Abstract
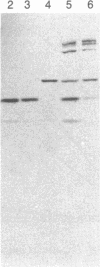
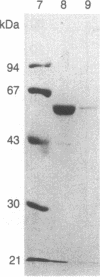
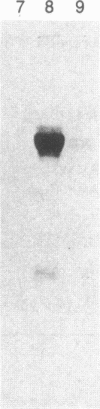
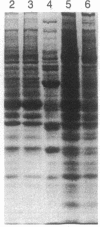
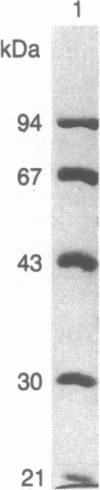
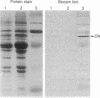
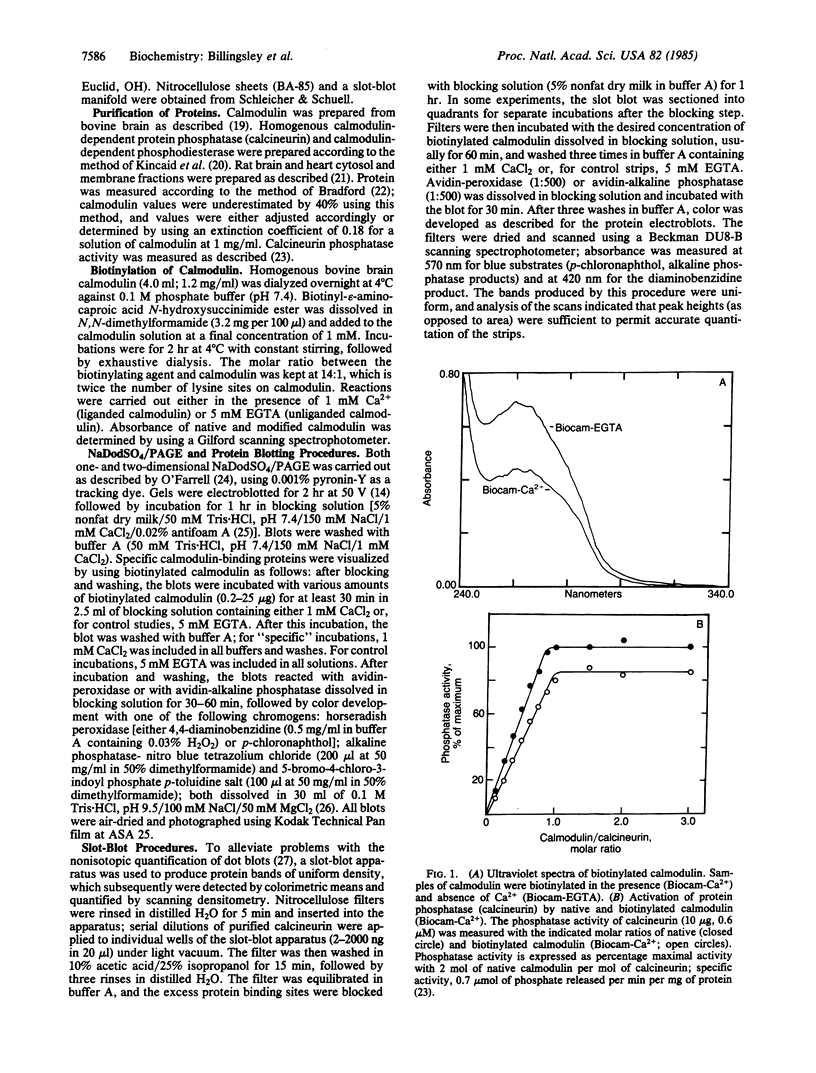
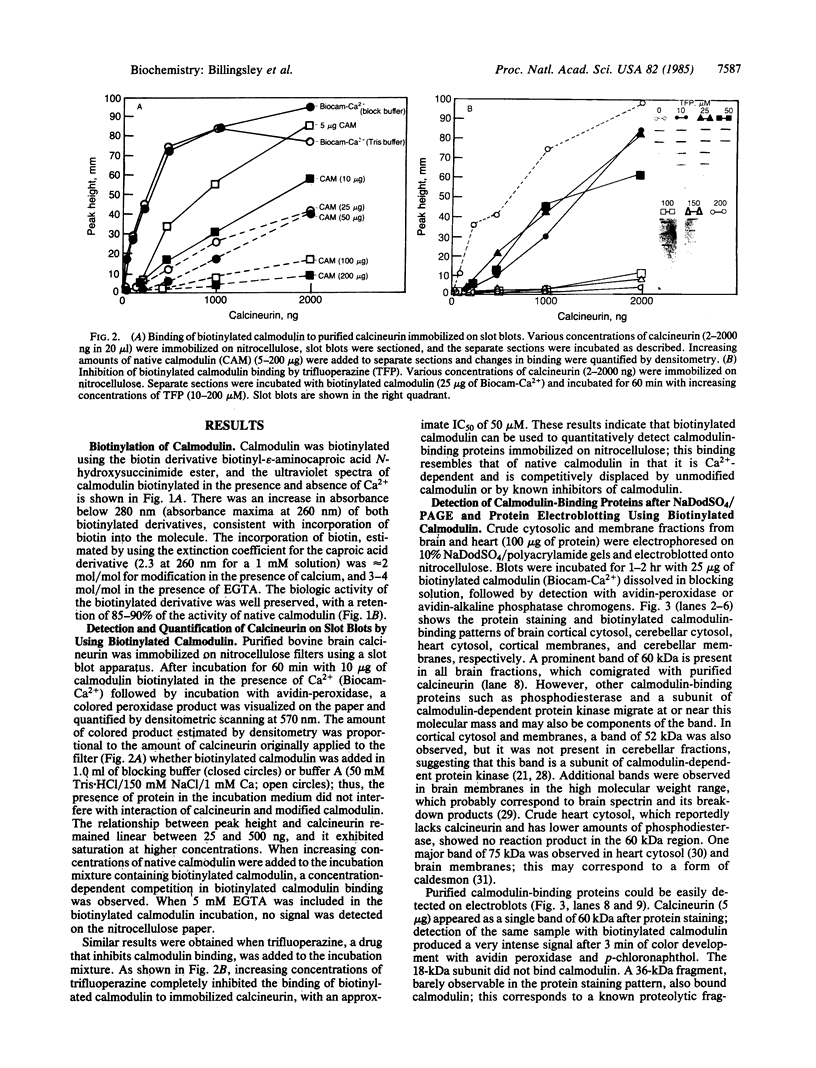
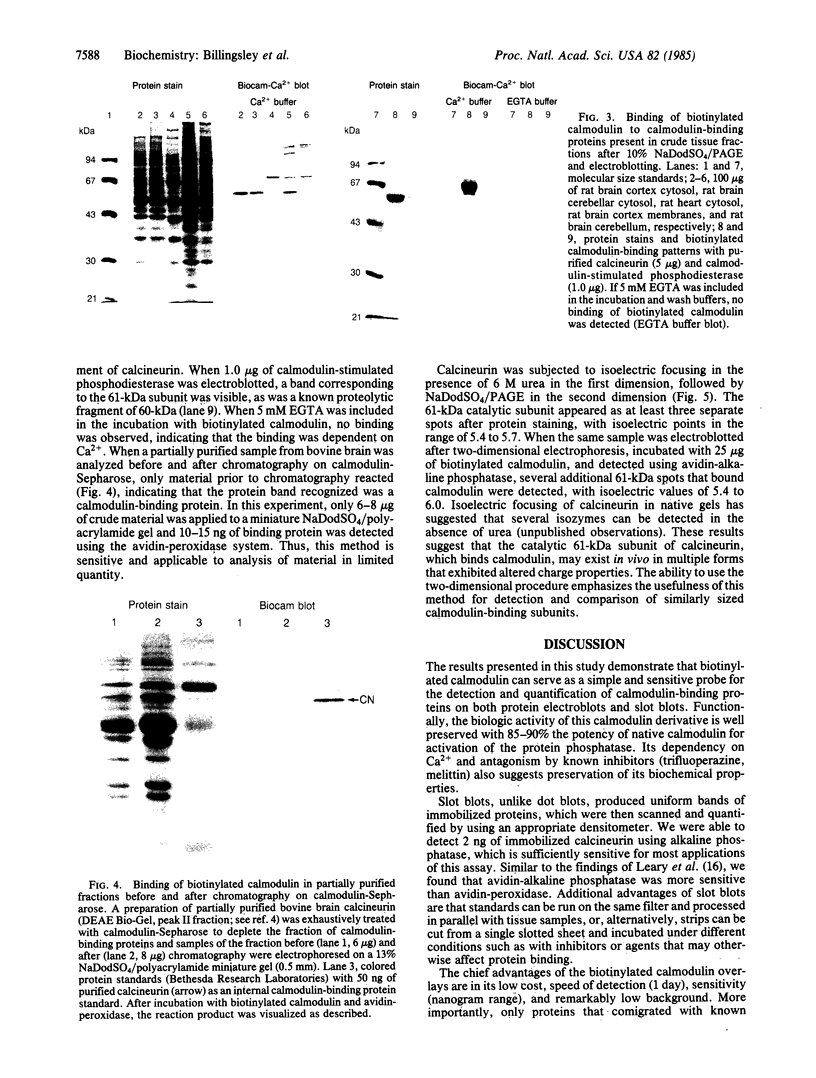
Purified bovine brain calmodulin was biotinylated with biotinyl-epsilon-aminocaproic acid N-hydroxysuccinimide. Biotinylated calmodulin was used to detect and quantify calmodulin-binding proteins following both protein blotting and slot-blot procedures by using alkaline phosphatase or peroxidase coupled to avidin. When purified bovine brain calcineurin, a calmodulin-dependent protein phosphatase, was immobilized on nitrocellulose slot blots, biotinylated calmodulin bound in a calcium-dependent saturable manner; these blots were then quantified by densitometry. Biotinylated calmodulin was able to detect as little as 10 ng of calcineurin, and the binding was competitively inhibited by addition of either native calmodulin or trifluoperazine. When biotinylated calmodulin was used to probe protein blots of crude brain cytosol and membrane preparations after gel electrophoresis, only protein bands characteristic of known calmodulin-binding proteins (i.e., calmodulin-dependent protein kinase, calcineurin, spectrin) were detected with avidin-peroxidase or avidin-alkaline phosphatase procedures. Purified calcineurin was subjected to one- and two-dimensional gel electrophoresis and protein blotting; as expected, only the 61-kDa calmodulin-binding subunit was detected. When the two-dimensional protein blot was incubated with biotinylated calmodulin and detected with avidin-alkaline phosphatase, several apparent forms of the 61-kDa catalytic subunit were detected, consistent with isozymic species of the enzyme. The results of these studies suggest that biotinylated calmodulin can be used as a simple, sensitive, and quantifiable probe for the study of calmodulin-binding proteins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billingsley M. L., Kincaid R. L., Lovenberg W. Stoichiometric methylation of calcineurin by protein carboxyl O-methyltransferase and its effects on calmodulin-stimulated phosphatase activity. