition to carnitine, substrates of OCTN1/Octn1 include TEA, quinidine, choline, nicotine, cimetidine, and clonidine (266). OCTN2 is localized in a number of tissues including the brain (267). OCTN2 mRNA has been found in neurons from the hippocampus, cerebellum, and cerebral cortex (250, 258, 267, 268) and OCTN2/Octn2 protein expression has been detected in primary cultures of brain capillary endothelial cells from a cows, pigs, rats, and humans (123). Functional studies involving transport of acetyl-L-carnitine indicate that OCTN2 is localized to the luminal side of the BBB (269, 270). OCTN2 has been characterized as a high affinity Na+ - dependent plasmalemmal carnitine electrogenic transporter (123, 271, 272). Octn3 is highly expressed in the liver and the testes but there is no known evidence to suggest that this transporter is expressed in the CNS (273).
Nucleoside Membrane Transport Systems
Nucleosides cannot be synthesized de novo in the brain. Therefore, brain cells must rely on recycling/salvaging pathways that require transport of nucleotides into CNS tissue. Since nucleosides function as second messengers in many signal transduction pathways, their regulation is critical for proper neuronal function (123). For example, nucleoside membrane transport systems regulate adenosine-mediated neuromodulation by controlling local concentrations of adenosine at adenosine receptor sites (169). Nucleoside membrane transporters are mechanistically and structurally unrelated and are categorized according to their Na+ dependence. Equilibrative nucleoside transporters (ENTs) are Na+ - independent and are members of the SLC29A transporter family (123). In contrast, concentrative nucleoside transporters (CNTs) are Na+ -dependent and are members of the SLC28A transporter family (104).
a) Equilibrative Nucleoside Transporters (ENTs/Ents)
ENTs are expressed in eukaryotes but no evidence of prokaryotic expression has been found to date (274). ENTs are ubiquitously expressed and four isoforms of ENTs have been discovered in both humans and rodents: ENT1/Ent1 (SLC29A1), ENT2/Ent2 (SLC29A2), ENT3/Ent3 (SLC29A3), and ENT4/Ent4 (SLC29A4) (275-277). These four ENT isoforms are grouped according to their sensitivity, or lack thereof, to nitrobenzylmercaptopurine (NBMPR), a competitive inhibitor of nucleoside transport. ENTs that are NBMPR sensitive are categorized as equilibrative sensitive (es) ENTs (i.e., ENT1/Ent1, ENT3/Ent3) while those that are NBMPR insensitive are categorized as equilibrative insensitive (ei) ENTs (i.e., ENT2/Ent2) (278). Despite this categorization, all ENT/Ent family members transport nucleosides in a bidirectional manner via a mechanism governed by the transmembrane concentration gradient. ENTs are predicted to possess 11 α-helical transmembrane domains with an intracellular N-terminus and extracellular C-terminus. In addition to a large cytoplasmic loop that connects TM6 and TM7, an N-glycosylation site is located on the extracellular loop connecting TM1 and TM2 (278, 279). Glycosylation of the extracellular loop is not necessary for transport function; however, it has been suggested that glycosylation may affect ENT binding affinity for transport inhibitors (188).
ENTs are critical within the CNS because they are responsible for salvaging nucleosides necessary for biosynthetic and regulatory pathways. ENT1/Ent1 (SLC29A1) is ubiquitously expressed in human and rodent tissues with mRNA and protein observed in both plasma and nuclear membranes (280). ENT2 (SLC29A2) mRNA is localized in a variety of human tissue types including brain (281). ENT1/Ent1 and ENT2/Ent2 are the most well characterized ENT/Ent isoforms and display broad substrate specificity for purine and pyrimidine nucleosides. For example, ENT1/Ent1 is a critical transport mechanism for antineoplastic drugs such as cladribine and cytarabine (282). ENT3/Ent3 is ubiquitously expressed in both rodent and human tissue. Unlike other ENTs that are expressed on cellular membranes, ENT3/Ent3 shows a predominant subcellular localization on lysozomal membranes. Its intracellular location has given rise to the idea that ENT3/Ent3 may function as an organellar transporter (277). Similar to ENT1 and ENT2, ENT4 is ubiquitously expressed, especially in brain tissue, and is localized to cell surface membranes (283). Moreover, the exact transport mechanism for ENT4/Ent4 has yet to be elucidated.
b) Concentrative Nucleoside Transporters (CNTs/Cnts)
Unlike ENTs, CNTs have been found in both prokaryotes and eukaryotes (275). CNTs are not expressed ubiquitously, rather, they are expressed in specialized cell types such as macrophages, microglia, leukemia cells, choroid plexus, and renal and gastroepithelia (276, 284-287). Six functionally different transport activities have been described for CNT transporters: cit (pyrimidine nucleosides and adenosine), cif (purine nucleosides and uridine), cib (purine and pyrimidine nucleosides), cit-like (pyrimidine selective but also transports adenosine and guanosine), cs (selective for adenosine and adenosine analogs) and csg (guanosine selective). To date three CNT isoforms have been identified: CNT1 (SLC28A1), CNT2 (SLC28A2), and CNT3 (SLC28A3) that have cit, cif, and cib activities respectively (123). All three known CNT transporters are insensitive to inhibition by NBMPR. CNT1 and CNT2 function as antiporters that transport nucleosides into the cell in exchange for sodium ions (288). CNT3 is also an antiporter but it transports nucleosides in exchange for either sodium ions or protons (288). In terms of membrane topology, CNTs are integral membrane proteins with 13 transmembrane α-helices and a large extracellular C-terminal region that is known to be glycosylated (289).
CNT1/Cnt1 is primarily expressed in epithelial tissues and is localized to the apical membrane of such polarized cells. Although Cnt1 mRNA transcripts are expressed in rodent brain, there is no evidence that Cnt1 protein is expressed at the BBB (290-292). Cnt1 mediates transport of a variety of nucleoside analogs that are routinely used to treat cancer and HIV-1 infection (293). CNT2 mRNA has been detected in various tissues including brain (291, 294, 295). Specifically, CNT2 protein has been identified at the luminal side of the BBB endothelium and the apical side of the choroid plexus epithelium. Measurement of radiolabeled adenosine uptake in rat brain endothelial cells (RBECs) and rat choroid plexus endothelial cells (RCPECs) cells demonstrated polarized transport activity, supporting the idea of polarized localization of Cnt2 (296). Non-physiological substrates of Cnt-2 include anti-retrovirals such as didanosine and ribavirin (191). CNT3/Cnt3 mRNA has been detected in various tissues including the brain (297) and is known to mediate transport of nucleoside analogs that are used as chemotherapeutics or drugs for treatment of HIV-1 infection. These analogs include cladribine, gemcitabine, and zidovudine (285).
Peptide Transporters
Peptide transporters are members of family 15 of the SLC superfamily (SLC15A). There are four members of the SLC15A family: peptide transporter-1 (PEPT1/Pept1; SLC15A1), peptide transporter-2 (PEPT2/Pept2; SLC15A2), Peptide/histidine transporter-1 (PHT1/Pht1; SLC15A4), and peptide/histidine transporter-2 (PHT2/Pht2; SLC15A3) (298-300). Such transporters are proton-dependent oligopeptide transporters that transport small peptides across biological membranes via an inwardly directed electrochemical proton gradient and a negative membrane potential (301-303). In mammals, the proton gradient necessary for transport activity is established by electroneutral proton-cation exchangers such as Na+/H+ antiporters (304, 305). The prototypical structure of peptide transporters consists of 12 α-helical transmembrane domains with both C- and N-termini located intracellularly. Extracellular loops can contain between 2 to 7 glycosylation sites while intracellular loops contain protein kinase A and C phosphorylation sites (85, 299).
Peptide transporters are expressed in several tissues including CNS (306-311). PEPT1/Pept1 tissue expression varies among different species but this transporter has not been detected within the CNS (300). In contrast, PEPT2/Pept2 has been detected in cerebral cortex, cerebellum, choroid plexus, and astrocytes (306, 309, 312, 313). PEPT2/Pept2 has a high affinity for a di- and tri-peptides such as Gly-Gln (314). Although Pept2 is expressed within rat cerebral cortex, its physiological role within the CNS is yet to be determined (309). It has been proposed that Pept2 plays a role in GSM metabolism by providing cysteineglycine (315). Pept2 is localized to the apical membrane of the choroid plexus epithelial cells (305). Its functional role at the choroid plexus includes efflux of neuropeptides, peptides, and peptidomimetics out of the CSF and into the blood (316-318).
In situ hybridization studies have detected Pht1 in rat brain at multiple locations including hippocampus, cerebellum, and cerebral cortex (319). Established substrates of PHT1/Pht1 include histidine, di- and tri-peptides as well as the antiviral drug valcyclovir (300, 320). Little is known about the specific functional role of PHT2/Pht2 other than that, like other peptide transporters, it has high affinity for di- and tri-peptides and plays a critical role in absorption and excretion of peptide nutrients (300). In addition to di- and tri-peptides, histidine and carnosine are well-established substrates of PHT2/Pht2 (300).
In addition to specific peptide transporters, brain uptake and distribution of peptides is also determined by transport systems that are endogenously expressed at the BBB endothelium. Of these systems, some are unidirectional (i.e., facilitate either blood-to-brain or brain-to-blood peptide transport) whereas others are bidirectional. For example, the peptide transport system-1 (PTS-1) has been found to mediate transport of tyrosinlylated analogues of the endogenous neuropeptide melanocyte inhibiting factor-1 (MIF-1), which is known to have opiate and antiopiate activity (321, 322). Analogs of pituitary adenylate cyclase-activating polypeptide (PACAP), a polypeptide with endocrine and vasodilatory properties, are transported across the BBB by PTS-6 (323-325). Specifically, blood-to-brain delivery of a 38 amino acid residue PACAP analog is facilitated by a saturable, carrier-mediated uptake component of PTS-6 (321) while CNS uptake of a 27 amino acid PACAP analog is determined by passive diffusion and a PTS-6 efflux component (324). In these studies, preservation of CNS concentrations of pharmacologically active peptides and/or enhancement of brain delivery of peptide therapeutics was achieved by blocking the efflux component of PTS-6 (321, 324).
Monocarboxylate transporters (MCTs/Mcts)
Monocarboxylate transporters (MCTs/Mcts) are members of solute carrier family 16 (SLC16), which is composed of 14 members. Of these 14 members, only six have been functionally characterized and include: MCT/Mct1-4, MCT8/Mct8, and the T-type amino acid transporter-1 (TAT-1/ MCT10) (326, 327). Of these transporters, MCT/Mct1-4 have been shown to be responsible for mediating transport of monocarboxylates (i.e. pyruvate, lactate, and ketone bodies) across plasma membranes, which is essential for metabolism of carbohydrates, fats, and amino acids (326, 328). MCT10/Mct10 was originally identified as an amino acid transporter in rat small intestine and transports aromatic amino acids, such as tryptophan, tyrosine, and phenylalanine across the plasma membrane (329). MCT8 expressed in Xenopus laevis oocytes has been characterized as a thyroid hormone transporter and has been shown to transport both T3 and T4 (330). Unlike MCT10, MCT8 does not appear to transport aromatic amino acids (330). The functions of the other 8 members of SLC16 are not known (328, 331).
MCT1 is expressed ubiquitously throughout the human body with higher expression in tissues that metabolize lactate (326). Immunocytochemical analysis using a polyclonal affinity-purified antibody to the C-terminal of Mct1 demonstrated localization of Mct1 to brain microvascular endothelium of adult and suckling rats (332). Staining associated with Mct1 immunoreactivity in adult rats was stronger than that observed in microvessels obtained from neonatal rats (332). A more recent study using electron microscopy employing the immunogold approach also demonstrated higher Mct1 expression in cerebral capillary endothelial cells from suckling rat pups as compared to adult rats. Mct1 staining was nearly equally distributed between the luminal and abluminal membranes and some light staining was detected at the choroid plexus epithelium (333). Mct1 mRNA has also been detected in primary cultures of rat astrocytes with only slight mRNA detection in neuron-rich cultures (334).
MCT2/Mct2 is expressed in a variety of tissues including the brain (326). Mct2 appears to be the only Mct isoform expressed in neurons and is believed to be the principal lactate uptake transporter present in rodent brain (334, 335). Western blot analysis revealed expression of Mct1 and Mct2 in shark brain tissue, where Mct1 was primarily localized to neuronal mitochondria while Mct2 was expressed at the neuronal plasma membrane (336). Mct2 mRNA is expressed in various rodent brain regions such as cerebral cortex, hippocampus, and cerebellum (334, 337). Protein expression patterns of this transporter is similar to its mRNA expression (335). There is some controversy as to whether MCT2/Mct2 is expressed in brain capillary endothelial cells. Koehler-Stec and colleagues detected Mct2 mRNA in mouse microvessels via Northern blot analysis (337); however, western blot analysis did not detect Mct2 protein in rat microvessels (338)
Studies using northern hybridization have found MCT3/Mct3 to be primarily expressed in retinal pigment epithelia with some expression in the choroid plexus epithelium (87, 335). Immunohistochemical analysis revealed MCT3/Mct3 to be expressed on the basolateral membrane of both epithelia (87). Few studies have been conducted to investigate the expression of MCT4/Mct4 in the CNS; however, Mct4 does appear to be expressed in astrocytes (335). Immunogold cytochemistry showed immunogold staining for Mct4 that was associated with glial cells in rat brain tissue (339). Studies investigating expression of MCT/Mct transporters in rat brain using immunoperoxidase and double immunofluorescence microscopy showed that Mct4 was present in astrocytes throughout all stages of postnatal rat development (340).
There are many similarities in MCT1/Mct1, MCT3/Mct3, and MCT4/Mct4 affinities for monocarboxylates across species; however, MCT2’s affinity for monocarboxylates differs considerably from its rodent orthologue Mct2 (326). MCT1/Mct1 exhibits a slightly higher affinity for pyruvate as compared to lactate in hamster Sf-9 cells, human and rat oocytes and mouse ELT cells. (341). Bovine and rat Mct4 exhibit a low affinity for lactate with Km values of 10 mM and 34 mM, respectively (341, 342). Human MCT4 has a greater affinity for lactate (Km 28 mM) compared to pyruvate (Km 153 mM) (343). Several studies have implicated MCTs/Mcts the transport of exogenous compounds including drugs. Drugs transported by MCTs/Mcts are weak organic acids that are monovalent and contain a relatively small R group that is relatively small (341). In vitro studies using primary cultures of bovine capillary endothelial cells demonstrated transport of radiolabeled HMG-CoA reductase inhibitors (i.e., simvastatin acid, lovastatin acid), which involved a carrier mediated transport system that transported organic anions with a monocarboxylic acid moiety (344). Inhibition of simvastatin acid transport by acetate indicates that simvastatin uptake may be mediated by MCT1/Mct1 because acetate is a well established pharmacological inhibitor for MCT1/Mct1 (326). In addition to statins, MCTs/Mcts have also been implicated in the transport of non-steroidal-anti inflammatory compounds and β-lactam antibiotics (i.e. phenethelicilin and propicillin) (326).
Drug Delivery to the Central Nervous System: strategies to circumvent brain barrier sites
The blood-brain barrier (BBB) is a formidable obstacle to drug delivery. Transcellular permeability of compounds across the BBB is complex and regulated by expression of various transport proteins. In fact, the overall balance of these transporters is a critical determinant in CNS permeation of multiple therapeutic drugs. Restricted entry of therapeutic compounds into the CNS results in ineffectual treatment of neurological disorders and/or traumas such as epilepsy, brain cancer, HIV-associated neurocognitive disease, cerebral hypoxia, ischemic stroke, and PIP. Therefore, several therapeutic strategies have been developed to circumvent the BBB and improve CNS drug delivery. Among those developed, some efforts have involved invasive procedures such as forced opening of the BBB that can cause undesirable side effects. Other efforts have focused on circumventing those efflux transporters (i.e., P-gp, MRPs/Mrps, BCRP/Bcrp) that severely limit entry of therapeutic compounds into the brain. While efflux transporter inhibition has achieved modest success in improving CNS drug permeability, their utility is greatly limited by adverse drug reactions that may occur due to increased drug concentrations in the brain and other peripheral tissues. Recently, there is a growing interest in exploiting other transport systems to improve drug delivery, including targeting endogenous influx transporters expressed at brain barrier sites. The following section will provide a brief overview of various methods for CNS drug delivery that have been developed to date as well as some advantages and disadvantages of each method.
Intracerebral implants/Intraventricular infusion
Early strategies adopted to circumvent the BBB involved invasive neurosurgery-based techniques, including intracerebral implants and acute or continuous intraventricular infusion of drugs directly into brain tissue have been used to treat various CNS disorder such as brain tumors and epilepsy (102).. In a rat epileptic model, delivery of phenytoin via implantation of polymers near the seizure focus resulted in decreased seizure activity (345). Similar results on seizure activity have been achieved using intraventricular infusion of anti-epileptic drugs. However, there was also an increase in CNS toxicity associated with increased brain drug concentrations (345-347). Direct delivery of CNS therapeutics via these techniques is not only invasive, but can lead to a host of unwanted adverse effects such as susceptibility to infection as well as unforeseen changes in BBB physiology and transporter expression.
Osmotic Opening of the Blood-Brain Barrier
Another strategy to bypass barrier transport systems is transient opening of the BBB through intracarotid infusion of hypertonic solutions such as those containing arabinose or mannitol. Under this approach, increased brain permeability of therapeutics is the result of vasodilation and shrinkage of cerebral capillary endothelial cells, events that open the paracellular route and enable drugs to freely diffuse across the BBB. Such vascular events may be facilitated by Ca2+- mediated contraction of the actin cytoskeleton (348). For example, exposure of cultured rat brain capillary endothelial cells to high concentrations (i.e., 1.4 M) of mannitol resulted in a rapid increase in intracellular concentration of Ca2+ with peak Ca2+ levels observed 10 seconds after exposure (349). Intracellular Ca2+ concentrations returned to basal levels approximately 200 s after initial mannitol exposure (349). Addition of a Na+/Ca2+ exchanger blocker did not interfere with the rapid increase in intracellular Ca2+ but it did prevent calcium levels from returning to baseline levels (349). Additional in vivo studies found that administration of a Na+/Ca2+ exchanger blocker prolonged osmotic opening of the BBB. BBB opening as assessed via intra-arterial perfusion of radiolabeled sucrose. Animals treated with the Na+/Ca2+ exchanger blocker exhibited a longer recovery time before BBB permeability levels returned to baseline levels. Animals treated with the Na+/Ca2+ exchanger blocker exhibited increased sucrose permeability across the BBB as compared to control animals (350). Osmotic BBB opening has been used in clinical settings to improve delivery of CNS therapeutics. For example, osmotic opening of the BBB using a 25% mannitol solution improved brain penetration of chemotherapeutics used to treat malignant primary or metastatic brain tumors (351).
Although effective at increasing permeability of therapeutics into the CNS, there are several drawbacks to this technique. Firstly, studies have suggested that different variables, such choice of anesthetic, can dramatically affect the degree of BBB opening. For example, the use of propofol/N2O resulted in greater osmotic BBB opening in rats compared to rats anesthetized with isofluorane/O2 (352). Transient opening of the BBB also results in increased intracranial pressure and brain edema (348, 353). Additionally, disruption of the BBB can leave the CNS vulnerable to infection from circulating pathogens or can allow entry of plasma proteins, which can trigger neuronal cell apoptosis (99, 347).
Blood-Brain Barrier Opening via Ultrasound and Electrical Stimulation
Focused ultrasound (FUS) is non-invasive disruptive technique that can be used to transiently increase BBB permeability and enhance delivery of therapeutics into the brain. FUS, concentrates acoustic energy that permeates through the skull and brain tissue onto small target area in the brain (354). Ultrasound frequencies used for BBB disruption range between 28 KHz to 8 Mhz for rodents, with clinical relevant frequencies falling between 0.2 and 1.5 MHz (354-356). Intravenous injection of microbubbles, typically used as a contrast agent (357), significantly reduces that amount of power necessary to open the BBB (358). The microbubbles expand and contract during sonication, a phenomena known as acoustic cavitation, and it is believed that disruption of the BBB is caused by this expansion and contraction that stretches the blood vessel walls (354). This opening of the blood-brain barrier is transient and has been found to reverse itself as early as 6 hours following FUS as long as no tissue damage has occurred (359). In vivo studies in rats investigating approaches to improve delivery of natural killer (NK) cells to metastatic brain tumors found that FUS improved delivery of NK cells to cerebral tumor sites (360). In a breast cancer metastasis model, FUS was shown to effectively enhance permeability at the BBB as well as the blood-tumor barrier, thus improving CNS delivery of the anti-cancer drug trastuzumab (361). Improved drug delivery resulted in decreased brain tumor volume as well as improved survival time (361).