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5612–5616. doi: 10.1073/pnas.82.17.5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brostrom C. O., Huang Y. C., Breckenridge B. M., Wolff D. J. Identification of a calcium-binding protein as a calcium-dependent regulator of brain adenylate cyclase. Proc Natl Acad Sci U S A. 1975 Jan;72(1):64–68. doi: 10.1073/pnas.72.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess W. H., Watterson D. M., Van Eldik L. J. Identification of calmodulin-binding proteins in chicken embryo fibroblasts. J Cell Biol. 1984 Aug;99(2):550–557. doi: 10.1083/jcb.99.2.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin R. K., Bartelt D. C., Siekevitz P. Identification of fodrin as a major calmodulin-binding protein in postsynaptic density preparations. J Cell Biol. 1983 Feb;96(2):443–448. doi: 10.1083/jcb.96.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin R. K., Grab D. J., Siekevitz P. Function of a calmodulin in postsynaptic densities. III. Calmodulin-binding proteins of the postsynaptic density. J Cell Biol. 1981 Jun;89(3):449–455. doi: 10.1083/jcb.89.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Demonstration of an activator. Biochem Biophys Res Commun. 1970 Feb 6;38(3):533–538. doi: 10.1016/0006-291x(70)90747-3. [DOI] [PubMed] [Google Scholar]
- DeLorenzo R. J., Freedman S. D., Yohe W. B., Maurer S. C. Stimulation of Ca2+-dependent neurotransmitter release and presynaptic nerve terminal protein phosphorylation by calmodulin and a calmodulin-like protein isolated from synaptic vesicles. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1838–1842. doi: 10.1073/pnas.76.4.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan S. D., Yost B. Calmodulin-binding proteins: visualization by 125I-calmodulin overlay on blots quenched with Tween 20 or bovine serum albumin and poly(ethylene oxide). Anal Biochem. 1984 Aug 1;140(2):510–519. doi: 10.1016/0003-2697(84)90202-1. [DOI] [PubMed] [Google Scholar]
- Flanagan S. D., Yost B., Crawford G. Putative 51,000-Mr protein marker for postsynaptic densities is virtually absent in cerebellum. J Cell Biol. 1982 Sep;94(3):743–748. doi: 10.1083/jcb.94.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenring J. R., Gonzalez B., McGuire J. S., Jr, DeLorenzo R. J. Purification and characterization of a calmodulin-dependent kinase from rat brain cytosol able to phosphorylate tubulin and microtubule-associated proteins. J Biol Chem. 1983 Oct 25;258(20):12632–12640. [PubMed] [Google Scholar]