Electrical stimulation has also been shown to be effective at increasing BBB permeability and improving delivery of therapeutics to the brain. For example, electrical stimulation of postganglionic parasympathetic fibers of the sphenopalatine ganglion (SPG) has been shown to increase BBB permeability to FITC-dextran, implying an increase in BBB permeability (72). Delivery of etoposide and HER2 monoclonal, both chemotherapeutic agents, was also improved by the use of electrical stimulation of postganglionic parasympathetic fibers of the SPG (72). Stimulation of the SPG has also been found to induce reperfusion and BBB protection in a rodent stroke model (362). Following focal cerebral ischemia (i.e., 15 min or 24 h), SPG stimulation was given for 3 h per day for four consecutive days. SPG-treated animals not only demonstrated increased cerebral blood flow, but also decreased BBB opening and lesion volumes as compared to untreated stroke animals (362).
Inhibition of Brain Barrier Efflux Transporters
In order to circumvent efflux transporters at brain barrier sites, particularly P-gp, pharmacological inhibitors have been developed with the intent of enabling greater drug penetration of into the CNS. Such studies have shown mixed results with regards to efficacy and safety of these inhibitory compounds. The first generation of P-gp inhibitors were identified in the early 1980’s. Despite their ability to inhibit P-gp transport activity and increase cellular drug permeation, the doses required to be effective inhibitors were extremely high and resulted in both toxicity and unwanted pharmacokinetic interactions (147). Second generation inhibitors, such as PSC833 (i.e., valspodar), are much more potent than their predecessors and do exhibit less toxicity. However, PSC833 demonstrated disappointing results in clinical studies, with only modest increases in CNS drug delivery (147). Additionally, this generation of inhibitors significantly inhibited metabolism and excretion of cytotoxic agents. These unexpected effects necessitated reduction in chemotherapy doses to levels that were no longer efficacious (147).
Recent work examining mechanisms that regulate changes in P-gp functional expression have elucidated discrete signaling pathways that can be targeted to impair P-gp function and improve CNS drug delivery. Targeting such pathways is an attractive alternative to global inhibition of P-gp as it can lead more precise control of this transporter in specific target tissues and/or preservation of basal P-gp activity, which is critical for neuroprotection. For example, the role of sphingolipid signaling in regulating basal levels of P-gp activity has been recently investigated. Using a confocal-based activity assay that used rat brain capillaries, investigators determined that basal activity levels of P-gp were regulated via signaling through the sphingosine-1-phosphate receptor (S1PR) (363). Exposure of brain capillaries to S1P, a bioactive lipid metabolite and endogenous ligand for S1PR1, resulted in a reduction in P-gp transport (363). Removal of S1P from the capillary media restored P-gp efflux activity to levels seen in the control group, demonstrating that signaling via S1PR can allow for transient modulation of P-gp-mediated efflux activity (363). Changes in P-gp function observed in isolated brain capillaries were validated in vivo using the in situ perfusion technique. Animals treated with S1PR antagonists (i.e., S1P, FTY720, or FTY729P) exhibited increased brain uptake of radiolabeled verapamil, loperamide, and paclitaxel, demonstrating reduced P-gp activity in vivo (363). The use of VPC, a S1PR1-specific antagonist, blocked this effect (363). While targeting the sphingolipid pathway may prove to be a useful method for controlling efflux transport at the BBB to facilitate CNS drug delivery, further studies are required to determine what type of dosing adjustments are needed for existing drugs when they are co-delivered with an S1P receptor agonist. Additionally, it is critical to determine what other efflux and influx transporters are affected by S1P receptor agonists in order to accurately assess the viability of targeting sphingolipid signaling for optimization of CNS drug delivery.
Studies investigating effects of PIP on P-gp trafficking at the BBB have revealed dynamic changes in P-gp trafficking and association with caveolin-1, a key scaffolding protein. Under conditions of peripheral inflammatory pain, disassembly of high-molecular weight disulfide-bonding P-gp containing structures was accompanied by an increase in ATPase activity, indicating that changes in protein trafficking and structure result in changes in activity that may be responsible for controlling drug delivery to the CNS (202). Additionally, demonstration of a rapid reduction of P-gp efflux function at the BBB through endocytosis further highlights the potential that therapeutic manipulation of a trafficking pathway may have in temporarily reducing P-glycoprotein efflux activity at the luminal membrane (364).
Targeting Receptor-mediated and Adsorptive-mediated transcytosis
In utilizing receptor-mediated transcytosis to circumvent the BBB, compounds are either conjugated to an endogenous substance that binds to an endogenous BBB receptor or are designed to directly bind a BBB receptor. Binding triggers internalization of the receptor and bound drug. Once internalized, the two moieties separate and the receptor travels back to the membrane while the drug is free to diffuse throughout the endothelial cell or cross the abluminal membrane and diffuse into the brain parenchyma (99, 102). An example of the use of receptor-mediated transcytosis to facilitate CNS delivery of a therapeutic is the use of the monoclonal antibody OX26, which was designed to recognize the transferrin receptor (102). OX26 has been used in several studies to deliver therapeutic peptides into the CNS (99) and may be useful in bypassing efflux transporter such as P-gp. Uptake of digoxin, a known P-gp substrate, by RBE4 rat brain capillary endothelial cells was increased (25-fold) by incorporating digoxin into OX26-immunolioposomes (365). Additionally, studies investigating immunoliposome uptake and transcytosis in hCMEC/D3 cells found that uptake and transcytosis of immunoliposome-associated dyes (FITC, trisodium 8-hydroxypyrene-1,3-6-trisulfonate) was higher as compared to control liposomes (366).
The clinical utility of targeting native receptor-mediated transport into the CNS is further elucidated by studies examining uptake of ANG1005, a novel paclitaxel derivative, into the CNS in order to treat brain metastasis of breast cancer. To improve CNS drug delivery, a 19-amino acid peptide was designed to bind to the low-density lipoprotein receptor-related protein-1 (LRP-1), one of several LRP receptors that mediate transcytosis at the BBB (367). Previous studies have shown that angiopep-2 enhances transcytosis in vitro as well as in vivo (368). Conjugation of ANG1005 to angiopep-2 resulted in increased in vivo uptake of radiolabelled ANG1005 in mice with brain metastases of breast cancer (369).
Cationic proteins can be taken into the brain via adsorption-mediated transcytosis. These proteins bind to the luminal membrane of capillary endothelial cells via electrostatic interactions with anionic sites on the membrane. These anionic sites are due to expression of acidic glycoproteins on the luminal membrane (102, 103). Binding to the membrane triggers endocytosis of the cationic compound into capillary endothelial cells where it can act on its intracellular target or it may be free to diffuse into brain parenchyma where it may have a pharmacological effect (99).
Targeting influx transporters expressed at the BBB
It is well established that BBB efflux transporters are a formidable barrier to drug delivery to the CNS. In fact, many research efforts have focused on developing inhibitors or uncovering regulatory signaling pathways that may be exploited to prevent increased function expression of efflux transporters (i.e., P-gp, MRPs/Mrps, BCRP/Bcrp). Understanding mechanisms that regulate trafficking of transporters, such as P-gp, to the luminal membrane would allow for inhibition of increased translocation of transporters to the membrane while maintaining basal expression levels that are critical for neuroprotection. Additionally increasing attention is being focused on exploring the possibility of exploiting the function of various influx transporters, such as LAT-1 and Oatp1a4, to improve CNS drug delivery.
The conventional pharmacological intervention for treatment of Parkinson’s disease is the use of L-DOPA, the metabolic precursor to dopamine. Delivery of dopamine to the CNS is mediated via LAT-1, which is endogenously expressed at the brain microvascular endothelium (370). In addition to L-DOPA, LAT-1 mediates transport of other therapeutics across the BBB. Melphalan, a nitrogen mustard derivative of the neutral amino acid L-phenylalanine and LAT-1 substrate, is used to treat brain cancer. Competitive inhibition studies done in rats implanted with experimental CNS tumors found that the administration of melphalan inhibited the transport of phenylalanine into the brain, indicating that melphalan is also a substrate for LAT-1 (371). LAT-1 has also been found to transport α-methyl-DOPA and gabapentin (370, 372).
In addition to LAT-1, another intriguing influx transporter family that can be targeted for optimization of CNS drug delivery are OATPs/oatps, which transports opioid analgesic peptides (211, 373, 374). Recently, we reported for the first time increased functional expression of Oatp1a4, a rodent orthologue of OATP1A2, at the BBB in rats subjected to PIP (12). Evidence for increased Oatp1a4 transport at the BBB included i) increased brain accumulation of taurocholate, a selective Oatp substrate; ii) attenuation of taurocholate uptake by Oatp transport inhibitors (i.e., digoxin, estrone-3-sulfate, fexofenadine); iii) increased in KIN for taurocholate during peripheral inflammatory pain, which implies increased blood-to-brain transport; and iv) an increase in taurocholate accumulation within brain interstitial fluid but no change in taurocholate sequestration within the BBB endothelium itself (12). In order to determine if Oatp1a4 could effectively facilitate CNS drug delivery, we studied BBB transport of DPDPE. Brain uptake of DPDPE is governed by multiple mechanisms in addition to Oatp1a4-mediated transport including transcytosis (375) and P-gp-mediated efflux (373). Although we showed increased Oatp1a4 functional expression at the BBB in animals subjected to PIP, we did not see any change in blood-to-brain DPDPE transport (12). In light of our previous work with P-gp (144), we proposed that Oatp1a4 influx transport was negated by P-gp efflux. This implies that the relative contribution of Oatp1a4 to overall brain uptake of DPDPE could only be determined in the absence of P-gp mediated transport activity. When we inhibited P-gp efflux transport using reversin 205, a selective P-gp inhibitory peptide, we observed that the relative contribution of Oatp1a4 to brain uptake of DPDPE increased from 56% in saline controls to 71% in animals subjected to PIP (12). These data are particularly critical because they showed, for the first time, that Oatp1a4 can be targeted for delivering opioids and/or opioid peptides to the brain.
In order to successfully target a transporter system for optimization of CNS drug delivery, it is crucial to determine how a transport of interest is regulated at the molecular level. In the context of PIP, this includes identification and characterization of biological mechanisms that enable peripheral pain to “transmit” signals upstream and alter BBB drug transporters. Based on our previous work (11), we proposed that this signal may involve cytokines such as TGF-β1, a critical regulator of brain microvascular homeostasis (376). Of particular significance, we showed that administration of diclofenac, a commonly prescribed NSAID, prevented decreases in serum TGF-β as well as reduced microvascular expression of ALK1/ALK5, suggesting that peripheral inflammation in the periphery is involved in overall regulation of the TGF-β signaling pathway (12). Furthermore, pharmacological inhibition of TGF-β/ALK5 signaling using SB431542 increased Oatp1a4 functional expression both in animals subjected to PIP and in corresponding saline controls (12). Although studies in immortalized mouse brain endothelial cells (MBE4) have shown involvement of ALK5-mediated signaling in P-gp regulation (377). Our work on TGF-β/ALK5 signaling demonstrated that this pathway can regulate permeability at the BBB both by increasing functional expression of an influx transporter (12). Furthermore, this studies highlight the potential of the TGF-β/ALK5 pathway as a pharmacological target that can be utilized to precisely control functional expression of a BBB influx transporter for optimization of CNS drug delivery.
Conclusion
The field of BBB biology, particularly study of endogenous xenobiotic transport systems, has rapidly advanced over the past two decades. It is now well-established that TJs between the capillary endothelial cells effectively limits paracellular drug diffusion while expression of various efflux transporters (i.e., P-gp, MRPs/Mrps, BCRP/Bcrp) interact with a multitude of therapeutic compounds, further restricting their entry into the brain parenchyma. Additionally, many previous studies reported on the controversial ability of drug transporters (i.e., Oatp1a4) to act as facilitators of brain drug uptake. Now, it is beginning to be appreciated that endogenous BBB transporters can facilitate uptake of xenobiotics from blood to the brain, thereby rendering these transport proteins potential targets for optimizing CNS drug delivery. Furthermore, molecular machinery involved in regulating endogenous BBB transport systems (i.e., TGF-β/ALK5 signaling, nuclear receptor systems) and mechanisms governing intracellular trafficking of BBB transporters are just now being identified and characterized. These critical discoveries have identified multiple molecular targets that can be exploited for optimization of CNS delivery of therapeutic agents. Such studies are particularly critical for newly developed therapeutics such as opioid analgesic peptides. In fact, many novel opioid peptides have been recently produced and have shown analgesic efficacy (380, 381); however, molecular and trafficking mechanisms involved in their CNS delivery have yet to be identified. Discovery of mechanisms that determine brain permeation of these peptides will undoubtedly enable more efficient analgesia and an improved utility of these compounds as potential therapeutics. Perhaps targeting of novel opioid peptides to influx transporters such as OATPs/Oatps, which is already known to be involved in opioid peptide transport at the BBB (12), will lead to significant advancements in the field of opioid pharmacology and pain management. Additionally, identification and characterization of intracellular signaling pathways and trafficking mechanisms that can regulate functional expression of uptake transporters provides additional approaches for pharmacological control of drug transporter systems in an effort to deliver therapeutics to the CNS. Future work will continue to provide more insight on the interplay of TJ protein complexes, transporters, and intracellular signaling pathways at the BBB and how these systems can be effectively targeted. Ultimately, data derived from these studies will enable achievement of more precise drug concentrations within the CNS and improved treatment for pathological conditions.
Figure 2.
Methods of xenobiotic transport across the blood-barrier
Figure 3.
Transport mechanisms expressed in cells comprising the neurovascular unit. A multitude of transporters are expressed on capillary endothelial cells, astrocytes, microglia, and neurons. Transporter systems aid in transport of nutrients, peptides, and ions into the brain parenchyma and as well as efflux of waste and potentially neurotoxic substance out of the brain. Arrows indicate the proposed direction of substrate transport.
Figure 4.
Transporter systems expressed on at the BCSF barrier in choroid plexus epithelial cells.
Table 2.
ABC transporter proteins and selected transport substrates
| Representative Substrates | References | |
|---|---|---|
|
P-gp (ABCB1) |
morphine, digoxin, verapamil, dexamethasone, saquinavir, nelfinavir, saquinavir, paclitaxel, loperamide, actinomycin D |
(123, 132, 144) |
|
BCRP (ABCG2) |
doxorubicin, topotecan, methotrexate, imatinib, pitavastatin, cerivastatin, zidovudine, mitoxanthrone |
(168, 382) |
|
MRP1 (ABCC1) |
sulfate conjugates, LTC4, vincristine, daunorubicin, doxorubicin, etoposide, MTX, GSH, GSH conjugates, Glucuronide conjugates |
(173, 175, 179, 181) |
|
MRP2 (ABCC2) |
bilirubin, cisplatin, prastatin, sulforhodamine 101 acid chloride (Texas Red), GSH, GSH conjugates, glucuronide conjugates |
|
|
MRP3 (ABCC3) |
monoanionic and conjugated bile acids, MTX, etoposide, teniposide |
|
|
MRP4 (ABCC4) |
cyclic nucleotides (cAMP, cGMP), nucleotide analogs (PMEA, PMEG), purine analogs, prostaglandins, MTX, unconjugated bile acids, sulfate conjugates, GSH, glucuronide conjugates |
|
|
MRP5 (ABCC5) |
cyclic nucleotides (cAMP, cGMP), nucleotide analogs (PMEA), stavudine monophosphate, GSH |
|
|
MRP6 (ABCC6) |
small peptides, etoposide, cisplatin, daunorubicin, doxorubicin, GSH conjugates |
References
- 1.Ronaldson PTD. Pharmacological Reviews. 2012. TP. Targeted Drug Delivery to Treat Pain and Cerebral Hypoxia. Epub in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiological reviews. 1997;77(3):731–58. doi: 10.1152/physrev.1997.77.3.731. Epub 1997/07/01. [DOI] [PubMed] [Google Scholar]
- 3.Oldendorf WH, Cornford ME, Brown WJ. The large apparent work capability of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Annals of neurology. 1977;1(5):409–17. doi: 10.1002/ana.410010502. Epub 1977/05/01. [DOI] [PubMed] [Google Scholar]
- 4.Takakura Y, Audus KL, Borchardt RT. Blood-brain barrier: transport studies in isolated brain capillaries and in cultured brain endothelial cells. Advances in pharmacology (San Diego, Calif) 1991;22:137–65. doi: 10.1016/s1054-3589(08)60034-4. Epub 1991/01/01. [DOI] [PubMed] [Google Scholar]
- 5.Fenstermacher J, Gross P, Sposito N, Acuff V, Pettersen S, Gruber K. Structural and functional variations in capillary systems within the brain. Annals of the New York Academy of Sciences. 1988;529:21–30. doi: 10.1111/j.1749-6632.1988.tb51416.x. [DOI] [PubMed] [Google Scholar]
- 6.Betz AL, Firth JA, Goldstein GW. Polarity of the blood-brain barrier: distribution of enzymes between the luminal and antiluminal membranes of brain capillary endothelial cells. Brain research. 1980;192(1):17–28. doi: 10.1016/0006-8993(80)91004-5. Epub 1980/06/16. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez del Pino MM, Peterson DR, Hawkins RA. Neutral amino acid transport characterization of isolated luminal and abluminal membranes of the blood-brain barrier. The Journal of biological chemistry. 1995;270(25):14913–8. doi: 10.1074/jbc.270.25.14913. Epub 1995/06/23. [DOI] [PubMed] [Google Scholar]
- 8.Vorbrodt AW, Dobrogowska DH. Molecular anatomy of intercellular junctions in brain endothelial and epithelial barriers: electron microscopist’s view. Brain research Brain research reviews. 2003;42(3):221–42. doi: 10.1016/s0165-0173(03)00177-2. Epub 2003/06/07. [DOI] [PubMed] [Google Scholar]
- 9.Bendayan R, Lee G, Bendayan M. Functional expression and localization of Pglycoprotein at the blood brain barrier. Microscopy research and technique. 2002;57(5):365–80. doi: 10.1002/jemt.10090. Epub 2002/07/12. [DOI] [PubMed] [Google Scholar]
- 10.Huber JD, Campos CR, Mark KS, Davis TP. Alterations in blood-brain barrier ICAM-1 expression and brain microglial activation after lambda-carrageenaninduced inflammatory pain. Am J Physiol Heart Circ Physiol. 2006;290(2):H732–40. doi: 10.1152/ajpheart.00747.2005. Epub 2005/10/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ronaldson PT, Demarco KM, Sanchez-Covarrubias L, Solinsky CM, Davis TP. Transforming growth factor-beta signaling alters substrate permeability and tight junction protein expression at the blood-brain barrier during inflammatory pain. J Cereb Blood Flow Metab. 2009;29(6):1084–98. doi: 10.1038/jcbfm.2009.32. Epub 2009/03/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ronaldson PT, Finch JD, DeMarco KM, Quigley CE, Davis TP. Inflammatory Pain Signals an Increase in Functional Expression of Organic Anion Transporting Polypeptide 1a4 at the Blood-Brain Barrier. J Pharmacol Exp Ther. 2011;336(3):827–39. doi: 10.1124/jpet.110.174151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartz AM, Bauer B, Fricker G, Miller DS. Rapid regulation of P-glycoprotein at the blood-brain barrier by endothelin-1. Molecular pharmacology. 2004;66(3):387–94. doi: 10.1124/mol.104.001503. Epub 2004/08/24. [DOI] [PubMed] [Google Scholar]
- 14.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57(2):173–85. doi: 10.1124/pr.57.2.4. Epub 2005/05/26. [DOI] [PubMed] [Google Scholar]
- 15.Abbruscato TJ, Davis TP. Combination of hypoxia/aglycemia compromises in vitro blood-brain barrier integrity. J Pharmacol Exp Ther. 1999;289(2):668–75. Epub 1999/04/24. [PubMed] [Google Scholar]
- 16.Iyer S, Ferreri DM, DeCocco NC, Minnear FL, Vincent PA. VE-cadherin-p120 interaction is required for maintenance of endothelial barrier function. American journal of physiology Lung cellular and molecular physiology. 2004;286(6):L1143–53. doi: 10.1152/ajplung.00305.2003. Epub 2003/12/16. [DOI] [PubMed] [Google Scholar]
- 17.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. Epub 2008/01/25. [DOI] [PubMed] [Google Scholar]
- 18.Butt AM, Jones HC, Abbott NJ. Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. The Journal of physiology. 1990;429:47–62. doi: 10.1113/jphysiol.1990.sp018243. Epub 1990/10/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ronaldson PT, Davis TP. Blood-brain barrier integrity and glial support: mechanisms that can be targeted for novel therapeutic approaches in stroke. Current pharmaceutical design. 2012;18(25):3624–44. doi: 10.2174/138161212802002625. Epub 2012/05/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dejana E, Lampugnani MG, Martinez-Estrada O, Bazzoni G. The molecular organization of endothelial junctions and their functional role in vascular morphogenesis and permeability. The International journal of developmental biology. 2000;44(6):743–8. Epub 2000/11/04. [PubMed] [Google Scholar]
- 21.Yeung D, Manias JL, Stewart DJ, Nag S. Decreased junctional adhesion molecule-A expression during blood-brain barrier breakdown. Acta neuropathologica. 2008;115(6):635–42. doi: 10.1007/s00401-008-0364-4. Epub 2008/03/22. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman WH, Stamatovic SM, Andjelkovic AV. Inflammatory mediators and blood brain barrier disruption in fatal brain edema of diabetic ketoacidosis. Brain research. 2009;1254:138–48. doi: 10.1016/j.brainres.2008.11.100. Epub 2008/12/24. [DOI] [PubMed] [Google Scholar]
- 23.Haarmann A, Deiss A, Prochaska J, Foerch C, Weksler B, Romero I, et al. Evaluation of soluble junctional adhesion molecule-A as a biomarker of human brain endothelial barrier breakdown. PloS one. 2010;5(10):e13568. doi: 10.1371/journal.pone.0013568. Epub 2010/11/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Del Maschio A, De Luigi A, Martin-Padura I, Brockhaus M, Bartfai T, Fruscella P, et al. Leukocyte recruitment in the cerebrospinal fluid of mice with experimental meningitis is inhibited by an antibody to junctional adhesion molecule (JAM) The Journal of experimental medicine. 1999;190(9):1351–6. doi: 10.1084/jem.190.9.1351. Epub 1999/11/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, et al. Occludin: a novel integral membrane protein localizing at tight junctions. The Journal of cell biology. 1993;123(6 Pt 2):1777–88. doi: 10.1083/jcb.123.6.1777. Epub 1993/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lippoldt A, Kniesel U, Liebner S, Kalbacher H, Kirsch T, Wolburg H, et al. Structural alterations of tight junctions are associated with loss of polarity in strokeprone spontaneously hypertensive rat blood-brain barrier endothelial cells. Brain research. 2000;885(2):251–61. doi: 10.1016/s0006-8993(00)02954-1. Epub 2000/12/05. [DOI] [PubMed] [Google Scholar]
- 27.McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, Rogers RA, et al. Occludin is a functional component of the tight junction. J Cell Sci. 1996;109:2287–98. doi: 10.1242/jcs.109.9.2287. [DOI] [PubMed] [Google Scholar]
- 28.Balda MS, Whitney JA, Flores C, Gonzalez S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. The Journal of cell biology. 1996;134(4):1031–49. doi: 10.1083/jcb.134.4.1031. Epub 1996/08/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCaffrey G, Staatz WD, Quigley CA, Nametz N, Seelbach MJ, Campos CR, et al. Tight junctions contain oligomeric protein assembly critical for maintaining bloodbrain barrier integrity in vivo. J Neurochem. 2007;103(6):2540–55. doi: 10.1111/j.1471-4159.2007.04943.x. Epub 2007/10/13. [DOI] [PubMed] [Google Scholar]
- 30.McCaffrey G, Seelbach MJ, Staatz WD, Nametz N, Quigley C, Campos CR, et al. Occludin oligomeric assembly at tight junctions of the blood-brain barrier is disrupted by peripheral inflammatory hyperalgesia. J Neurochem. 2008;106(6):2395–409. doi: 10.1111/j.1471-4159.2008.05582.x. Epub 2008/07/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lochhead JJ, McCaffrey G, Quigley CE, Finch J, DeMarco KM, Nametz N, et al. Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J Cereb Blood Flow Metab. 2010;30(9):1625–36. doi: 10.1038/jcbfm.2010.29. Epub 2010/03/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lochhead JJ, McCaffrey G, Sanchez-Covarrubias L, Finch JD, Demarco KM, Quigley CE, et al. Tempol modulates changes in xenobiotic permeability and occludin oligomeric assemblies at the blood-brain barrier during inflammatory pain. Am J Physiol Heart Circ Physiol. 2012;302(3):H582–93. doi: 10.1152/ajpheart.00889.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bauer H-CT,A, Bauer H. Proteins of the tight junction in the blood brain barrier. In: Sharma HSW,J, editor. Blood-Spinal Chord and Brain Barriers in Health and Disease. Elsevier; San Diego: 2004. [Google Scholar]
- 34.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. The Journal of cell biology. 1998;141(7):1539–50. doi: 10.1083/jcb.141.7.1539. Epub 1998/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. The Journal of cell biology. 1999;147(4):891–903. doi: 10.1083/jcb.147.4.891. Epub 1999/11/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kubota K, Furuse M, Sasaki H, Sonoda N, Fujita K, Nagafuchi A, et al. Ca(2+)-independent cell-adhesion activity of claudins, a family of integral membrane proteins localized at tight junctions. Current biology : CB. 1999;9(18):1035–8. doi: 10.1016/s0960-9822(99)80452-7. Epub 1999/10/06. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez-Mariscal L, Betanzos A, Avila-Flores A. MAGUK proteins: structure and role in the tight junction. Seminars in cell & developmental biology. 2000;11(4):315–24. doi: 10.1006/scdb.2000.0178. Epub 2000/09/01. [DOI] [PubMed] [Google Scholar]
- 38.Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. The Journal of cell biology. 1986;103(3):755–66. doi: 10.1083/jcb.103.3.755. Epub 1986/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howarth AG, Hughes MR, Stevenson BR. Detection of the tight junctionassociated protein ZO-1 in astrocytes and other nonepithelial cell types. The American journal of physiology. 1992;262(2 Pt 1):C461–9. doi: 10.1152/ajpcell.1992.262.2.C461. Epub 1992/02/01. [DOI] [PubMed] [Google Scholar]
- 40.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. The Journal of biological chemistry. 1998;273(45):29745–53. doi: 10.1074/jbc.273.45.29745. Epub 1998/10/29. [DOI] [PubMed] [Google Scholar]
- 41.Abbruscato TJ, Lopez SP, Mark KS, Hawkins BT, Davis TP. Nicotine and cotinine modulate cerebral microvascular permeability and protein expression of ZO-1 through nicotinic acetylcholine receptors expressed on brain endothelial cells. J Pharm Sci. 2002;91(12):2525–38. doi: 10.1002/jps.10256. Epub 2002/11/16. [DOI] [PubMed] [Google Scholar]
- 42.Mark KS, Davis TP. Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol. 2002;282(4):H1485–94. doi: 10.1152/ajpheart.00645.2001. Epub 2002/03/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Umeda K, Matsui T, Nakayama M, Furuse K, Sasaki H, Furuse M, et al. Establishment and characterization of cultured epithelial cells lacking expression of ZO-1. The Journal of biological chemistry. 2004;279(43):44785–94. doi: 10.1074/jbc.M406563200. Epub 2004/08/05. [DOI] [PubMed] [Google Scholar]
- 44.Inoko A, Itoh M, Tamura A, Matsuda M, Furuse M, Tsukita S. Expression and distribution of ZO-3, a tight junction MAGUK protein, in mouse tissues. Genes to cells : devoted to molecular & cellular mechanisms. 2003;8(11):837–45. doi: 10.1046/j.1365-2443.2003.00681.x. Epub 2003/11/19. [DOI] [PubMed] [Google Scholar]
- 45.Raff MC, Abney ER, Cohen J, Lindsay R, Noble M. Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1983;3(6):1289–300. doi: 10.1523/JNEUROSCI.03-06-01289.1983. Epub 1983/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. Epub 2005/12/24. [DOI] [PubMed] [Google Scholar]
- 47.Kacem K, Lacombe P, Seylaz J, Bonvento G. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: a confocal microscopy study. Glia. 1998;23(1):1–10. Epub 1998/04/30. [PubMed] [Google Scholar]
- 48.Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325(6101):253–7. doi: 10.1038/325253a0. Epub 1987/01/15. [DOI] [PubMed] [Google Scholar]
- 49.Willis CL, Leach L, Clarke GJ, Nolan CC, Ray DE. Reversible disruption of tight junction complexes in the rat blood-brain barrier, following transitory focal astrocyte loss. Glia. 2004;48(1):1–13. doi: 10.1002/glia.20049. Epub 2004/08/25. [DOI] [PubMed] [Google Scholar]
- 50.Yi JH, Hazell AS. Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochemistry international. 2006;48(5):394–403. doi: 10.1016/j.neuint.2005.12.001. Epub 2006/02/14. [DOI] [PubMed] [Google Scholar]
- 51.Pardridge WM, Golden PL, Kang YS, Bickel U. Brain microvascular and astrocyte localization of P-glycoprotein. J Neurochem. 1997;68(3):1278–85. doi: 10.1046/j.1471-4159.1997.68031278.x. Epub 1997/03/01. [DOI] [PubMed] [Google Scholar]
- 52.van Vliet EA, Redeker S, Aronica E, Edelbroek PM, Gorter JA. Expression of multidrug transporters MRP1, MRP2, and BCRP shortly after status epilepticus, during the latent period, and in chronic epileptic rats. Epilepsia. 2005;46(10):1569–80. doi: 10.1111/j.1528-1167.2005.00250.x. Epub 2005/09/30. [DOI] [PubMed] [Google Scholar]
- 53.Aronica E, Gorter JA, Redeker S, van Vliet EA, Ramkema M, Scheffer GL, et al. Localization of breast cancer resistance protein (BCRP) in microvessel endothelium of human control and epileptic brain. Epilepsia. 2005;46(6):849–57. doi: 10.1111/j.1528-1167.2005.66604.x. Epub 2005/06/11. [DOI] [PubMed] [Google Scholar]
- 54.Ronaldson PT, Persidsky Y, Bendayan R. Regulation of ABC membrane transporters in glial cells: relevance to the pharmacotherapy of brain HIV-1 infection. Glia. 2008;56(16):1711–35. doi: 10.1002/glia.20725. Epub 2008/07/24. [DOI] [PubMed] [Google Scholar]
- 55.Kofler J, Wiley CA. Microglia: key innate immune cells of the brain. Toxicologic pathology. 2011;39(1):103–14. doi: 10.1177/0192623310387619. Epub 2010/11/17. [DOI] [PubMed] [Google Scholar]
- 56.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiological reviews. 2011;91(2):461–553. doi: 10.1152/physrev.00011.2010. Epub 2011/04/30. [DOI] [PubMed] [Google Scholar]
- 57.Berezowski V, Landry C, Dehouck MP, Cecchelli R, Fenart L. Contribution of glial cells and pericytes to the mRNA profiles of P-glycoprotein and multidrug resistance-associated proteins in an in vitro model of the blood-brain barrier. Brain research. 2004;1018(1):1–9. doi: 10.1016/j.brainres.2004.05.092. Epub 2004/07/21. [DOI] [PubMed] [Google Scholar]
- 58.Gibson CJ, Hossain M, Richardson JR, Aleksunes LM. Inflammatory Regulation of ABC Efflux Transporter Expression and Function in Microglia. J Pharmacol Exp Ther. 2012 doi: 10.1124/jpet.112.196543. Epub 2012/09/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sa-Pereira I, Brites D, Brito MA. Neurovascular unit: a focus on pericytes. Molecular neurobiology. 2012;45(2):327–47. doi: 10.1007/s12035-012-8244-2. Epub 2012/03/01. [DOI] [PubMed] [Google Scholar]
- 60.Dore-Duffy P, Cleary K. Morphology and properties of pericytes. Methods Mol Biol. 2011;686:49–68. doi: 10.1007/978-1-60761-938-3_2. Epub 2010/11/18. [DOI] [PubMed] [Google Scholar]
- 61.Hori S, Ohtsuki S, Hosoya K, Nakashima E, Terasaki T. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie-2 activation in vitro. J Neurochem. 2004;89(2):503–13. doi: 10.1111/j.1471-4159.2004.02343.x. Epub 2004/04/02. [DOI] [PubMed] [Google Scholar]
- 62.Al Ahmad A, Taboada CB, Gassmann M, Ogunshola OO. Astrocytes and pericytes differentially modulate blood-brain barrier characteristics during development and hypoxic insult. J Cereb Blood Flow Metab. 2011;31(2):693–705. doi: 10.1038/jcbfm.2010.148. Epub 2010/09/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for bloodbrain barrier integrity during embryogenesis. Nature. 2010;468(7323):562–6. doi: 10.1038/nature09513. Epub 2010/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bendayan R, Ronaldson PT, Gingras D, Bendayan M. In situ localization of Pglycoprotein (ABCB1) in human and rat brain. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2006;54(10):1159–67. doi: 10.1369/jhc.5A6870.2006. Epub 2006/06/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saint-Pol J, Vandenhaute E, Boucau MC, Candela P, Dehouck L, Cecchelli R, et al. Brain pericytes ABCA1 expression mediates cholesterol efflux but not cellular amyloid-beta peptide accumulation. Journal of Alzheimer’s disease : JAD. 2012;30(3):489–503. doi: 10.3233/JAD-2012-112090. Epub 2012/03/22. [DOI] [PubMed] [Google Scholar]
- 66.Ben-Menachem E, Johansson BB, Svensson TH. Increased vulnerability of the blood-brain barrier to acute hypertension following depletion of brain noradrenaline. Journal of neural transmission. 1982;53(2-3):159–67. doi: 10.1007/BF01243407. Epub 1982/01/01. [DOI] [PubMed] [Google Scholar]