- Gopalakrishna R., Anderson W. B. Ca2+-induced hydrophobic site on calmodulin: application for purification of calmodulin by phenyl-Sepharose affinity chromatography. Biochem Biophys Res Commun. 1982 Jan 29;104(2):830–836. doi: 10.1016/0006-291x(82)90712-4. [DOI] [PubMed] [Google Scholar]
- Guesdon J. L., Ternynck T., Avrameas S. The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem. 1979 Aug;27(8):1131–1139. doi: 10.1177/27.8.90074. [DOI] [PubMed] [Google Scholar]
- Jahn R., Schiebler W., Greengard P. A quantitative dot-immunobinding assay for proteins using nitrocellulose membrane filters. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1684–1687. doi: 10.1073/pnas.81.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid R. L., Manganiello V. C., Odya C. E., Osborne J. C., Jr, Stith-Coleman I. E., Danello M. A., Vaughan M. Purification and properties of calmodulin-stimulated phosphodiesterase from mammalian brain. J Biol Chem. 1984 Apr 25;259(8):5158–5166. [PubMed] [Google Scholar]
- Kincaid R. L., Vaughan M., Osborne J. C., Jr, Tkachuk V. A. Ca2+-dependent interaction of 5-dimethylaminonaphthalene-1-sulfonyl-calmodulin with cyclic nucleotide phosphodiesterase, calcineurin, and troponin I. J Biol Chem. 1982 Sep 25;257(18):10638–10643. [PubMed] [Google Scholar]
- Klee C. B., Haiech J. Concerted role of calmodulin and calcineurin in calcium regulation. Ann N Y Acad Sci. 1980;356:43–54. doi: 10.1111/j.1749-6632.1980.tb29598.x. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Krinks M. H. Purification of cyclic 3',5'-nucleotide phosphodiesterase inhibitory protein by affinity chromatography on activator protein coupled to Sepharose. Biochemistry. 1978 Jan 10;17(1):120–126. doi: 10.1021/bi00594a017. [DOI] [PubMed] [Google Scholar]
- LaPorte D. C., Toscano W. A., Jr, Storm D. R. Cross-linking of iodine-125-labeled, calcium-dependent regulatory protein to the Ca2+-sensitive phosphodiesterase purified from bovine heart. Biochemistry. 1979 Jun 26;18(13):2820–2825. doi: 10.1021/bi00580a021. [DOI] [PubMed] [Google Scholar]