- 67.Cohen Z, Molinatti G, Hamel E. Astroglial and vascular interactions of noradrenaline terminals in the rat cerebral cortex. J Cereb Blood Flow Metab. 1997;17(8):894–904. doi: 10.1097/00004647-199708000-00008. Epub 1997/08/01. [DOI] [PubMed] [Google Scholar]
- 68.Cohen Z, Bonvento G, Lacombe P, Hamel E. Serotonin in the regulation of brain microcirculation. Progress in neurobiology. 1996;50(4):335–62. doi: 10.1016/s0301-0082(96)00033-0. Epub 1996/11/01. [DOI] [PubMed] [Google Scholar]
- 69.Tong XK, Hamel E. Regional cholinergic denervation of cortical microvessels and nitric oxide synthase-containing neurons in Alzheimer’s disease. Neuroscience. 1999;92(1):163–75. doi: 10.1016/s0306-4522(98)00750-7. Epub 1999/07/07. [DOI] [PubMed] [Google Scholar]
- 70.Vaucher E, Hamel E. Cholinergic basal forebrain neurons project to cortical microvessels in the rat: electron microscopic study with anterogradely transported Phaseolus vulgaris leucoagglutinin and choline acetyltransferase immunocytochemistry. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15(11):7427–41. doi: 10.1523/JNEUROSCI.15-11-07427.1995. Epub 1995/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vaucher E, Tong XK, Cholet N, Lantin S, Hamel E. GABA neurons provide a rich input to microvessels but not nitric oxide neurons in the rat cerebral cortex: a means for direct regulation of local cerebral blood flow. The Journal of comparative neurology. 2000;421(2):161–71. Epub 2000/05/17. [PubMed] [Google Scholar]
- 72.Yarnitsky D, Gross Y, Lorian A, Shalev A, Lamensdorf I, Bornstein R, et al. Blood-brain barrier opened by stimulation of the parasympathetic sphenopalatine ganglion: a new method for macromolecule delivery to the brain. Journal of neurosurgery. 2004;101(2):303–9. doi: 10.3171/jns.2004.101.2.0303. Epub 2004/08/18. [DOI] [PubMed] [Google Scholar]
- 73.Lopez NE, Krzyzaniak MJ, Costantini TW, Putnam J, Hageny AM, Eliceiri B, et al. Vagal nerve stimulation decreases blood-brain barrier disruption after traumatic brain injury. The journal of trauma and acute care surgery. 2012;72(6):1562–6. doi: 10.1097/TA.0b013e3182569875. Epub 2012/06/15. [DOI] [PubMed] [Google Scholar]
- 74.Rosenstein JM, Krum JM, Ruhrberg C. VEGF in the nervous system. Organogenesis. 2010;6(2):107–14. doi: 10.4161/org.6.2.11687. Epub 2010/10/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davis B, Tang J, Zhang L, Mu D, Jiang X, Biran V, et al. Role of vasodilator stimulated phosphoprotein in VEGF induced blood-brain barrier permeability in endothelial cell monolayers. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2010;28(6):423–8. doi: 10.1016/j.ijdevneu.2010.06.010. Epub 2010/07/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Seminars in immunopathology. 2009;31(4):497–511. doi: 10.1007/s00281-009-0177-0. Epub 2009/09/26. [DOI] [PubMed] [Google Scholar]
- 77.Strazielle N, Ghersi-Egea JF. Choroid plexus in the central nervous system: biology and physiopathology. Journal of neuropathology and experimental neurology. 2000;59(7):561–74. doi: 10.1093/jnen/59.7.561. Epub 2000/07/20. [DOI] [PubMed] [Google Scholar]
- 78.Johanson CE, Stopa EG, McMillan PN. The blood-cerebrospinal fluid barrier: structure and functional significance. Methods Mol Biol. 2011;686:101–31. doi: 10.1007/978-1-60761-938-3_4. Epub 2010/11/18. [DOI] [PubMed] [Google Scholar]
- 79.Davson H, Hollingsworth G, Segal MB. The mechanism of drainage of the cerebrospinal fluid. Brain : a journal of neurology. 1970;93(4):665–78. doi: 10.1093/brain/93.4.665. Epub 1970/01/01. [DOI] [PubMed] [Google Scholar]
- 80.Saunders NR, Habgood MD, Dziegielewska KM. Barrier mechanisms in the brain, I. Adult brain. Clinical and experimental pharmacology & physiology. 1999;26(1):11–9. doi: 10.1046/j.1440-1681.1999.02986.x. Epub 1999/02/23. [DOI] [PubMed] [Google Scholar]
- 81.Felgenhauer K. Protein size and cerebrospinal fluid composition. Klinische Wochenschrift. 1974;52(24):1158–64. doi: 10.1007/BF01466734. Epub 1974/12/15. [DOI] [PubMed] [Google Scholar]
- 82.Azzi G, Bernaudin JF, Bouchaud C, Bellon B, Fleury-Feith J. Permeability of the normal rat brain, spinal cord and dorsal root ganglia microcirculations to immunoglobulins G. Biology of the cell / under the auspices of the European Cell Biology Organization. 1990;68(1):31–6. doi: 10.1111/j.1768-322x.1990.tb00890.x. Epub 1990/01/01. [DOI] [PubMed] [Google Scholar]
- 83.Tachikawa M, Ozeki G, Higuchi T, Akanuma SI, Tsuji K, Hosoya KI. Role of the blood-cerebrospinal fluid barrier transporter as a cerebral clearance system for prostaglandin E(2) produced in the brain. J Neurochem. 2012 doi: 10.1111/jnc.12018. Epub 2012/09/18. [DOI] [PubMed] [Google Scholar]
- 84.Choudhuri S, Cherrington NJ, Li N, Klaassen CD. Constitutive expression of various xenobiotic and endobiotic transporter mRNAs in the choroid plexus of rats. Drug metabolism and disposition: the biological fate of chemicals. 2003;31(11):1337–45. doi: 10.1124/dmd.31.11.1337. Epub 2003/10/23. [DOI] [PubMed] [Google Scholar]
- 85.Smith DE, Johanson CE, Keep RF. Peptide and peptide analog transport systems at the blood-CSF barrier. Advanced drug delivery reviews. 2004;56(12):1765–91. doi: 10.1016/j.addr.2004.07.008. Epub 2004/09/24. [DOI] [PubMed] [Google Scholar]
- 86.Roberts LM, Black DS, Raman C, Woodford K, Zhou M, Haggerty JE, et al. Subcellular localization of transporters along the rat blood-brain barrier and bloodcerebral-spinal fluid barrier by in vivo biotinylation. Neuroscience. 2008;155(2):423–38. doi: 10.1016/j.neuroscience.2008.06.015. Epub 2008/07/16. [DOI] [PubMed] [Google Scholar]
- 87.Philp NJ, Yoon H, Lombardi L. Mouse MCT3 gene is expressed preferentially in retinal pigment and choroid plexus epithelia. American journal of physiology Cell physiology. 2001;280(5):C1319–26. doi: 10.1152/ajpcell.2001.280.5.C1319. Epub 2001/04/05. [DOI] [PubMed] [Google Scholar]
- 88.Rao VV, Dahlheimer JL, Bardgett ME, Snyder AZ, Finch RA, Sartorelli AC, et al. Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance-associated protein contribute to the blood-cerebrospinal-fluid drugpermeability barrier. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(7):3900–5. doi: 10.1073/pnas.96.7.3900. Epub 1999/03/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Halwachs S, Lakoma C, Schafer I, Seibel P, Honscha W. The antiepileptic drugs phenobarbital and carbamazepine reduce transport of methotrexate in rat choroid plexus by down-regulation of the reduced folate carrier. Molecular pharmacology. 2011;80(4):621–9. doi: 10.1124/mol.111.072421. Epub 2011/07/09. [DOI] [PubMed] [Google Scholar]
- 90.Xia LZ M, Wang J. Nucleoside Transporters: CNTs and ENTs. In: GMME You., editor. Drug Transportes: molecular characterization and role in drug disposition. John Wiley & Sons, Inc; Hoboken, NJ: 2007. pp. 171–200. [Google Scholar]
- 91.Habgood MD, Begley DJ, Abbott NJ. Determinants of passive drug entry into the central nervous system. Cellular and molecular neurobiology. 2000;20(2):231–53. doi: 10.1023/A:1007001923498. Epub 2000/03/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wolka AM, Huber JD, Davis TP. Pain and the blood-brain barrier: obstacles to drug delivery. Advanced drug delivery reviews. 2003;55(8):987–1006. doi: 10.1016/s0169-409x(03)00100-5. Epub 2003/08/26. [DOI] [PubMed] [Google Scholar]
- 93.Walsh CTS-B. R. Levine’s Pharmacology: Drug interactions and reactions. Seventh ed. Taylor & Francis; United Kingdon: 2005. p. 545. 2005. [Google Scholar]
- 94.De Gregori S, De Gregori M, Ranzani GN, Allegri M, Minella C, Regazzi M. Morphine metabolism, transport and brain disposition. Metabolic brain disease. 2012;27(1):1–5. doi: 10.1007/s11011-011-9274-6. Epub 2011/12/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Au-Yeung SC, Rurak DW, Gruber N, Riggs KW. A pharmacokinetic study of diphenhydramine transport across the blood-brain barrier in adult sheep: potential involvement of a carrier-mediated mechanism. Drug metabolism and disposition: the biological fate of chemicals. 2006;34(6):955–60. doi: 10.1124/dmd.105.007898. Epub 2006/03/03. [DOI] [PubMed] [Google Scholar]
- 96.Uldry M, Thorens B. The SLC2 family of facilitated hexose and polyol transporters. Pflügers Archiv European Journal of Physiology. 2004;447(5):480–9. doi: 10.1007/s00424-003-1085-0. [DOI] [PubMed] [Google Scholar]
- 97.del Amo EM, Urtti A, Yliperttula M. Pharmacokinetic role of L-type amino acid transporters LAT1 and LAT2. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2008;35(3):161–74. doi: 10.1016/j.ejps.2008.06.015. Epub 2008/07/29. [DOI] [PubMed] [Google Scholar]
- 98.Simionescu M, Gafencu A, Antohe F. Transcytosis of plasma macromolecules in endothelial cells: a cell biological survey. Microscopy research and technique. 2002;57(5):269–88. doi: 10.1002/jemt.10086. Epub 2002/07/12. [DOI] [PubMed] [Google Scholar]
- 99.Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab. 2012 doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fishman JB, Rubin JB, Handrahan JV, Connor JR, Fine RE. Receptor-mediated transcytosis of transferrin across the blood-brain barrier. Journal of neuroscience research. 1987;18(2):299–304. doi: 10.1002/jnr.490180206. Epub 1987/01/01. [DOI] [PubMed] [Google Scholar]
- 101.King GL, Johnson SM. Receptor-mediated transport of insulin across endothelial cells. Science (New York, NY) 1985;227(4694):1583–6. doi: 10.1126/science.3883490. Epub 1985/03/29. [DOI] [PubMed] [Google Scholar]
- 102.Scherrmann JM. Drug delivery to brain via the blood-brain barrier. Vascular pharmacology. 2002;38(6):349–54. doi: 10.1016/s1537-1891(02)00202-1. Epub 2003/01/18. [DOI] [PubMed] [Google Scholar]
- 103.Tamai I, Tsuji A. Transporter-mediated permeation of drugs across the blood-brain barrier. J Pharm Sci. 2000;89(11):1371–88. doi: 10.1002/1520-6017(200011)89:11<1371::aid-jps1>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 104.Boron WF, Boulep EL. Medical Physiology. Elsevier Saunders; Philedelphia, PA: 2005. p. 1269. [Google Scholar]
- 105.Abbott NJ, Romero IA. Transporting therapeutics across the blood-brain barrier. Mol Med Today. 1996;2(3):106–13. doi: 10.1016/1357-4310(96)88720-x. [DOI] [PubMed] [Google Scholar]
- 106.Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome research. 2001;11(7):1156–66. doi: 10.1101/gr.184901. Epub 2001/07/04. [DOI] [PubMed] [Google Scholar]
- 107.Gottesman MM, Hrycyna CA, Schoenlein PV, Germann UA, Pastan I. Genetic analysis of the multidrug transporter. Annual review of genetics. 1995;29:607–49. doi: 10.1146/annurev.ge.29.120195.003135. Epub 1995/01/01. [DOI] [PubMed] [Google Scholar]
- 108.Hyde SC, Emsley P, Hartshorn MJ, Mimmack MM, Gileadi U, Pearce SR, et al. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature. 1990;346(6282):362–5. doi: 10.1038/346362a0. Epub 1990/07/26. [DOI] [PubMed] [Google Scholar]
- 109.Zhou SF. Structure, function and regulation of P-glycoprotein and its clinical relevance in drug disposition. Xenobiotica; the fate of foreign compounds in biological systems. 2008;38(7-8):802–32. doi: 10.1080/00498250701867889. Epub 2008/08/01. [DOI] [PubMed] [Google Scholar]
- 110.Hennessy M, Spiers JP. A primer on the mechanics of P-glycoprotein the multidrug transporter. Pharmacological research : the official journal of the Italian Pharmacological Society. 2007;55(1):1–15. doi: 10.1016/j.phrs.2006.10.007. Epub 2006/11/11. [DOI] [PubMed] [Google Scholar]
- 111.Leslie EM, Deeley RG, Cole SP. Multidrug resistance proteins: role of Pglycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicology and applied pharmacology. 2005;204(3):216–37. doi: 10.1016/j.taap.2004.10.012. Epub 2005/04/23. [DOI] [PubMed] [Google Scholar]
- 112.You GMME. Overview of Drug Transporter Families. In: You GMME, editor. Drug Transporters: molecular characterization and role in drug disposition. John Wiley & Sons, Inc.; Hoboken, NJ: 2007. pp. 1–10. [Google Scholar]
- 113.Dean M, Hamon Y, Chimini G. The human ATP-binding cassette (ABC) transporter superfamily. Journal of lipid research. 2001;42(7):1007–17. Epub 2001/07/07. [PubMed] [Google Scholar]
- 114.Santamarina-Fojo S, Remaley AT, Neufeld EB, Brewer HB., Jr. Regulation and intracellular trafficking of the ABCA1 transporter. Journal of lipid research. 2001;42(9):1339–45. Epub 2001/08/24. [PubMed] [Google Scholar]
- 115.Puntoni M, Sbrana F, Bigazzi F, Sampietro T. Tangier disease: epidemiology, pathophysiology, and management. American journal of cardiovascular drugs : drugs, devices, and other interventions. 2012;12(5):303–11. doi: 10.2165/11634140-000000000-00000. Epub 2012/08/24. [DOI] [PubMed] [Google Scholar]
- 116.Glaser B, Blech I, Krakinovsky Y, Ekstein J, Gillis D, Mazor-Aronovitch K, et al. ABCC8 mutation allele frequency in the Ashkenazi Jewish population and risk of focal hyperinsulinemic hypoglycemia. Genetics in medicine : official journal of the American College of Medical Genetics. 2011;13(10):891–4. doi: 10.1097/GIM.0b013e31821fea33. Epub 2011/07/01. [DOI] [PubMed] [Google Scholar]
- 117.Fournet JC, Mayaud C, de Lonlay P, Gross-Morand MS, Verkarre V, Castanet M, et al. Unbalanced expression of 11p15 imprinted genes in focal forms of congenital hyperinsulinism: association with a reduction to homozygosity of a mutation in ABCC8 or KCNJ11. The American journal of pathology. 2001;158(6):2177–84. doi: 10.1016/S0002-9440(10)64689-5. Epub 2001/06/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wada M, Toh S, Taniguchi K, Nakamura T, Uchiumi T, Kohno K, et al. Mutations in the canilicular multispecific organic anion transporter (cMOAT) gene, a novel ABC transporter, in patients with hyperbilirubinemia II/Dubin-Johnson syndrome. Human molecular genetics. 1998;7(2):203–7. doi: 10.1093/hmg/7.2.203. Epub 1998/03/21. [DOI] [PubMed] [Google Scholar]
- 119.Cohen TS, Prince A. Cystic fibrosis: a mucosal immunodeficiency syndrome. Nature medicine. 2012;18(4):509–19. doi: 10.1038/nm.2715. Epub 2012/04/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wanders RJ, Visser WF, van Roermund CW, Kemp S, Waterham HR. The peroxisomal ABC transporter family. Pflugers Archiv : European journal of physiology. 2007;453(5):719–34. doi: 10.1007/s00424-006-0142-x. Epub 2006/10/14. [DOI] [PubMed] [Google Scholar]
- 121.Cappa M, Bizzarri C, Vollono C, Petroni A, Banni S. Adrenoleukodystrophy. Endocrine development. 2011;20:149–60. doi: 10.1159/000321236. Epub 2010/12/18. [DOI] [PubMed] [Google Scholar]
- 122.Tian Y, Han X, Tian DL. The biological regulation of ABCE1. IUBMB life. 2012;64(10):795–800. doi: 10.1002/iub.1071. Epub 2012/09/26. [DOI] [PubMed] [Google Scholar]
- 123.Ronaldson PTBKB. R. Drug Transport in the Brain. In: You GMME, editor. Drug Transporters: molecular characterization and role in drug disposition. John Wiley & Sons, Inc.; Hoboken, N.J.: 2007. pp. 411–62. [Google Scholar]
- 124.Chen CJ, Chin JE, Ueda K, Clark DP, Pastan I, Gottesman MM, et al. Internal duplication and homology with bacterial transport proteins in the mdr1 (Pglycoprotein) gene from multidrug-resistant human cells. Cell. 1986;47(3):381–9. doi: 10.1016/0092-8674(86)90595-7. Epub 1986/11/07. [DOI] [PubMed] [Google Scholar]
- 125.Roninson IB, Chin JE, Choi KG, Gros P, Housman DE, Fojo A, et al. Isolation of human mdr DNA sequences amplified in multidrug-resistant KB carcinoma cells. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(12):4538–42. doi: 10.1073/pnas.83.12.4538. Epub 1986/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ueda K, Cardarelli C, Gottesman MM, Pastan I. Expression of a full-length cDNA for the human "MDR1" gene confers resistance to colchicine, doxorubicin, and vinblastine. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(9):3004–8. doi: 10.1073/pnas.84.9.3004. Epub 1987/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Smit JJ, Schinkel AH, Oude Elferink RP, Groen AK, Wagenaar E, van Deemter L, et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75(3):451–62. doi: 10.1016/0092-8674(93)90380-9. Epub 1993/11/05. [DOI] [PubMed] [Google Scholar]
- 128.Gribar JJ, Ramachandra M, Hrycyna CA, Dey S, Ambudkar SV. Functional characterization of glycosylation-deficient human P-glycoprotein using a vaccinia virus expression system. The Journal of membrane biology. 2000;173(3):203–14. doi: 10.1007/s002320001020. Epub 2000/02/10. [DOI] [PubMed] [Google Scholar]
- 129.Xie Y, Burcu M, Linn DE, Qiu Y, Baer MR. Pim-1 kinase protects Pglycoprotein from degradation and enables its glycosylation and cell surface expression. Molecular pharmacology. 2010;78(2):310–8. doi: 10.1124/mol.109.061713. Epub 2010/05/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ling V, Thompson LH. Reduced permeability in CHO cells as a mechanism of resistance to colchicine. Journal of cellular physiology. 1974;83(1):103–16. doi: 10.1002/jcp.1040830114. Epub 1974/02/01. [DOI] [PubMed] [Google Scholar]
- 131.Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochimica et biophysica acta. 1976;455(1):152–62. doi: 10.1016/0005-2736(76)90160-7. Epub 1976/11/11. [DOI] [PubMed] [Google Scholar]
- 132.Sharom FJ. Multidrug Resistance Protein: P-glycoprotein. In: GYaME Morris., editor. Drug Transporters: Molecular Characterization and Role in Drug Disposition. John Wiley & Sons, Inc.; Hoboken, NJ: 2007. pp. 263–318. [Google Scholar]
- 133.Schinkel AH, Smit JJM, Vantellingen O, Beijnen JH, Wagenaar E, Vandeemter L, et al. Disruption of the Mouse Mdr1a P-Glycoprotein Gene Leads to a Deficiency in the Blood-Brain-Barrier and to Increased Sensitivity to Drugs. Cell. 1994;77(4):491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 134.Doran A, Obach RS, Smith BJ, Hosea NA, Becker S, Callegari E, et al. The impact of P-glycoprotein on the disposition of drugs targeted for indications of the central nervous system: evaluation using the MDR1A/1B knockout mouse model. Drug metabolism and disposition: the biological fate of chemicals. 2005;33(1):165–74. doi: 10.1124/dmd.104.001230. Epub 2004/10/27. [DOI] [PubMed] [Google Scholar]
- 135.Golden PL, Pardridge WM. P-Glycoprotein on astrocyte foot processes of unfixed isolated human brain capillaries. Brain research. 1999;819(1-2):143–6. doi: 10.1016/s0006-8993(98)01305-5. Epub 1999/03/20. [DOI] [PubMed] [Google Scholar]
- 136.Schlachetzki F, Pardridge WM. P-glycoprotein and caveolin-1 alpha in endothelium and astrocytes of primate brain. Neuroreport. 2003;14(16):2041–6. doi: 10.1097/00001756-200311140-00007. [DOI] [PubMed] [Google Scholar]
- 137.Ronaldson PT, Bendayan M, Gingras D, Piquette-Miller M, Bendayan R. Cellular localization and functional expression of P-glycoprotein in rat astrocyte cultures. J Neurochem. 2004;89(3):788–800. doi: 10.1111/j.1471-4159.2004.02417.x. [DOI] [PubMed] [Google Scholar]
- 138.Lee G, Dallas S, Hong M, Bendayan R. Drug transporters in the central nervous system: Brain barriers and brain parenchyma considerations. Pharmacol Rev. 2001;53(4):569–96. [PubMed] [Google Scholar]
- 139.Volk HA, Burkhardt K, Potschka H, Chen J, Becker A, Loscher W. Neuronal expression of the drug efflux transporter P-glycoprotein in the rat hippocampus after limbic seizures. Neuroscience. 2004;123(3):751–9. doi: 10.1016/j.neuroscience.2003.10.012. Epub 2004/01/07. [DOI] [PubMed] [Google Scholar]
- 140.Sun J, He ZG, Cheng G, Wang SJ, Hao XH, Zou MJ. Multidrug resistance Pglycoprotein: crucial significance in drug disposition and interaction. Medical science monitor : international medical journal of experimental and clinical research. 2004;10(1):RA5–14. Epub 2004/01/06. [PubMed] [Google Scholar]
- 141.Demeule M, Regina A, Jodoin J, Laplante A, Dagenais C, Berthelet F, et al. Drug transport to the brain: Key roles for the efflux pump P-glycoprotein in the bloodbrain barrier. Vasc Pharmacol. 2002;38(6):339–48. doi: 10.1016/s1537-1891(02)00201-x. [DOI] [PubMed] [Google Scholar]
- 142.Shirasaka Y, Suzuki K, Shichiri M, Nakanishi T, Tamai I. Intestinal absorption of HMG-CoA reductase inhibitor pitavastatin mediated by organic anion transporting polypeptide and P-glycoprotein/multidrug resistance 1. Drug metabolism and pharmacokinetics. 2011;26(2):171–9. doi: 10.2133/dmpk.dmpk-10-rg-073. Epub 2011/01/06. [DOI] [PubMed] [Google Scholar]
- 143.Shirasaka Y, Suzuki K, Nakanishi T, Tamai I. Differential effect of grapefruit juice on intestinal absorption of statins due to inhibition of organic anion transporting polypeptide and/or P-glycoprotein. J Pharm Sci. 2011;100(9):3843–53. doi: 10.1002/jps.22586. Epub 2011/04/27. [DOI] [PubMed] [Google Scholar]
- 144.Seelbach MJ, Brooks TA, Egleton RD, Davis TP. Peripheral inflammatory hyperalgesia modulates morphine delivery to the brain: a role for P-glycoprotein. J Neurochem. 2007;102(5):1677–90. doi: 10.1111/j.1471-4159.2007.04644.x. [DOI] [PubMed] [Google Scholar]
- 145.Chen C, Pollack GM. Altered disposition and antinociception of [Dpenicillamine( 2,5)] enkephalin in mdr1a-gene-deficient mice. J Pharmacol Exp Ther. 1998;287(2):545–52. Epub 1998/11/10. [PubMed] [Google Scholar]
- 146.Chen C, Pollack GM. Extensive biliary excretion of the model opioid peptide [D-PEN2,5] enkephalin in rats. Pharmaceutical research. 1997;14(3):345–50. doi: 10.1023/a:1012054222845. Epub 1997/03/01. [DOI] [PubMed] [Google Scholar]
- 147.Thomas H, Coley HM. Overcoming multidrug resistance in cancer: an update on the clinical strategy of inhibiting p-glycoprotein. Cancer control : journal of the Moffitt Cancer Center. 2003;10(2):159–65. doi: 10.1177/107327480301000207. Epub 2003/04/25. [DOI] [PubMed] [Google Scholar]
- 148.Goard CA, Mather RG, Vinepal B, Clendening JW, Martirosyan A, Boutros PC, et al. Differential interactions between statins and P-glycoprotein: implications for exploiting statins as anticancer agents. International journal of cancer Journal international du cancer. 2010;127(12):2936–48. doi: 10.1002/ijc.25295. Epub 2011/02/26. [DOI] [PubMed] [Google Scholar]
- 149.Chen YN, Mickley LA, Schwartz AM, Acton EM, Hwang JL, Fojo AT. Characterization of adriamycin-resistant human breast cancer cells which display overexpression of a novel resistance-related membrane protein. The Journal of biological chemistry. 1990;265(17):10073–80. Epub 1990/06/15. [PubMed] [Google Scholar]
- 150.Lee JS, Scala S, Matsumoto Y, Dickstein B, Robey R, Zhan Z, et al. Reduced drug accumulation and multidrug resistance in human breast cancer cells without associated P-glycoprotein or MRP overexpression. Journal of cellular biochemistry. 1997;65(4):513–26. Epub 1997/06/15. [PubMed] [Google Scholar]
- 151.Nakagawa M, Schneider E, Dixon KH, Horton J, Kelley K, Morrow C, et al. Reduced intracellular drug accumulation in the absence of P-glycoprotein (mdr1) overexpression in mitoxantrone-resistant human MCF-7 breast cancer cells. Cancer research. 1992;52(22):6175–81. Epub 1992/11/15. [PubMed] [Google Scholar]
- 152.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(26):15665–70. doi: 10.1073/pnas.95.26.15665. Epub 1998/12/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Diop NK, Hrycyna CA. N-Linked glycosylation of the human ABC transporter ABCG2 on asparagine 596 is not essential for expression, transport activity, or trafficking to the plasma membrane. Biochemistry. 2005;44(14):5420–9. doi: 10.1021/bi0479858. Epub 2005/04/06. [DOI] [PubMed] [Google Scholar]
- 154.Mohrmann K, van Eijndhoven MA, Schinkel AH, Schellens JH. Absence of Nlinked glycosylation does not affect plasma membrane localization of breast cancer resistance protein (BCRP/ABCG2) Cancer chemotherapy and pharmacology. 2005;56(4):344–50. doi: 10.1007/s00280-005-1004-5. Epub 2005/05/06. [DOI] [PubMed] [Google Scholar]
- 155.Graf GA, Li WP, Gerard RD, Gelissen I, White A, Cohen JC, et al. Coexpression of ATP-binding cassette proteins ABCG5 and ABCG8 permits their transport to the apical surface. The Journal of clinical investigation. 2002;110(5):659–69. doi: 10.1172/JCI16000. Epub 2002/09/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Graf GA, Yu L, Li WP, Gerard R, Tuma PL, Cohen JC, et al. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. The Journal of biological chemistry. 2003;278(48):48275–82. doi: 10.1074/jbc.M310223200. Epub 2003/09/25. [DOI] [PubMed] [Google Scholar]
- 157.Fetsch PA, Abati A, Litman T, Morisaki K, Honjo Y, Mittal K, et al. Localization of the ABCG2 mitoxantrone resistance-associated protein in normal tissues. Cancer letters. 2006;235(1):84–92. doi: 10.1016/j.canlet.2005.04.024. Epub 2005/07/02. [DOI] [PubMed] [Google Scholar]
- 158.Eisenblatter T, Huwel S, Galla HJ. Characterisation of the brain multidrug resistance protein (BMDP/ABCG2/BCRP) expressed at the blood-brain barrier. Brain research. 2003;971(2):221–31. doi: 10.1016/s0006-8993(03)02401-6. Epub 2003/04/23. [DOI] [PubMed] [Google Scholar]
- 159.Zhang W, Mojsilovic-Petrovic J, Andrade MF, Zhang H, Ball M, Stanimirovic DB. The expression and functional characterization of ABCG2 in brain endothelial cells and vessels. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17(14):2085–7. doi: 10.1096/fj.02-1131fje. Epub 2003/09/06. [DOI] [PubMed] [Google Scholar]
- 160.Lee G, Babakhanian K, Ramaswamy M, Prat A, Wosik K, Bendayan R. Expression of the ATP-binding cassette membrane transporter, ABCG2, in human and rodent brain microvessel endothelial and glial cell culture systems. Pharmaceutical research. 2007;24(7):1262–74. doi: 10.1007/s11095-007-9244-1. Epub 2007/03/24. [DOI] [PubMed] [Google Scholar]
- 161.Fujiyoshi M, Ohtsuki S, Hori S, Tachikawa M, Terasaki T. 24S-hydroxycholesterol induces cholesterol release from choroid plexus epithelial cells in an apical- and apoE isoform-dependent manner concomitantly with the induction of ABCA1 and ABCG1 expression. J Neurochem. 2007;100(4):968–78. doi: 10.1111/j.1471-4159.2006.04240.x. Epub 2006/11/15. [DOI] [PubMed] [Google Scholar]
- 162.Hori S, Ohtsuki S, Tachikawa M, Kimura N, Kondo T, Watanabe M, et al. Functional expression of rat ABCG2 on the luminal side of brain capillaries and its enhancement by astrocyte-derived soluble factor(s) J Neurochem. 2004;90(3):526–36. doi: 10.1111/j.1471-4159.2004.02537.x. Epub 2004/07/17. [DOI] [PubMed] [Google Scholar]
- 163.Cooray HC, Janvilisri T, van Veen HW, Hladky SB, Barrand MA. Interaction of the breast cancer resistance protein with plant polyphenols. Biochemical and biophysical research communications. 2004;317(1):269–75. doi: 10.1016/j.bbrc.2004.03.040. Epub 2004/03/30. [DOI] [PubMed] [Google Scholar]
- 164.Zhang S, Yang X, Morris ME. Flavonoids are inhibitors of breast cancer resistance protein (ABCG2)-mediated transport. Molecular pharmacology. 2004;65(5):1208–16. doi: 10.1124/mol.65.5.1208. Epub 2004/04/23. [DOI] [PubMed] [Google Scholar]
- 165.Lee YJ, Kusuhara H, Jonker JW, Schinkel AH, Sugiyama Y. Investigation of efflux transport of dehydroepiandrosterone sulfate and mitoxantrone at the mouse blood-brain barrier: a minor role of breast cancer resistance protein. J Pharmacol Exp Ther. 2005;312(1):44–52. doi: 10.1124/jpet.104.073320. Epub 2004/09/28. [DOI] [PubMed] [Google Scholar]
- 166.Breedveld P, Pluim D, Cipriani G, Wielinga P, van Tellingen O, Schinkel AH, et al. effect of Bcrp1 (Abcg2) on the in vivo pharmacokinetics and brain penetration of imatinib mesylate (Gleevec): implications for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patients. Cancer research. 2005;65(7):2577–82. doi: 10.1158/0008-5472.CAN-04-2416. Epub 2005/04/05. [DOI] [PubMed] [Google Scholar]
- 167.Staud F, Pavek P. Breast cancer resistance protein (BCRP/ABCG2) The international journal of biochemistry & cell biology. 2005;37(4):720–5. doi: 10.1016/j.biocel.2004.11.004. Epub 2005/02/08. [DOI] [PubMed] [Google Scholar]
- 168.Mao Q, Unadkat JD. Role of the breast cancer resistance protein (ABCG2) in drug transport. The AAPS journal. 2005;7(1):E118–33. doi: 10.1208/aapsj070112. Epub 2005/09/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kellner U, Hutchinson L, Seidel A, Lage H, Danks MK, Dietel M, et al. Decreased drug accumulation in a mitoxantrone-resistant gastric carcinoma cell line in the absence of P-glycoprotein. International journal of cancer Journal international du cancer. 1997;71(5):817–24. doi: 10.1002/(sici)1097-0215(19970529)71:5<817::aid-ijc20>3.0.co;2-3. Epub 1997/05/29. [DOI] [PubMed] [Google Scholar]
- 170.Rabindran SK, He H, Singh M, Brown E, Collins KI, Annable T, et al. Reversal of a novel multidrug resistance mechanism in human colon carcinoma cells by fumitremorgin C. Cancer research. 1998;58(24):5850–8. Epub 1998/12/29. [PubMed] [Google Scholar]
- 171.Allen JD, Brinkhuis RF, Wijnholds J, Schinkel AH. The mouse Bcrp1/Mxr/Abcp gene: amplification and overexpression in cell lines selected for resistance to topotecan, mitoxantrone, or doxorubicin. Cancer research. 1999;59(17):4237–41. Epub 1999/09/15. [PubMed] [Google Scholar]
- 172.Zhang Y, Han H, Elmquist WF, Miller DW. Expression of various multidrug resistance-associated protein (MRP) homologues in brain microvessel endothelial cells. Brain research. 2000;876(1-2):148–53. doi: 10.1016/s0006-8993(00)02628-7. Epub 2000/09/06. [DOI] [PubMed] [Google Scholar]
- 173.Borst P, Evers R, Kool M, Wijnholds J. A family of drug transporters: the multidrug resistance-associated proteins. Journal of the National Cancer Institute. 2000;92(16):1295–302. doi: 10.1093/jnci/92.16.1295. Epub 2000/08/17. [DOI] [PubMed] [Google Scholar]
- 174.Lee YJ, Kusuhara H, Sugiyama Y. Do multidrug resistance-associated protein-1 and -2 play any role in the elimination of estradiol-17 beta-glucuronide and 2,4-dinitrophenyl-S-glutathione across the blood-cerebrospinal fluid barrier? J Pharm Sci. 2004;93(1):99–107. doi: 10.1002/jps.10521. Epub 2003/12/04. [DOI] [PubMed] [Google Scholar]
- 175.Dallas S, Miller DS, Bendayan R. Multidrug resistance-associated proteins: expression and function in the central nervous system. Pharmacol Rev. 2006;58(2):140–61. doi: 10.1124/pr.58.2.3. Epub 2006/05/23. [DOI] [PubMed] [Google Scholar]
- 176.Hirrlinger J, Konig J, Dringen R. Expression of mRNAs of multidrug resistance proteins (Mrps) in cultured rat astrocytes, oligodendrocytes, microglial cells and neurones. J Neurochem. 2002;82(3):716–9. doi: 10.1046/j.1471-4159.2002.01082.x. Epub 2002/08/03. [DOI] [PubMed] [Google Scholar]
- 177.Ballerini P, Di Iorio P, Ciccarelli R, Nargi E, D’Alimonte I, Traversa U, et al. Glial cells express multiple ATP binding cassette proteins which are involved in ATP release. Neuroreport. 2002;13(14):1789–92. doi: 10.1097/00001756-200210070-00019. Epub 2002/10/24. [DOI] [PubMed] [Google Scholar]
- 178.Leslie EM, Deeley RG, Cole SP. Toxicological relevance of the multidrug resistance protein 1, MRP1 (ABCC1) and related transporters. Toxicology. 2001;167(1):3–23. doi: 10.1016/s0300-483x(01)00454-1. Epub 2001/09/15. [DOI] [PubMed] [Google Scholar]
- 179.Kruh GD, Belinsky MG. The MRP family of drug efflux pumps. Oncogene. 2003;22(47):7537–52. doi: 10.1038/sj.onc.1206953. [DOI] [PubMed] [Google Scholar]
- 180.Leslie EM, Deeley RG, Cole SP. Bioflavonoid stimulation of glutathione transport by the 190-kDa multidrug resistance protein 1 (MRP1) Drug metabolism and disposition: the biological fate of chemicals. 2003;31(1):11–5. doi: 10.1124/dmd.31.1.11. Epub 2002/12/18. [DOI] [PubMed] [Google Scholar]
- 181.Nies ATR,M, Keppler D. Mutlidrug Resistance Proteins of the ABCC Subfamily. In: You GMME, editor. Drug Transporters: Molecular Characterization and Role in Drug Disposition. John Wiley & Sons, Inc.; Hoboken, NJ: 2007. pp. 263–318. [Google Scholar]
- 182.Bart J, Groen HJ, Hendrikse NH, van der Graaf WT, Vaalburg W, de Vries EG. The blood-brain barrier and oncology: new insights into function and modulation. Cancer treatment reviews. 2000;26(6):449–62. doi: 10.1053/ctrv.2000.0194. Epub 2001/01/05. [DOI] [PubMed] [Google Scholar]
- 183.Zelcer N, Saeki T, Reid G, Beijnen JH, Borst P. Characterization of drug transport by the human multidrug resistance protein 3 (ABCC3) The Journal of biological chemistry. 2001;276(49):46400–7. doi: 10.1074/jbc.M107041200. Epub 2001/10/03. [DOI] [PubMed] [Google Scholar]
- 184.Rius M, Nies AT, Hummel-Eisenbeiss J, Jedlitschky G, Keppler D. Cotransport of reduced glutathione with bile salts by MRP4 (ABCC4) localized to the basolateral hepatocyte membrane. Hepatology (Baltimore, Md) 2003;38(2):374–84. doi: 10.1053/jhep.2003.50331. Epub 2003/07/29. [DOI] [PubMed] [Google Scholar]
- 185.Nies AT, Jedlitschky G, Konig J, Herold-Mende C, Steiner HH, Schmitt HP, et al. Expression and immunolocalization of the multidrug resistance proteins, MRP1-MRP6 (ABCC1-ABCC6), in human brain. Neuroscience. 2004;129(2):349–60. doi: 10.1016/j.neuroscience.2004.07.051. Epub 2004/10/27. [DOI] [PubMed] [Google Scholar]
- 186.Wijnholds J, Mol CA, van Deemter L, de Haas M, Scheffer GL, Baas F, et al. Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(13):7476–81. doi: 10.1073/pnas.120159197. Epub 2000/06/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kusuhara H, Sugiyama Y. Active efflux across the blood-brain barrier: role of the solute carrier family. NeuroRx : the journal of the American Society for Experimental NeuroTherapeutics. 2005;2(1):73–85. doi: 10.1602/neurorx.2.1.73. Epub 2005/02/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kong W, Engel K, Wang J. Mammalian nucleoside transporters. Current drug metabolism. 2004;5(1):63–84. doi: 10.2174/1389200043489162. Epub 2004/02/18. [DOI] [PubMed] [Google Scholar]
- 189.Hediger MA, Romero MF, Peng JB, Rolfs A, Takanaga H, Bruford EA. The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteinsIntroduction. Pflugers Archiv : European journal of physiology. 2004;447(5):465–8. doi: 10.1007/s00424-003-1192-y. Epub 2003/11/19. [DOI] [PubMed] [Google Scholar]
- 190.Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Archiv : European journal of physiology. 2004;447(5):653–65. doi: 10.1007/s00424-003-1168-y. Epub 2003/10/28. [DOI] [PubMed] [Google Scholar]
- 191.Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. British journal of pharmacology. 2012;165(5):1260–87. doi: 10.1111/j.1476-5381.2011.01724.x. Epub 2011/10/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Westholm DE, Rumbley JN, Salo DR, Rich TP, Anderson GW. Organic aniontransporting polypeptides at the blood-brain and blood-cerebrospinal fluid barriers. Current topics in developmental biology. 2008;80:135–70. doi: 10.1016/S0070-2153(07)80004-4. Epub 2007/10/24. [DOI] [PubMed] [Google Scholar]
- 193.Sai Y, Kaneko Y, Ito S, Mitsuoka K, Kato Y, Tamai I, et al. Predominant contribution of organic anion transporting polypeptide OATP-B (OATP2B1) to apical uptake of estrone-3-sulfate by human intestinal Caco-2 cells. Drug metabolism and disposition: the biological fate of chemicals. 2006;34(8):1423–31. doi: 10.1124/dmd.106.009530. Epub 2006/05/23. [DOI] [PubMed] [Google Scholar]
- 194.Varma MV, Rotter CJ, Chupka J, Whalen KM, Duignan DB, Feng B, et al. pHsensitive interaction of HMG-CoA reductase inhibitors (statins) with organic anion transporting polypeptide 2B1. Molecular pharmaceutics. 2011;8(4):1303–13. doi: 10.1021/mp200103h. Epub 2011/06/30. [DOI] [PubMed] [Google Scholar]
- 195.Nozawa T, Sugiura S, Nakajima M, Goto A, Yokoi T, Nezu JI, et al. Involvement of organic anion transporting polypeptides in the transport of troglitazone sulfate: Implications for understanding troglitazone hepatotoxicity. Drug Metabolism and Disposition. 2004;32(3):291–4. doi: 10.1124/dmd.32.3.291. [DOI] [PubMed] [Google Scholar]
- 196.Leuthold S, Hagenbuch B, Mohebbi N, Wagner CA, Meier PJ, Stieger B. Mechanisms of pH-gradient driven transport mediated by organic anion polypeptide transporters. American journal of physiology Cell physiology. 2009;296(3):C570–82. doi: 10.1152/ajpcell.00436.2008. Epub 2009/01/09. [DOI] [PubMed] [Google Scholar]
- 197.Martinez-Becerra P, Briz O, Romero MR, Macias RIR, Perez MJ, Sancho-Mateo C, et al. Further Characterization of the Electrogenicity and pH Sensitivity of the Human Organic Anion-Transporting Polypeptides OATP1B1 and OATP1B3. Molecular pharmacology. 2011;79(3):596–607. doi: 10.1124/mol.110.069971. [DOI] [PubMed] [Google Scholar]
- 198.Mahagita C, Grassl SM, Piyachaturawat P, Ballatori N. Human organic anion transporter 1B1 and 1B3 function as bidirectional carriers and do not mediate GSHbile acid cotransport. Am J Physiol-Gastr L. 2007;293(1):G271–G8. doi: 10.1152/ajpgi.00075.2007. [DOI] [PubMed] [Google Scholar]