- Leary J. J., Brigati D. J., Ward D. C. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4045–4049. doi: 10.1073/pnas.80.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means A. R., Dedman J. R. Calmodulin--an intracellular calcium receptor. Nature. 1980 May 8;285(5760):73–77. doi: 10.1038/285073a0. [DOI] [PubMed] [Google Scholar]
- Miller S. G., Kennedy M. B. Distinct forebrain and cerebellar isozymes of type II Ca2+/calmodulin-dependent protein kinase associate differently with the postsynaptic density fraction. J Biol Chem. 1985 Jul 25;260(15):9039–9046. [PubMed] [Google Scholar]
- Molla A., Hincke M. T., Katz S., Lazaro R. Azidocalmodulin derivatives. Activation of, and binding to, three target proteins: aorta myosin light-chain kinase, erythrocyte (Mg2+ + Ca2+)-dependent ATPase and cardiac sarcoplasmic-reticulum kinase. Biochem J. 1983 Dec 1;215(3):475–482. doi: 10.1042/bj2150475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Owada M. K., Hakura A., Iida K., Yahara I., Sobue K., Kakiuchi S. Occurrence of caldesmon (a calmodulin-binding protein) in cultured cells: comparison of normal and transformed cells. Proc Natl Acad Sci U S A. 1984 May;81(10):3133–3137. doi: 10.1073/pnas.81.10.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallen C. J., Wang J. H. Calmodulin-stimulated dephosphorylation of p-nitrophenyl phosphate and free phosphotyrosine by calcineurin. J Biol Chem. 1983 Jul 25;258(14):8550–8553. [PubMed] [Google Scholar]
- Sarkar D., Erlichman J., Rubin C. S. Identification of a calmodulin-binding protein that co-purifies with the regulatory subunit of brain protein kinase II. J Biol Chem. 1984 Aug 10;259(15):9840–9846. [PubMed] [Google Scholar]
- Schulman H., Greengard P. Stimulation of brain membrane protein phosphorylation by calcium and an endogenous heat-stable protein. Nature. 1978 Feb 2;271(5644):478–479. doi: 10.1038/271478a0. [DOI] [PubMed] [Google Scholar]
- Stewart A. A., Ingebritsen T. S., Manalan A., Klee C. B., Cohen P. Discovery of a Ca2+- and calmodulin-dependent protein phosphatase: probable identity with calcineurin (CaM-BP80). FEBS Lett. 1982 Jan 11;137(1):80–84. doi: 10.1016/0014-5793(82)80319-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler M. A., Merat D. L., Tallant E. A., Hawkins S., Cheung W. Y. Catalytic site of calmodulin-dependent protein phosphatase from bovine brain resides in subunit A. Proc Natl Acad Sci U S A. 1984 May;81(10):3054–3058. doi: 10.1073/pnas.81.10.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]