- 199.Lee W, Glaeser H, Smith LH, Roberts RL, Moeckel GW, Gervasini G, et al. Polymorphisms in human organic anion-transporting polypeptide 1A2 (OATP1A2): implications for altered drug disposition and central nervous system drug entry. The Journal of biological chemistry. 2005;280(10):9610–7. doi: 10.1074/jbc.M411092200. Epub 2005/01/06. [DOI] [PubMed] [Google Scholar]
- 200.Uchida Y, Ohtsuki S, Katsukura Y, Ikeda C, Suzuki T, Kamiie J, et al. Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J Neurochem. 2011;117(2):333–45. doi: 10.1111/j.1471-4159.2011.07208.x. Epub 2011/02/05. [DOI] [PubMed] [Google Scholar]
- 201.Hau VS, Huber JD, Campos CR, Davis RT, Davis TP. Effect of lambdacarrageenan-induced inflammatory pain on brain uptake of codeine and antinociception. Brain research. 2004;1018(2):257–64. doi: 10.1016/j.brainres.2004.05.081. Epub 2004/07/28. [DOI] [PubMed] [Google Scholar]
- 202.McCaffrey G, Staatz WD, Sanchez-Covarrubias L, Finch JD, Demarco K, Laracuente ML, et al. P-glycoprotein trafficking at the blood-brain barrier altered by peripheral inflammatory hyperalgesia. J Neurochem. 2012;122(5):962–75. doi: 10.1111/j.1471-4159.2012.07831.x. Epub 2012/06/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Huber RD, Gao B, Sidler Pfandler MA, Zhang-Fu W, Leuthold S, Hagenbuch B, et al. Characterization of two splice variants of human organic anion transporting polypeptide 3A1 isolated from human brain. American journal of physiology Cell physiology. 2007;292(2):C795–806. doi: 10.1152/ajpcell.00597.2005. Epub 2006/09/15. [DOI] [PubMed] [Google Scholar]
- 204.Pizzagalli F, Hagenbuch B, Stieger B, Klenk U, Folkers G, Meier PJ. Identification of a novel human organic anion transporting polypeptide as a high affinity thyroxine transporter. Molecular endocrinology (Baltimore, Md) 2002;16(10):2283–96. doi: 10.1210/me.2001-0309. Epub 2002/09/28. [DOI] [PubMed] [Google Scholar]
- 205.Kullak-Ublick GA, Ismair MG, Stieger B, Landmann L, Huber R, Pizzagalli F, et al. Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology. 2001;120(2):525–33. doi: 10.1053/gast.2001.21176. Epub 2001/02/13. [DOI] [PubMed] [Google Scholar]
- 206.Bronger H, Konig J, Kopplow K, Steiner HH, Ahmadi R, Herold-Mende C, et al. ABCC drug efflux pumps and organic anion uptake transporters in human gliomas and the blood-tumor barrier. Cancer research. 2005;65(24):11419–28. doi: 10.1158/0008-5472.CAN-05-1271. Epub 2005/12/17. [DOI] [PubMed] [Google Scholar]
- 207.Ohtsuki S, Takizawa T, Takanaga H, Terasaki N, Kitazawa T, Sasaki M, et al. In vitro study of the functional expression of organic anion transporting polypeptide 3 at rat choroid plexus epithelial cells and its involvement in the cerebrospinal fluidto-blood transport of estrone-3-sulfate. Molecular pharmacology. 2003;63(3):532–7. doi: 10.1124/mol.63.3.532. Epub 2003/02/28. [DOI] [PubMed] [Google Scholar]
- 208.Ronaldson PT, Davis TP. Targeted drug delivery to treat pain and cerebral hypoxia. Pharmacol Rev. 2013;65(1):291–314. doi: 10.1124/pr.112.005991. Epub 2013/01/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Angeletti RH, Novikoff PM, Juvvadi SR, Fritschy JM, Meier PJ, Wolkoff AW. The choroid plexus epithelium is the site of the organic anion transport protein in the brain. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(1):283–6. doi: 10.1073/pnas.94.1.283. Epub 1997/01/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hagenbuch B, Meier PJ. The superfamily of organic anion transporting polypeptides. Biochimica et biophysica acta. 2003;1609(1):1–18. doi: 10.1016/s0005-2736(02)00633-8. Epub 2003/01/01. [DOI] [PubMed] [Google Scholar]
- 211.Gao B, Hagenbuch B, Kullak-Ublick GA, Benke D, Aguzzi A, Meier PJ. Organic anion-transporting polypeptides mediate transport of opioid peptides across bloodbrain barrier. J Pharmacol Exp Ther. 2000;294(1):73–9. Epub 2000/06/28. [PubMed] [Google Scholar]
- 212.Tokui T, Nakai D, Nakagomi R, Yawo H, Abe T, Sugiyama Y. Pravastatin, an HMG-CoA reductase inhibitor, is transported by rat organic anion transporting polypeptide, oatp2. Pharmaceutical research. 1999;16(6):904–8. doi: 10.1023/a:1018838405987. Epub 1999/07/09. [DOI] [PubMed] [Google Scholar]
- 213.Sugiyama D, Kusuhara H, Taniguchi H, Ishikawa S, Nozaki Y, Aburatani H, et al. Functional characterization of rat brain-specific organic anion transporter (Oatp14) at the blood-brain barrier: high affinity transporter for thyroxine. The Journal of biological chemistry. 2003;278(44):43489–95. doi: 10.1074/jbc.M306933200. Epub 2003/08/19. [DOI] [PubMed] [Google Scholar]
- 214.Mikkaichi T, Suzuki T, Onogawa T, Tanemoto M, Mizutamari H, Okada M, et al. Isolation and characterization of a digoxin transporter and its rat homologue expressed in the kidney. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(10):3569–74. doi: 10.1073/pnas.0304987101. Epub 2004/03/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kis B, Isse T, Snipes JA, Chen L, Yamashita H, Ueta Y, et al. Effects of LPS stimulation on the expression of prostaglandin carriers in the cells of the bloodbrain and blood-cerebrospinal fluid barriers. J Appl Physiol. 2006;100(4):1392–9. doi: 10.1152/japplphysiol.01259.2005. Epub 2005/12/03. [DOI] [PubMed] [Google Scholar]
- 216.Simonson GD, Vincent AC, Roberg KJ, Huang Y, Iwanij V. Molecular cloning and characterization of a novel liver-specific transport protein. J Cell Sci. 1994;107(Pt 4):1065–72. doi: 10.1242/jcs.107.4.1065. Epub 1994/04/01. [DOI] [PubMed] [Google Scholar]
- 217.Mori K, Ogawa Y, Ebihara K, Aoki T, Tamura N, Sugawara A, et al. Kidneyspecific expression of a novel mouse organic cation transporter-like protein. FEBS letters. 1997;417(3):371–4. doi: 10.1016/s0014-5793(97)01325-2. Epub 1997/12/31. [DOI] [PubMed] [Google Scholar]
- 218.Brady KP, Dushkin H, Fornzler D, Koike T, Magner F, Her H, et al. A novel putative transporter maps to the osteosclerosis (oc) mutation and is not expressed in the oc mutant mouse. Genomics. 1999;56(3):254–61. doi: 10.1006/geno.1998.5722. Epub 1999/03/24. [DOI] [PubMed] [Google Scholar]
- 219.Kusuhara H, Sekine T, Utsunomiya-Tate N, Tsuda M, Kojima R, Cha SH, et al. Molecular cloning and characterization of a new multispecific organic anion transporter from rat brain. The Journal of biological chemistry. 1999;274(19):13675–80. doi: 10.1074/jbc.274.19.13675. Epub 1999/05/01. [DOI] [PubMed] [Google Scholar]
- 220.Cha SH, Sekine T, Kusuhara H, Yu E, Kim JY, Kim DK, et al. Molecular cloning and characterization of multispecific organic anion transporter 4 expressed in the placenta. The Journal of biological chemistry. 2000;275(6):4507–12. doi: 10.1074/jbc.275.6.4507. Epub 2000/02/08. [DOI] [PubMed] [Google Scholar]
- 221.Lopez-Nieto CE, You G, Bush KT, Barros EJ, Beier DR, Nigam SK. Molecular cloning and characterization of NKT, a gene product related to the organic cation transporter family that is almost exclusively expressed in the kidney. The Journal of biological chemistry. 1997;272(10):6471–8. doi: 10.1074/jbc.272.10.6471. Epub 1997/03/07. [DOI] [PubMed] [Google Scholar]
- 222.Sekine T, Watanabe N, Hosoyamada M, Kanai Y, Endou H. Expression cloning and characterization of a novel multispecific organic anion transporter. The Journal of biological chemistry. 1997;272(30):18526–9. doi: 10.1074/jbc.272.30.18526. Epub 1997/07/25. [DOI] [PubMed] [Google Scholar]
- 223.Sweet DH, Pritchard JB. The molecular biology of renal organic anion and organic cation transporters. Cell biochemistry and biophysics. 1999;31(1):89–118. doi: 10.1007/BF02738157. Epub 1999/10/03. [DOI] [PubMed] [Google Scholar]
- 224.Eraly SA, Nigam SK. Novel human cDNAs homologous to Drosophila Orct and mammalian carnitine transporters. Biochemical and biophysical research communications. 2002;297(5):1159–66. doi: 10.1016/s0006-291x(02)02343-4. Epub 2002/10/10. [DOI] [PubMed] [Google Scholar]
- 225.Monte JC, Nagle MA, Eraly SA, Nigam SK. Identification of a novel murine organic anion transporter family member, OAT6, expressed in olfactory mucosa. Biochemical and biophysical research communications. 2004;323(2):429–36. doi: 10.1016/j.bbrc.2004.08.112. Epub 2004/09/17. [DOI] [PubMed] [Google Scholar]
- 226.Sekine T, Cha SH, Endou H. The multispecific organic anion transporter (OAT) family. Pflugers Archiv : European journal of physiology. 2000;440(3):337–50. doi: 10.1007/s004240000297. Epub 2000/08/23. [DOI] [PubMed] [Google Scholar]
- 227.Emami Riedmaier A, Nies AT, Schaeffeler E, Schwab M. Organic anion transporters and their implications in pharmacotherapy. Pharmacol Rev. 2012;64(3):421–49. doi: 10.1124/pr.111.004614. Epub 2012/03/30. [DOI] [PubMed] [Google Scholar]
- 228.Youngblood GL, Sweet DH. Identification and functional assessment of the novel murine organic anion transporter Oat5 (Slc22a19) expressed in kidney. American journal of physiology Renal physiology. 2004;287(2):F236–44. doi: 10.1152/ajprenal.00012.2004. Epub 2004/04/08. [DOI] [PubMed] [Google Scholar]
- 229.Burckhardt BC, Burckhardt G. Transport of organic anions across the basolateral membrane of proximal tubule cells. Reviews of physiology, biochemistry and pharmacology. 2003;146:95–158. doi: 10.1007/s10254-002-0003-8. Epub 2003/02/28. [DOI] [PubMed] [Google Scholar]
- 230.Sekine T, Cha SH, Tsuda M, Apiwattanakul N, Nakajima N, Kanai Y, et al. Identification of multispecific organic anion transporter 2 expressed predominantly in the liver. FEBS letters. 1998;429(2):179–82. doi: 10.1016/s0014-5793(98)00585-7. Epub 1998/07/03. [DOI] [PubMed] [Google Scholar]
- 231.Sweet DH, Miller DS, Pritchard JB, Fujiwara Y, Beier DR, Nigam SK. Impaired organic anion transport in kidney and choroid plexus of organic anion transporter 3 (Oat3 (Slc22a8)) knockout mice. The Journal of biological chemistry. 2002;277(30):26934–43. doi: 10.1074/jbc.M203803200. Epub 2002/05/16. [DOI] [PubMed] [Google Scholar]
- 232.Nagata Y, Kusuhara H, Endou H, Sugiyama Y. Expression and functional characterization of rat organic anion transporter 3 (rOat3) in the choroid plexus. Molecular pharmacology. 2002;61(5):982–8. doi: 10.1124/mol.61.5.982. Epub 2002/04/19. [DOI] [PubMed] [Google Scholar]
- 233.Kikuchi R, Kusuhara H, Sugiyama D, Sugiyama Y. Contribution of organic anion transporter 3 (Slc22a8) to the elimination of p-aminohippuric acid and benzylpenicillin across the blood-brain barrier. J Pharmacol Exp Ther. 2003;306(1):51–8. doi: 10.1124/jpet.103.049197. Epub 2003/04/10. [DOI] [PubMed] [Google Scholar]
- 234.Xu G, Bhatnagar V, Wen G, Hamilton BA, Eraly SA, Nigam SK. Analyses of coding region polymorphisms in apical and basolateral human organic anion transporter (OAT) genes [OAT1 (NKT), OAT2, OAT3, OAT4, URAT (RST)] Kidney international. 2005;68(4):1491–9. doi: 10.1111/j.1523-1755.2005.00612.x. Epub 2005/09/17. [DOI] [PubMed] [Google Scholar]
- 235.Kusch-Poddar M, Drewe J, Fux I, Gutmann H. Evaluation of the immortalized human brain capillary endothelial cell line BB19 as a human cell culture model for the blood-brain barrier. Brain research. 2005;1064(1-2):21–31. doi: 10.1016/j.brainres.2005.10.014. Epub 2005/11/18. [DOI] [PubMed] [Google Scholar]
- 236.Kimura H, Takeda M, Narikawa S, Enomoto A, Ichida K, Endou H. Human organic anion transporters and human organic cation transporters mediate renal transport of prostaglandins. J Pharmacol Exp Ther. 2002;301(1):293–8. doi: 10.1124/jpet.301.1.293. Epub 2002/03/22. [DOI] [PubMed] [Google Scholar]
- 237.Babu E, Takeda M, Narikawa S, Kobayashi Y, Enomoto A, Tojo A, et al. Role of human organic anion transporter 4 in the transport of ochratoxin A. Biochimica et biophysica acta. 2002;1590(1-3):64–75. doi: 10.1016/s0167-4889(02)00187-8. Epub 2002/06/14. [DOI] [PubMed] [Google Scholar]
- 238.Jonker JW, Schinkel AH. Pharmacological and physiological functions of the polyspecific organic cation transporters: OCT1, 2, and 3 (SLC22A1-3) J Pharmacol Exp Ther. 2004;308(1):2–9. doi: 10.1124/jpet.103.053298. Epub 2003/10/25. [DOI] [PubMed] [Google Scholar]
- 239.Zhang L, Gorset W, Dresser MJ, Giacomini KM. The interaction of ntetraalkylammonium compounds with a human organic cation transporter, hOCT1. J Pharmacol Exp Ther. 1999;288(3):1192–8. Epub 1999/02/23. [PubMed] [Google Scholar]
- 240.Koepsell H, Gorboulev V, Arndt P. Molecular pharmacology of organic cation transporters in kidney. The Journal of membrane biology. 1999;167(2):103–17. doi: 10.1007/s002329900475. Epub 1999/01/23. [DOI] [PubMed] [Google Scholar]
- 241.Koepsell H. Organic cation transporters in intestine, kidney, liver, and brain. Annual review of physiology. 1998;60:243–66. doi: 10.1146/annurev.physiol.60.1.243. Epub 1998/04/29. [DOI] [PubMed] [Google Scholar]
- 242.Dresser MJ, Leabman MK, Giacomini KM. Transporters involved in the elimination of drugs in the kidney: organic anion transporters and organic cation transporters. J Pharm Sci. 2001;90(4):397–421. doi: 10.1002/1520-6017(200104)90:4<397::aid-jps1000>3.0.co;2-d. Epub 2001/02/15. [DOI] [PubMed] [Google Scholar]
- 243.Grundemann D, Gorboulev V, Gambaryan S, Veyhl M, Koepsell H. Drug excretion mediated by a new prototype of polyspecific transporter. Nature. 1994;372(6506):549–52. doi: 10.1038/372549a0. Epub 1994/12/08. [DOI] [PubMed] [Google Scholar]
- 244.Grundemann D, Babin-Ebell J, Martel F, Ording N, Schmidt A, Schomig E. Primary structure and functional expression of the apical organic cation transporter from kidney epithelial LLC-PK1 cells. The Journal of biological chemistry. 1997;272(16):10408–13. doi: 10.1074/jbc.272.16.10408. Epub 1997/04/18. [DOI] [PubMed] [Google Scholar]
- 245.Gorboulev V, Ulzheimer JC, Akhoundova A, Ulzheimer-Teuber I, Karbach U, Quester S, et al. Cloning and characterization of two human polyspecific organic cation transporters. DNA and cell biology. 1997;16(7):871–81. doi: 10.1089/dna.1997.16.871. Epub 1997/07/01. [DOI] [PubMed] [Google Scholar]
- 246.Ullrich KJ. Specificity of transporters for ‘organic anions’ and ‘organic cations’ in the kidney. Biochimica et biophysica acta. 1994;1197(1):45–62. doi: 10.1016/0304-4157(94)90018-3. Epub 1994/04/05. [DOI] [PubMed] [Google Scholar]
- 247.Ciarimboli G, Schlatter E. Regulation of organic cation transport. Pflugers Archiv : European journal of physiology. 2005;449(5):423–41. doi: 10.1007/s00424-004-1355-5. Epub 2005/02/03. [DOI] [PubMed] [Google Scholar]
- 248.Pao SS, Paulsen IT, Saier MH., Jr. Major facilitator superfamily. Microbiology and molecular biology reviews : MMBR. 1998;62(1):1–34. doi: 10.1128/mmbr.62.1.1-34.1998. Epub 1998/04/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Dickens D, Owen A, Alfirevic A, Giannoudis A, Davies A, Weksler B, et al. Lamotrigine is a substrate for OCT1 in brain endothelial cells. Biochemical pharmacology. 2012;83(6):805–14. doi: 10.1016/j.bcp.2011.12.032. Epub 2012/01/10. [DOI] [PubMed] [Google Scholar]
- 250.Slitt AL, Cherrington NJ, Hartley DP, Leazer TM, Klaassen CD. Tissue distribution and renal developmental changes in rat organic cation transporter mRNA levels. Drug metabolism and disposition: the biological fate of chemicals. 2002;30(2):212–9. doi: 10.1124/dmd.30.2.212. Epub 2002/01/17. [DOI] [PubMed] [Google Scholar]
- 251.Grundemann D, Schechinger B, Rappold GA, Schomig E. Molecular identification of the corticosterone-sensitive extraneuronal catecholamine transporter. Nat Neurosci. 1998;1(5):349–51. doi: 10.1038/1557. Epub 1999/04/10. [DOI] [PubMed] [Google Scholar]
- 252.Busch AE, Karbach U, Miska D, Gorboulev V, Akhoundova A, Volk C, et al. Human neurons express the polyspecific cation transporter hOCT2, which translocates monoamine neurotransmitters, amantadine, and memantine. Molecular pharmacology. 1998;54(2):342–52. doi: 10.1124/mol.54.2.342. Epub 1998/08/04. [DOI] [PubMed] [Google Scholar]
- 253.Wu X, Huang W, Ganapathy ME, Wang H, Kekuda R, Conway SJ, et al. Structure, function, and regional distribution of the organic cation transporter OCT3 in the kidney. American journal of physiology Renal physiology. 2000;279(3):F449–58. doi: 10.1152/ajprenal.2000.279.3.F449. Epub 2000/09/01. [DOI] [PubMed] [Google Scholar]
- 254.Kekuda R, Prasad PD, Wu X, Wang H, Fei YJ, Leibach FH, et al. Cloning and functional characterization of a potential-sensitive, polyspecific organic cation transporter (OCT3) most abundantly expressed in placenta. The Journal of biological chemistry. 1998;273(26):15971–9. doi: 10.1074/jbc.273.26.15971. Epub 1998/06/20. [DOI] [PubMed] [Google Scholar]
- 255.Grundemann D, Koster S, Kiefer N, Breidert T, Engelhardt M, Spitzenberger F, et al. Transport of monoamine transmitters by the organic cation transporter type 2, OCT2. The Journal of biological chemistry. 1998;273(47):30915–20. doi: 10.1074/jbc.273.47.30915. Epub 1998/11/13. [DOI] [PubMed] [Google Scholar]
- 256.Wu X, Kekuda R, Huang W, Fei YJ, Leibach FH, Chen J, et al. Identity of the organic cation transporter OCT3 as the extraneuronal monoamine transporter (uptake2) and evidence for the expression of the transporter in the brain. The Journal of biological chemistry. 1998;273(49):32776–86. doi: 10.1074/jbc.273.49.32776. Epub 1998/11/26. [DOI] [PubMed] [Google Scholar]
- 257.Schmitt A, Mossner R, Gossmann A, Fischer IG, Gorboulev V, Murphy DL, et al. Organic cation transporter capable of transporting serotonin is up-regulated in serotonin transporter-deficient mice. Journal of neuroscience research. 2003;71(5):701–9. doi: 10.1002/jnr.10521. Epub 2003/02/14. [DOI] [PubMed] [Google Scholar]
- 258.Kristufek D, Rudorfer W, Pifl C, Huck S. Organic cation transporter mRNA and function in the rat superior cervical ganglion. The Journal of physiology. 2002;543(Pt 1):117–34. doi: 10.1113/jphysiol.2002.021170. Epub 2002/08/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Okura T, Hattori A, Takano Y, Sato T, Hammarlund-Udenaes M, Terasaki T, et al. Involvement of the pyrilamine transporter, a putative organic cation transporter, in blood-brain barrier transport of oxycodone. Drug metabolism and disposition: the biological fate of chemicals. 2008;36(10):2005–13. doi: 10.1124/dmd.108.022087. Epub 2008/07/09. [DOI] [PubMed] [Google Scholar]
- 260.Koepsell H, Endou H. The SLC22 drug transporter family. Pflugers Archiv : European journal of physiology. 2004;447(5):666–76. doi: 10.1007/s00424-003-1089-9. Epub 2003/07/29. [DOI] [PubMed] [Google Scholar]
- 261.Wawrzenczyk A, Nalecz KA, Nalecz MJ. Effect of externally added carnitine on the synthesis of acetylcholine in rat cerebral cortex cells. Neurochemistry international. 1995;26(6):635–41. doi: 10.1016/0197-0186(94)00162-n. Epub 1995/06/01. [DOI] [PubMed] [Google Scholar]
- 262.Tamai I, Ohashi R, Nezu JI, Sai Y, Kobayashi D, Oku A, et al. Molecular and functional characterization of organic cation/carnitine transporter family in mice. The Journal of biological chemistry. 2000;275(51):40064–72. doi: 10.1074/jbc.M005340200. Epub 2000/09/30. [DOI] [PubMed] [Google Scholar]
- 263.Koepsell H, Schmitt BM, Gorboulev V. Organic cation transporters. Reviews of physiology, biochemistry and pharmacology. 2003;150:36–90. doi: 10.1007/s10254-003-0017-x. Epub 2003/06/27. [DOI] [PubMed] [Google Scholar]
- 264.Tamai I, Yabuuchi H, Nezu J, Sai Y, Oku A, Shimane M, et al. Cloning and characterization of a novel human pH-dependent organic cation transporter, OCTN1. FEBS letters. 1997;419(1):107–11. doi: 10.1016/s0014-5793(97)01441-5. Epub 1998/01/13. [DOI] [PubMed] [Google Scholar]
- 265.Wu X, George RL, Huang W, Wang H, Conway SJ, Leibach FH, et al. Structural and functional characteristics and tissue distribution pattern of rat OCTN1, an organic cation transporter, cloned from placenta. Biochimica et biophysica acta. 2000;1466(1-2):315–27. doi: 10.1016/s0005-2736(00)00189-9. Epub 2000/05/29. [DOI] [PubMed] [Google Scholar]
- 266.Yabuuchi H, Tamai I, Nezu J, Sakamoto K, Oku A, Shimane M, et al. Novel membrane transporter OCTN1 mediates multispecific, bidirectional, and pHdependent transport of organic cations. J Pharmacol Exp Ther. 1999;289(2):768–73. Epub 1999/04/24. [PubMed] [Google Scholar]
- 267.Tamai I, Ohashi R, Nezu J, Yabuuchi H, Oku A, Shimane M, et al. Molecular and functional identification of sodium ion-dependent, high affinity human carnitine transporter OCTN2. The Journal of biological chemistry. 1998;273(32):20378–82. doi: 10.1074/jbc.273.32.20378. Epub 1998/08/01. [DOI] [PubMed] [Google Scholar]
- 268.Wu X, Huang W, Prasad PD, Seth P, Rajan DP, Leibach FH, et al. Functional characteristics and tissue distribution pattern of organic cation transporter 2 (OCTN2), an organic cation/carnitine transporter. J Pharmacol Exp Ther. 1999;290(3):1482–92. Epub 1999/08/24. [PubMed] [Google Scholar]
- 269.Kido Y, Tamai I, Ohnari A, Sai Y, Kagami T, Nezu J, et al. Functional relevance of carnitine transporter OCTN2 to brain distribution of L-carnitine and acetyl-Lcarnitine across the blood-brain barrier. J Neurochem. 2001;79(5):959–69. doi: 10.1046/j.1471-4159.2001.00621.x. Epub 2001/12/12. [DOI] [PubMed] [Google Scholar]
- 270.Inano A, Sai Y, Nikaido H, Hasimoto N, Asano M, Tsuji A, et al. Acetyl-Lcarnitine permeability across the blood-brain barrier and involvement of carnitine transporter OCTN2. Biopharmaceutics & drug disposition. 2003;24(8):357–65. doi: 10.1002/bdd.371. Epub 2003/11/05. [DOI] [PubMed] [Google Scholar]
- 271.Wagner CA, Lukewille U, Kaltenbach S, Moschen I, Broer A, Risler T, et al. Functional and pharmacological characterization of human Na(+)-carnitine cotransporter hOCTN2. American journal of physiology Renal physiology. 2000;279(3):F584–91. doi: 10.1152/ajprenal.2000.279.3.F584. Epub 2000/09/01. [DOI] [PubMed] [Google Scholar]
- 272.Ohashi R, Tamai I, Yabuuchi H, Nezu JI, Oku A, Sai Y, et al. Na(+)-dependent carnitine transport by organic cation transporter (OCTN2): its pharmacological and toxicological relevance. J Pharmacol Exp Ther. 1999;291(2):778–84. Epub 1999/10/19. [PubMed] [Google Scholar]
- 273.Lamhonwah A-MTI, Ackerly CA, Tilups A, Edwards VD, Wanders RJ. OCTN3 is a mammalian peroxisomal membrane carnitine transporter. Biochemical and biophysical research communications. 2005;338:1966–72. doi: 10.1016/j.bbrc.2005.10.170. [DOI] [PubMed] [Google Scholar]
- 274.Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, Young JD. The equilibrative nucleoside transporter family, SLC29. Pflugers Archiv : European journal of physiology. 2004;447(5):735–43. doi: 10.1007/s00424-003-1103-2. Epub 2003/07/03. [DOI] [PubMed] [Google Scholar]
- 275.Cabrita MA, Baldwin SA, Young JD, Cass CE. Molecular biology and regulation of nucleoside and nucleobase transporter proteins in eukaryotes and prokaryotes. Biochemistry and cell biology = Biochimie et biologie cellulaire. 2002;80(5):623–38. doi: 10.1139/o02-153. Epub 2002/11/21. [DOI] [PubMed] [Google Scholar]
- 276.Plagemann PG, Aran JM. Characterization of Na(+)-dependent, active nucleoside transport in rat and mouse peritoneal macrophages, a mouse macrophage cell line and normal rat kidney cells. Biochimica et biophysica acta. 1990;1028(3):289–98. doi: 10.1016/0005-2736(90)90178-q. Epub 1990/10/19. [DOI] [PubMed] [Google Scholar]
- 277.Baldwin SA, Yao SY, Hyde RJ, Ng AM, Foppolo S, Barnes K, et al. Functional characterization of novel human and mouse equilibrative nucleoside transporters (hENT3 and mENT3) located in intracellular membranes. The Journal of biological chemistry. 2005;280(16):15880–7. doi: 10.1074/jbc.M414337200. Epub 2005/02/11. [DOI] [PubMed] [Google Scholar]
- 278.Sundaram M, Yao SY, Ingram JC, Berry ZA, Abidi F, Cass CE, et al. Topology of a human equilibrative, nitrobenzylthioinosine (NBMPR)-sensitive nucleoside transporter (hENT1) implicated in the cellular uptake of adenosine and anti-cancer drugs. The Journal of biological chemistry. 2001;276(48):45270–5. doi: 10.1074/jbc.M107169200. Epub 2001/10/05. [DOI] [PubMed] [Google Scholar]
- 279.Hyde RJ, Cass CE, Young JD, Baldwin SA. The ENT family of eukaryote nucleoside and nucleobase transporters: recent advances in the investigation of structure/function relationships and the identification of novel isoforms. Molecular membrane biology. 2001;18(1):53–63. Epub 2001/06/09. [PubMed] [Google Scholar]
- 280.Mani RS, Hammond JR, Marjan JM, Graham KA, Young JD, Baldwin SA, et al. Demonstration of equilibrative nucleoside transporters (hENT1 and hENT2) in nuclear envelopes of cultured human choriocarcinoma (BeWo) cells by functional reconstitution in proteoliposomes. The Journal of biological chemistry. 1998;273(46):30818–25. doi: 10.1074/jbc.273.46.30818. Epub 1998/11/07. [DOI] [PubMed] [Google Scholar]
- 281.Crawford CR, Patel DH, Naeve C, Belt JA. Cloning of the human equilibrative, nitrobenzylmercaptopurine riboside (NBMPR)-insensitive nucleoside transporter ei by functional expression in a transport-deficient cell line. The Journal of biological chemistry. 1998;273(9):5288–93. doi: 10.1074/jbc.273.9.5288. Epub 1998/03/28. [DOI] [PubMed] [Google Scholar]
- 282.Pastor-Anglada M, Felipe A, Casado FJ. Transport and mode of action of nucleoside derivatives used in chemical and antiviral therapies. Trends in pharmacological sciences. 1998;19(10):424–30. doi: 10.1016/s0165-6147(98)01253-x. Epub 1998/11/06. [DOI] [PubMed] [Google Scholar]
- 283.Engel K, Zhou M, Wang J. Identification and characterization of a novel monoamine transporter in the human brain. The Journal of biological chemistry. 2004;279(48):50042–9. doi: 10.1074/jbc.M407913200. Epub 2004/09/28. [DOI] [PubMed] [Google Scholar]
- 284.Ritzel MW, Yao SY, Ng AM, Mackey JR, Cass CE, Young JD. Molecular cloning, functional expression and chromosomal localization of a cDNA encoding a human Na+/nucleoside cotransporter (hCNT2) selective for purine nucleosides and uridine. Molecular membrane biology. 1998;15(4):203–11. doi: 10.3109/09687689709044322. Epub 1999/03/24. [DOI] [PubMed] [Google Scholar]
- 285.Ritzel MW, Ng AM, Yao SY, Graham K, Loewen SK, Smith KM, et al. Molecular identification and characterization of novel human and mouse concentrative Na+-nucleoside cotransporter proteins (hCNT3 and mCNT3) broadly selective for purine and pyrimidine nucleosides (system cib) The Journal of biological chemistry. 2001;276(4):2914–27. doi: 10.1074/jbc.M007746200. Epub 2000/10/18. [DOI] [PubMed] [Google Scholar]
- 286.Wu X, Yuan G, Brett CM, Hui AC, Giacomini KM. Sodium-dependent nucleoside transport in choroid plexus from rabbit. Evidence for a single transporter for purine and pyrimidine nucleosides. The Journal of biological chemistry. 1992;267(13):8813–8. Epub 1992/05/05. [PubMed] [Google Scholar]
- 287.Crawford CR, Ng CY, Noel LD, Belt JA. Nucleoside transport in L1210 murine leukemia cells. Evidence for three transporters. The Journal of biological chemistry. 1990;265(17):9732–6. Epub 1990/06/15. [PubMed] [Google Scholar]
- 288.Smith KM, Slugoski MD, Loewen SK, Ng AM, Yao SY, Chen XZ, et al. The broadly selective human Na+/nucleoside cotransporter (hCNT3) exhibits novel cation-coupled nucleoside transport characteristics. The Journal of biological chemistry. 2005;280(27):25436–49. doi: 10.1074/jbc.M409454200. Epub 2005/05/05. [DOI] [PubMed] [Google Scholar]
- 289.Hamilton SR, Yao SY, Ingram JC, Hadden DA, Ritzel MW, Gallagher MP, et al. Subcellular distribution and membrane topology of the mammalian concentrative Na+-nucleoside cotransporter rCNT1. The Journal of biological chemistry. 2001;276(30):27981–8. doi: 10.1074/jbc.M100518200. Epub 2001/05/29. [DOI] [PubMed] [Google Scholar]
- 290.Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx : the journal of the American Society for Experimental NeuroTherapeutics. 2005;2(1):3–14. doi: 10.1602/neurorx.2.1.3. Epub 2005/02/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Anderson CM, Xiong W, Young JD, Cass CE, Parkinson FE. Demonstration of the existence of mRNAs encoding N1/cif and N2/cit sodium/nucleoside cotransporters in rat brain. Brain research Molecular brain research. 1996;42(2):358–61. doi: 10.1016/s0169-328x(96)00244-6. Epub 1996/12/01. [DOI] [PubMed] [Google Scholar]
- 292.Lu H, Chen C, Klaassen C. Tissue distribution of concentrative and equilibrative nucleoside transporters in male and female rats and mice. Drug metabolism and disposition: the biological fate of chemicals. 2004;32(12):1455–61. doi: 10.1124/dmd.104.001123. Epub 2004/09/17. [DOI] [PubMed] [Google Scholar]
- 293.Graham KA, Leithoff J, Coe IR, Mowles D, Mackey JR, Young JD, et al. Differential transport of cytosine-containing nucleosides by recombinant human concentrative nucleoside transporter protein hCNT1. Nucleosides, nucleotides & nucleic acids. 2000;19(1-2):415–34. doi: 10.1080/15257770008033018. Epub 2000/04/25. [DOI] [PubMed] [Google Scholar]
- 294.Pennycooke M, Chaudary N, Shuralyova I, Zhang Y, Coe IR. Differential expression of human nucleoside transporters in normal and tumor tissue. Biochemical and biophysical research communications. 2001;280(3):951–9. doi: 10.1006/bbrc.2000.4205. Epub 2001/02/13. [DOI] [PubMed] [Google Scholar]
- 295.Nagai K, Nagasawa K, Fujimoto S. Transport mechanisms for adenosine and uridine in primary-cultured rat cortical neurons and astrocytes. Biochemical and biophysical research communications. 2005;334(4):1343–50. doi: 10.1016/j.bbrc.2005.07.032. Epub 2005/07/27. [DOI] [PubMed] [Google Scholar]
- 296.Redzic ZB, Biringer J, Barnes K, Baldwin SA, Al-Sarraf H, Nicola PA, et al. Polarized distribution of nucleoside transporters in rat brain endothelial and choroid plexus epithelial cells. J Neurochem. 2005;94(5):1420–6. doi: 10.1111/j.1471-4159.2005.03312.x. Epub 2005/08/23. [DOI] [PubMed] [Google Scholar]
- 297.Ritzel MW, Ng AM, Yao SY, Graham K, Loewen SK, Smith KM, et al. Recent molecular advances in studies of the concentrative Na+-dependent nucleoside transporter (CNT) family: identification and characterization of novel human and mouse proteins (hCNT3 and mCNT3) broadly selective for purine and pyrimidine nucleosides (system cib) Molecular membrane biology. 2001;18(1):65–72. doi: 10.1080/09687680010026313. Epub 2001/06/09. [DOI] [PubMed] [Google Scholar]
- 298.Herrera-Ruiz D, Knipp GT. Current perspectives on established and putative mammalian oligopeptide transporters. J Pharm Sci. 2003;92(4):691–714. doi: 10.1002/jps.10303. Epub 2003/03/28. [DOI] [PubMed] [Google Scholar]
- 299.Daniel H, Kottra G. The proton oligopeptide cotransporter family SLC15 in physiology and pharmacology. Pflugers Archiv : European journal of physiology. 2004;447(5):610–8. doi: 10.1007/s00424-003-1101-4. Epub 2003/08/09. [DOI] [PubMed] [Google Scholar]
- 300.Carl SMH-R,D, Bhardwaj RK, Gudmundsson O, Knipp GT. Mammalian Oligopeptide Transporters. In: You GMME, editor. Drug Transporters: molecular characterization and role in drug disposition. John Wiley & Sons, Inc.; Hoboken, NJ: 2007. pp. 105–46. [Google Scholar]
- 301.Ocheltree SM, Shen H, Hu Y, Keep RF, Smith DE. Role and relevance of peptide transporter 2 (PEPT2) in the kidney and choroid plexus: in vivo studies with glycylsarcosine in wild-type and PEPT2 knockout mice. J Pharmacol Exp Ther. 2005;315(1):240–7. doi: 10.1124/jpet.105.089359. Epub 2005/07/01. [DOI] [PubMed] [Google Scholar]
- 302.Brandsch M, Brandsch C, Prasad PD, Ganapathy V, Hopfer U, Leibach FH. Identification of a renal cell line that constitutively expresses the kidney-specific high-affinity H+/peptide cotransporter. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1995;9(14):1489–96. doi: 10.1096/fasebj.9.14.7589991. Epub 1995/11/01. [DOI] [PubMed] [Google Scholar]
- 303.Ganapathy V, Leibach FH. Role of pH gradient and membrane potential in dipeptide transport in intestinal and renal brush-border membrane vesicles from the rabbit. Studies with L-carnosine and glycyl-L-proline. The Journal of biological chemistry. 1983;258(23):14189–92. Epub 1983/12/10. [PubMed] [Google Scholar]
- 304.Thwaites DT, Kennedy DJ, Raldua D, Anderson CM, Mendoza ME, Bladen CL, et al. H/dipeptide absorption across the human intestinal epithelium is controlled indirectly via a functional Na/H exchanger. Gastroenterology. 2002;122(5):1322–33. doi: 10.1053/gast.2002.32992. Epub 2002/05/02. [DOI] [PubMed] [Google Scholar]
- 305.Kennedy DJ, Leibach FH, Ganapathy V, Thwaites DT. Optimal absorptive transport of the dipeptide glycylsarcosine is dependent on functional Na+/H+ exchange activity. Pflugers Archiv : European journal of physiology. 2002;445(1):139–46. doi: 10.1007/s00424-002-0910-1. Epub 2002/10/25. [DOI] [PubMed] [Google Scholar]
- 306.Fujita T, Kishida T, Okada N, Ganapathy V, Leibach FH, Yamamoto A. Interaction of kyotorphin and brain peptide transporter in synaptosomes prepared from rat cerebellum: implication of high affinity type H+/peptide transporter PEPT2 mediated transport system. Neuroscience letters. 1999;271(2):117–20. doi: 10.1016/s0304-3940(99)00540-6. Epub 1999/09/07. [DOI] [PubMed] [Google Scholar]
- 307.Shen H, Smith DE, Keep RF, Brosius FC., 3rd. Immunolocalization of the proton-coupled oligopeptide transporter PEPT2 in developing rat brain. Molecular pharmaceutics. 2004;1(4):248–56. doi: 10.1021/mp049944b. Epub 2005/06/29. [DOI] [PubMed] [Google Scholar]
- 308.Knutter I, Rubio-Aliaga I, Boll M, Hause G, Daniel H, Neubert K, et al. H+-peptide cotransport in the human bile duct epithelium cell line SK-ChA-1. American journal of physiology Gastrointestinal and liver physiology. 2002;283(1):G222–9. doi: 10.1152/ajpgi.00534.2001. Epub 2002/06/18. [DOI] [PubMed] [Google Scholar]
- 309.Fujita T, Kishida T, Wada M, Okada N, Yamamoto A, Leibach FH, et al. Functional characterization of brain peptide transporter in rat cerebral cortex: identification of the high-affinity type H+/peptide transporter PEPT2. Brain research. 2004;997(1):52–61. doi: 10.1016/j.brainres.2003.10.049. Epub 2004/01/13. [DOI] [PubMed] [Google Scholar]
- 310.Boll M, Markovich D, Weber WM, Korte H, Daniel H, Murer H. Expression cloning of a cDNA from rabbit small intestine related to proton-coupled transport of peptides, beta-lactam antibiotics and ACE-inhibitors. Pflugers Archiv : European journal of physiology. 1994;429(1):146–9. doi: 10.1007/BF02584043. Epub 1994/11/01. [DOI] [PubMed] [Google Scholar]
- 311.Boll M, Herget M, Wagener M, Weber WM, Markovich D, Biber J, et al. Expression cloning and functional characterization of the kidney cortex high-affinity proton-coupled peptide transporter. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(1):284–9. doi: 10.1073/pnas.93.1.284. Epub 1996/01/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Xiang J, Chiang PP, Hu Y, Smith DE, Keep RF. Role of PEPT2 in glycylsarcosine transport in astrocyte and glioma cultures. Neuroscience letters. 2006;396(3):225–9. doi: 10.1016/j.neulet.2005.11.037. Epub 2005/12/21. [DOI] [PubMed] [Google Scholar]
- 313.Berger UV, Hediger MA. Distribution of peptide transporter PEPT2 mRNA in the rat nervous system. Anatomy and embryology. 1999;199(5):439–49. doi: 10.1007/s004290050242. Epub 1999/04/30. [DOI] [PubMed] [Google Scholar]
- 314.Daniel H, Herget M. Cellular and molecular mechanisms of renal peptide transport. The American journal of physiology. 1997;273(1 Pt 2):F1–8. doi: 10.1152/ajprenal.1997.273.1.F1. Epub 1997/07/01. [DOI] [PubMed] [Google Scholar]
- 315.Dringen R, Hamprecht B, Broer S. The peptide transporter PepT2 mediates the uptake of the glutathione precursor CysGly in astroglia-rich primary cultures. J Neurochem. 1998;71(1):388–93. doi: 10.1046/j.1471-4159.1998.71010388.x. Epub 1998/07/02. [DOI] [PubMed] [Google Scholar]
- 316.Teuscher NS, Novotny A, Keep RF, Smith DE. Functional evidence for presence of PEPT2 in rat choroid plexus: studies with glycylsarcosine. J Pharmacol Exp Ther. 2000;294(2):494–9. Epub 2000/07/20. [PubMed] [Google Scholar]
- 317.Shu C, Shen H, Teuscher NS, Lorenzi PJ, Keep RF, Smith DE. Role of PEPT2 in peptide/mimetic trafficking at the blood-cerebrospinal fluid barrier: studies in rat choroid plexus epithelial cells in primary culture. J Pharmacol Exp Ther. 2002;301(3):820–9. doi: 10.1124/jpet.301.3.820. Epub 2002/05/23. [DOI] [PubMed] [Google Scholar]
- 318.Novotny A, Xiang J, Stummer W, Teuscher NS, Smith DE, Keep RF. Mechanisms of 5-aminolevulinic acid uptake at the choroid plexus. J Neurochem. 2000;75(1):321–8. doi: 10.1046/j.1471-4159.2000.0750321.x. Epub 2000/06/15. [DOI] [PubMed] [Google Scholar]
- 319.Yamashita T, Shimada S, Guo W, Sato K, Kohmura E, Hayakawa T, et al. Cloning and functional expression of a brain peptide/histidine transporter. The Journal of biological chemistry. 1997;272(15):10205–11. doi: 10.1074/jbc.272.15.10205. Epub 1997/04/11. [DOI] [PubMed] [Google Scholar]
- 320.Guo A, Hu P, Balimane PV, Leibach FH, Sinko PJ. Interactions of a nonpeptidic drug, valacyclovir, with the human intestinal peptide transporter (hPEPT1) expressed in a mammalian cell line. J Pharmacol Exp Ther. 1999;289(1):448–54. Epub 1999/03/23. [PubMed] [Google Scholar]
- 321.Banks WA, Kastin AJ, Ehrensing CA. Endogenous peptide Tyr-Pro-Trp-Gly-NH2 (Tyr-W-MIF-1) is transported from the brain to the blood by peptide transport system-1. Journal of neuroscience research. 1993;35(6):690–5. doi: 10.1002/jnr.490350611. Epub 1993/08/15. [DOI] [PubMed] [Google Scholar]
- 322.Banks WA, Kastin AJ, Harrison LM, Zadina JE. Perinatal treatment of rats with opiates affects the development of the blood-brain barrier transport system PTS-1. Neurotoxicology and teratology. 1996;18(6):711–5. doi: 10.1016/s0892-0362(96)00128-6. Epub 1996/11/01. [DOI] [PubMed] [Google Scholar]
- 323.Banks WA, Kastin AJ, Komaki G, Arimura A. Passage of pituitary adenylate cyclase activating polypeptide1-27 and pituitary adenylate cyclase activating polypeptide1-38 across the blood-brain barrier. J Pharmacol Exp Ther. 1993;267(2):690–6. Epub 1993/11/01. [PubMed] [Google Scholar]
- 324.Dogrukol-Ak D, Kumar VB, Ryerse JS, Farr SA, Verma S, Nonaka N, et al. Isolation of peptide transport system-6 from brain endothelial cells: therapeutic effects with antisense inhibition in Alzheimer and stroke models. J Cereb Blood Flow Metab. 2009;29(2):411–22. doi: 10.1038/jcbfm.2008.131. Epub 2008/11/13. [DOI] [PubMed] [Google Scholar]
- 325.Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000;52(2):269–324. Epub 2000/06/02. [PubMed] [Google Scholar]
- 326.Spanier JAD. L.R. Monocarboxylate Transporters. In: You GMME, editor. Drug Transporters: Molecular Characterization and Role in Drug Disposition. John Wiley & Sons, Inc.; Hoboken, NJ: 2007. pp. 147–70. [Google Scholar]
- 327.Kim DK, Kanai Y, Matsuo H, Kim JY, Chairoungdua A, Kobayashi Y, et al. The human T-type amino acid transporter-1: characterization, gene organization, and chromosomal location. Genomics. 2002;79(1):95–103. doi: 10.1006/geno.2001.6678. Epub 2002/02/06. [DOI] [PubMed] [Google Scholar]
- 328.Halestrap AP. The monocarboxylate transporter family--Structure and functional characterization. IUBMB life. 2012;64(1):1–9. doi: 10.1002/iub.573. Epub 2011/12/02. [DOI] [PubMed] [Google Scholar]
- 329.Kim DK, Kanai Y, Chairoungdua A, Matsuo H, Cha SH, Endou H. Expression cloning of a Na+-independent aromatic amino acid transporter with structural similarity to H+/monocarboxylate transporters. The Journal of biological chemistry. 2001;276(20):17221–8. doi: 10.1074/jbc.M009462200. Epub 2001/03/30. [DOI] [PubMed] [Google Scholar]
- 330.Friesema EC, Ganguly S, Abdalla A, Manning Fox JE, Halestrap AP, Visser TJ. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. The Journal of biological chemistry. 2003;278(41):40128–35. doi: 10.1074/jbc.M300909200. Epub 2003/07/23. [DOI] [PubMed] [Google Scholar]
- 331.Meredith D, Christian HC. The SLC16 monocaboxylate transporter family. Xenobiotica; the fate of foreign compounds in biological systems. 2008;38(7-8):1072–106. doi: 10.1080/00498250802010868. Epub 2008/08/01. [DOI] [PubMed] [Google Scholar]
- 332.Gerhart DZ, Enerson BE, Zhdankina OY, Leino RL, Drewes LR. Expression of monocarboxylate transporter MCT1 by brain endothelium and glia in adult and suckling rats. The American journal of physiology. 1997;273(1 Pt 1):E207–13. doi: 10.1152/ajpendo.1997.273.1.E207. Epub 1997/07/01. [DOI] [PubMed] [Google Scholar]
- 333.Leino RL, Gerhart DZ, Drewes LR. Monocarboxylate transporter (MCT1) abundance in brains of suckling and adult rats: a quantitative electron microscopic immunogold study. Brain research Developmental brain research. 1999;113(1-2):47–54. doi: 10.1016/s0165-3806(98)00188-6. Epub 1999/03/05. [DOI] [PubMed] [Google Scholar]
- 334.Broer S, Rahman B, Pellegri G, Pellerin L, Martin JL, Verleysdonk S, et al. Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT 1) expressing Xenopus laevis oocytes. Expression of two different monocarboxylate transporters in astroglial cells and neurons. The Journal of biological chemistry. 1997;272(48):30096–102. doi: 10.1074/jbc.272.48.30096. Epub 1997/12/31. [DOI] [PubMed] [Google Scholar]
- 335.Pierre K, Pellerin L. Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J Neurochem. 2005;94(1):1–14. doi: 10.1111/j.1471-4159.2005.03168.x. Epub 2005/06/15. [DOI] [PubMed] [Google Scholar]
- 336.Balmaceda-Aguilera C, Cortes-Campos C, Cifuentes M, Peruzzo B, Mack L, Tapia JC, et al. Glucose transporter 1 and monocarboxylate transporters 1, 2, and 4 localization within the glial cells of shark blood-brain-barriers. PloS one. 2012;7(2):e32409. doi: 10.1371/journal.pone.0032409. Epub 2012/03/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Koehler-Stec EM, Simpson IA, Vannucci SJ, Landschulz KT, Landschulz WH. Monocarboxylate transporter expression in mouse brain. The American journal of physiology. 1998;275(3 Pt 1):E516–24. doi: 10.1152/ajpendo.1998.275.3.E516. Epub 1998/09/02. [DOI] [PubMed] [Google Scholar]
- 338.Vannucci SJ, Simpson IA. Developmental switch in brain nutrient transporter expression in the rat. American journal of physiology Endocrinology and metabolism. 2003;285(5):E1127–34. doi: 10.1152/ajpendo.00187.2003. Epub 2003/10/10. [DOI] [PubMed] [Google Scholar]
- 339.Bergersen L, Waerhaug O, Helm J, Thomas M, Laake P, Davies AJ, et al. A novel postsynaptic density protein: the monocarboxylate transporter MCT2 is co-localized with delta-glutamate receptors in postsynaptic densities of parallel fiber-Purkinje cell synapses. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 2001;136(4):523–34. doi: 10.1007/s002210000600. Epub 2001/04/09. [DOI] [PubMed] [Google Scholar]
- 340.Rafiki A, Boulland JL, Halestrap AP, Ottersen OP, Bergersen L. Highly differential expression of the monocarboxylate transporters MCT2 and MCT4 in the developing rat brain. Neuroscience. 2003;122(3):677–88. doi: 10.1016/j.neuroscience.2003.08.040. Epub 2003/11/19. [DOI] [PubMed] [Google Scholar]
- 341.Enerson BE, Drewes LR. Molecular features, regulation, and function of monocarboxylate transporters: implications for drug delivery. J Pharm Sci. 2003;92(8):1531–44. doi: 10.1002/jps.10389. Epub 2003/07/29. [DOI] [PubMed] [Google Scholar]
- 342.Dimmer KS, Friedrich B, Lang F, Deitmer JW, Broer S. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem J. 2000;350(Pt 1):219–27. Epub 2000/08/06. [PMC free article] [PubMed] [Google Scholar]
- 343.Manning Fox JE, Meredith D, Halestrap AP. Characterisation of human monocarboxylate transporter 4 substantiates its role in lactic acid efflux from skeletal muscle. The Journal of physiology. 2000;529(Pt 2):285–93. doi: 10.1111/j.1469-7793.2000.00285.x. Epub 2000/12/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Tsuji A, Saheki A, Tamai I, Terasaki T. Transport mechanism of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors at the blood-brain barrier. J Pharmacol Exp Ther. 1993;267(3):1085–90. Epub 1993/12/01. [PubMed] [Google Scholar]
- 345.Tamargo RJ, Rossell LA, Kossoff EH, Tyler BM, Ewend MG, Aryanpur JJ. The intracerebral administration of phenytoin using controlled-release polymers reduces experimental seizures in rats. Epilepsy research. 2002;48(3):145–55. doi: 10.1016/s0920-1211(01)00330-8. Epub 2002/03/21. [DOI] [PubMed] [Google Scholar]
- 346.Barcia JA, Gallego JM. Intraventricular and intracerebral delivery of antiepileptic drugs in the kindling model. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2009;6(2):337–43. doi: 10.1016/j.nurt.2009.01.015. Epub 2009/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Pathan SA, Iqbal Z, Zaidi SM, Talegaonkar S, Vohra D, Jain GK, et al. CNS drug delivery systems: novel approaches. Recent Pat Drug Deliv Formul. 2009;3(1):71–89. doi: 10.2174/187221109787158355. [DOI] [PubMed] [Google Scholar]
- 348.Rapoport SI. Osmotic opening of the blood-brain barrier: principles, mechanism, and therapeutic applications. Cellular and molecular neurobiology. 2000;20(2):217–30. doi: 10.1023/A:1007049806660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Nagashima T, Ikeda K, Wu S, Kondo T, Yamaguchi M, Tamaki N. The mechanism of reversible osmotic opening of the blood-brain barrier: role of intracellular calcium ion in capillary endothelial cells. Acta neurochirurgica Supplement. 1997;70:231–3. doi: 10.1007/978-3-7091-6837-0_71. Epub 1997/01/01. [DOI] [PubMed] [Google Scholar]
- 350.Bhattacharjee AK, Nagashima T, Kondoh T, Tamaki N. The effects of the Na(+)/Ca(++) exchange blocker on osmotic blood-brain barrier disruption. Brain research. 2001;900(2):157–62. doi: 10.1016/s0006-8993(01)02253-3. Epub 2001/05/04. [DOI] [PubMed] [Google Scholar]
- 351.Haluska M, Anthony ML. Osmotic blood-brain barrier modification for the treatment of malignant brain tumors. Clinical journal of oncology nursing. 2004;8(3):263–7. doi: 10.1188/04.CJON.263-267. Epub 2004/06/24. [DOI] [PubMed] [Google Scholar]
- 352.Remsen LG, Pagel MA, McCormick CI, Fiamengo SA, Sexton G, Neuwelt EA. The influence of anesthetic choice, PaCO2, and other factors on osmotic blood-brain barrier disruption in rats with brain tumor xenografts. Anesthesia and analgesia. 1999;88(3):559–67. doi: 10.1097/00000539-199903000-00018. Epub 1999/03/11. [DOI] [PubMed] [Google Scholar]
- 353.Rapoport SI. Advances in osmotic opening of the blood-brain barrier to enhance CNS chemotherapy. Expert opinion on investigational drugs. 2001;10(10):1809–18. doi: 10.1517/13543784.10.10.1809. Epub 2002/01/05. [DOI] [PubMed] [Google Scholar]
- 354.Burgess A, Hynynen K. Noninvasive and Targeted Drug Delivery to the Brain Using Focused Ultrasound. ACS chemical neuroscience. 2013 doi: 10.1021/cn300191b. Epub 2013/02/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Bing KF, Howles GP, Qi Y, Palmeri ML, Nightingale KR. Blood-brain barrier (BBB) disruption using a diagnostic ultrasound scanner and Definity in Mice. Ultrasound in medicine & biology. 2009;35(8):1298–308. doi: 10.1016/j.ultrasmedbio.2009.03.012. Epub 2009/06/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Liu HL, Pan CH, Ting CY, Hsiao MJ. Opening of the blood-brain barrier by lowfrequency (28-kHz) ultrasound: a novel pinhole-assisted mechanical scanning device. Ultrasound in medicine & biology. 2010;36(2):325–35. doi: 10.1016/j.ultrasmedbio.2009.10.004. Epub 2009/12/19. [DOI] [PubMed] [Google Scholar]
- 357.Blomley MJ, Cooke JC, Unger EC, Monaghan MJ, Cosgrove DO. Microbubble contrast agents: a new era in ultrasound. BMJ (Clinical research ed) 2001;322(7296):1222–5. doi: 10.1136/bmj.322.7296.1222. Epub 2001/05/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220(3):640–6. doi: 10.1148/radiol.2202001804. Epub 2001/08/30. [DOI] [PubMed] [Google Scholar]
- 359.Mesiwala AH, Farrell L, Wenzel HJ, Silbergeld DL, Crum LA, Winn HR, et al. High-intensity focused ultrasound selectively disrupts the blood-brain barrier in vivo. Ultrasound in medicine & biology. 2002;28(3):389–400. doi: 10.1016/s0301-5629(01)00521-x. Epub 2002/04/30. [DOI] [PubMed] [Google Scholar]
- 360.Alkins RD, Burgess A, Ganguly M, Francia G, Kerbel RS, Wels WS, et al. Focused Ultrasound Delivers Targeted Immune Cells To Metastatic Brain Tumors. Cancer research. 2013 doi: 10.1158/0008-5472.CAN-12-2609. Epub 2013/01/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Park EJ, Zhang YZ, Vykhodtseva N, McDannold N. Ultrasound-mediated blood-brain/blood-tumor barrier disruption improves outcomes with trastuzumab in a breast cancer brain metastasis model. Journal of controlled release : official journal of the Controlled Release Society. 2012;163(3):277–84. doi: 10.1016/j.jconrel.2012.09.007. Epub 2012/09/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Levi H, Schoknecht K, Prager O, Chassidim Y, Weissberg I, Serlin Y, et al. Stimulation of the sphenopalatine ganglion induces reperfusion and blood-brain barrier protection in the photothrombotic stroke model. PloS one. 2012;7(6):e39636. doi: 10.1371/journal.pone.0039636. Epub 2012/06/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Cannon RE, Peart JC, Hawkins BT, Campos CR, Miller DS. Targeting bloodbrain barrier sphingolipid signaling reduces basal P-glycoprotein activity and improves drug delivery to the brain. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(39):15930–5. doi: 10.1073/pnas.1203534109. Epub 2012/09/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Hawkins BT, Rigor RR, Miller DS. Rapid loss of blood-brain barrier Pglycoprotein activity through transporter internalization demonstrated using a novel in situ proteolysis protection assay. J Cereb Blood Flow Metab. 2010;30(9):1593–7. doi: 10.1038/jcbfm.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Huwyler J, Cerletti A, Fricker G, Eberle AN, Drewe J. By-passing of Pglycoprotein using immunoliposomes. Journal of drug targeting. 2002;10(1):73–9. doi: 10.1080/10611860290007559. Epub 2002/05/09. [DOI] [PubMed] [Google Scholar]
- 366.Markoutsa E, Pampalakis G, Niarakis A, Romero IA, Weksler B, Couraud PO, et al. Uptake and permeability studies of BBB-targeting immunoliposomes using the hCMEC/D3 cell line. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2011;77(2):265–74. doi: 10.1016/j.ejpb.2010.11.015. Epub 2010/12/02. [DOI] [PubMed] [Google Scholar]
- 367.Pan W, Kastin AJ, Zankel TC, van Kerkhof P, Terasaki T, Bu G. Efficient transfer of receptor-associated protein (RAP) across the blood-brain barrier. J Cell Sci. 2004;117(Pt 21):5071–8. doi: 10.1242/jcs.01381. Epub 2004/09/24. [DOI] [PubMed] [Google Scholar]
- 368.Demeule M, Currie JC, Bertrand Y, Che C, Nguyen T, Regina A, et al. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector angiopep-2. J Neurochem. 2008;106(4):1534–44. doi: 10.1111/j.1471-4159.2008.05492.x. Epub 2008/05/21. [DOI] [PubMed] [Google Scholar]
- 369.Thomas FC, Taskar K, Rudraraju V, Goda S, Thorsheim HR, Gaasch JA, et al. Uptake of ANG1005, a novel paclitaxel derivative, through the blood-brain barrier into brain and experimental brain metastases of breast cancer. Pharmaceutical research. 2009;26(11):2486–94. doi: 10.1007/s11095-009-9964-5. Epub 2009/09/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Pardridge WM. Blood-brain barrier drug targeting: the future of brain drug development. Molecular interventions. 2003;3(2):90–105. doi: 10.1124/mi.3.2.90. 51. Epub 2004/03/03. [DOI] [PubMed] [Google Scholar]
- 371.Cornford EM, Young D, Paxton JW, Finlay GJ, Wilson WR, Pardridge WM. Melphalan penetration of the blood-brain barrier via the neutral amino acid transporter in tumor-bearing brain. Cancer research. 1992;52(1):138–43. Epub 1992/01/01. [PubMed] [Google Scholar]
- 372.Uchino H, Kanai Y, Kim DK, Wempe MF, Chairoungdua A, Morimoto E, et al. Transport of amino acid-related compounds mediated by L-type amino acid transporter 1 (LAT1): insights into the mechanisms of substrate recognition. Molecular pharmacology. 2002;61(4):729–37. doi: 10.1124/mol.61.4.729. Epub 2002/03/20. [DOI] [PubMed] [Google Scholar]
- 373.Dagenais C, Graff CL, Pollack GM. Variable modulation of opioid brain uptake by P-glycoprotein in mice. Biochemical pharmacology. 2004;67(2):269–76. doi: 10.1016/j.bcp.2003.08.027. Epub 2003/12/31. [DOI] [PubMed] [Google Scholar]
- 374.Ose A, Kusuhara H, Endo C, Tohyama K, Miyajima M, Kitamura S, et al. Functional characterization of mouse organic anion transporting peptide 1a4 in the uptake and efflux of drugs across the blood-brain barrier. Drug metabolism and disposition: the biological fate of chemicals. 2010;38(1):168–76. doi: 10.1124/dmd.109.029454. Epub 2009/10/17. [DOI] [PubMed] [Google Scholar]
- 375.Egleton RD, Davis TP. Transport of the delta-opioid receptor agonist [Dpenicillamine2,5] enkephalin across the blood-brain barrier involves transcytosis1. J Pharm Sci. 1999;88(4):392–7. doi: 10.1021/js980410+. Epub 1999/04/03. [DOI] [PubMed] [Google Scholar]
- 376.Lebrin F, Deckers M, Bertolino P, Ten Dijke P. TGF-beta receptor function in the endothelium. Cardiovascular research. 2005;65(3):599–608. doi: 10.1016/j.cardiores.2004.10.036. Epub 2005/01/25. [DOI] [PubMed] [Google Scholar]
- 377.Dohgu S, Yamauchi A, Takata F, Naito M, Tsuruo T, Higuchi S, et al. Transforming growth factor-beta1 upregulates the tight junction and P-glycoprotein of brain microvascular endothelial cells. Cellular and molecular neurobiology. 2004;24(3):491–7. doi: 10.1023/B:CEMN.0000022776.47302.ce. Epub 2004/06/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Zhang LL, Lu L, Jin S, Jing XY, Yao D, Hu N, et al. Tissue-specific alterations in expression and function of P-glycoprotein in streptozotocin-induced diabetic rats. Acta pharmacologica Sinica. 2011;32(7):956–66. doi: 10.1038/aps.2011.33. Epub 2011/06/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Bauer B, Hartz AM, Pekcec A, Toellner K, Miller DS, Potschka H. Seizureinduced up-regulation of P-glycoprotein at the blood-brain barrier through glutamate and cyclooxygenase-2 signaling. Molecular pharmacology. 2008;73(5):1444–53. doi: 10.1124/mol.107.041210. Epub 2007/12/21. [DOI] [PubMed] [Google Scholar]
- 380.Largent-Milnes TM, Yamamoto T, Nair P, Moulton JW, Hruby VJ, Lai J, et al. Spinal or systemic TY005, a peptidic opioid agonist/neurokinin 1 antagonist, attenuates pain with reduced tolerance. British journal of pharmacology. 2010;161(5):986–1001. doi: 10.1111/j.1476-5381.2010.00824.x. Epub 2010/10/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Yamamoto T, Nair P, Jacobsen NE, Vagner J, Kulkarni V, Davis P, et al. Improving metabolic stability by glycosylation: bifunctional peptide derivatives that are opioid receptor agonists and neurokinin 1 receptor antagonists. Journal of medicinal chemistry. 2009;52(16):5164–75. doi: 10.1021/jm900473p. Epub 2010/06/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Robey RWP,O, Deeken J, To KKW, Bates SE. Breast Cancer Resistance Protein. In: You GMME, editor. Drug Transporters: Molecular Characterization and Role in Drug Disposition. John Wiley & Sons, Inc; Hoboken, NJ: 2007. pp. 319–58. [Google Scholar]