Abstract
Prolactin controls the development and function of milk-producing breast epithelia but also supports growth and differentiation of breast cancer, especially luminal subtypes. A principal signaling mediator of prolactin, Stat5, promotes cellular differentiation of breast cancer cells in vitro, and loss of active Stat5 in tumors is associated with anti-estrogen therapy failure in patients. In luminal breast cancer progesterone induces a cytokeratin-5 (CK5)-positive basal cell-like population. This population possesses characteristics of tumor stem cells including quiescence, therapy-resistance, and tumor-initiating capacity. Here we report that prolactin counteracts induction of the CK5-positive population by the synthetic progestin R5020 in luminal breast cancer cells both in vitro and in vivo. CK5-positive cells were chemoresistant as determined by four-fold reduced rate of apoptosis following docetaxel exposure. Progestin-induction of CK5 was preceded by marked up-regulation of BCL6, an oncogene and transcriptional repressor critical for the maintenance of leukemia-initiating cells. Knockdown of BCL6 prevented induction of CK5-positive cell population by progestin. Prolactin suppressed progestin-induced BCL6 through Jak2-Stat5 but not Erk- or Akt-dependent pathways. In premenopausal but not postmenopausal patients with hormone receptor-positive breast cancer, tumor protein levels of CK5 correlated positively with BCL6, and high BCL6 or CK5 protein levels were associated with unfavorable clinical outcome. Suppression of progestin-induction of CK5-positive cells represents a novel pro-differentiation effect of prolactin in breast cancer. The present progress may have direct implications for breast cancer progression and therapy since loss of prolactin receptor-Stat5 signaling occurs frequently and BCL6 inhibitors currently being evaluated for lymphomas may have value for breast cancer.
Keywords: breast cancer, CK5, BCL6, prolactin, progesterone, Stat5
Introduction
Eighty percent of newly diagnosed breast cancers are estrogen receptor (ER)-positive and classified as luminal subtypes (1). Although luminal breast cancers are associated with more favorable prognosis than ER-negative breast cancers and may be effectively treated by surgery and hormone therapy, long-term drug resistance and tumor recurrence remain significant obstacles (2). Emergence of cancer cell populations with stem cell features of drug resistance and tumor-initiating capacity is one proposed mechanism for inherent or acquired chemoresistance in cancer (3). Tumor-initiating cells often express high levels of drug transporters and tend to survive and increase in number when breast cancers are exposed to chemotherapy (4–6).
In addition to ER expression, luminal breast cancers are characterized by frequent but not obligate expression of progesterone receptors (PR) and typically lack broad expression of basal cytokeratins such as CK5. However, within some luminal breast cancers a subpopulation of ER-negative/PR-negative/CK5-positive cells that expresses basal-like markers has been identified (7, 8). This subpopulation of cells display stem-like features of drug resistance and tumor-initiating ability as supported by in vitro and in vivo data (9, 10). Importantly, the CK5-positive cell population is up-regulated in patients whose luminal breast cancer develops resistance to chemo- and hormone therapies (9, 10). In experimental breast cancer models, CK5-positive cells expand in number in response to antiestrogen or hormone withdrawal therapies, further supporting the notion that this cell population is causally related to therapy resistance in luminal breast cancer (10). Initial evidence implicates progesterone as an inducer of the CK5-positive cell population (7, 8).
There is limited knowledge about hormonal control of progesterone-induced CK5-positive cells in breast cancer. Despite extensive interactions between progesterone and prolactin in the control of normal breast epithelial expansion and differentiation during pregnancy, both opposing and cooperative, surprisingly little is known about prolactin and progesterone interactions in luminal breast cancer (11). The pituitary protein hormone prolactin is a potent activator of Stat5a and to a lesser extent Stat5b in breast epithelia (12, 13). Loss of Stat5 signaling in breast cancer is associated with poor clinical outcome including increased risk of unresponsiveness to antiestrogen therapy (14–18). In addition, prolactin maintains cellular differentiation and suppresses invasive features of luminal breast cancer cell lines in vitro (19–21). We undertook these studies to determine whether prolactin might modulate progesterone-induced expansion of the CK5-positive breast cancer cell population in luminal breast cancer.
We now report that prolactin blocked progesterone receptor-mediated induction of CK5-positive cells in luminal breast cancer. This is supported by mRNA and protein analysis in extracts and in individual cells. This progestin (Pg)-induced CK5-positive cell population was resistant to chemotherapy-induced apoptosis. Importantly, we provide novel evidence that Pg rapidly up-regulated the transcriptional repressor BCL6 prior to CK5-induction, and that Pg-driven BCL6 expression was required for induction of CK5-positive cells. Furthermore, prolactin effectively blocked Pg-induction of BCL6, providing a mechanism for negative regulation by prolactin of a novel progesterone receptor-BCL6 axis. Stat5 but not Erk- or Akt-dependent pathways, was important for prolactin suppression of Pg-induction of CK5. Finally, in situ quantitative immunofluorescence analyses of clinical specimens revealed that protein levels of CK5 and BCL6 were positively correlated in hormone receptor-positive tumors from premenopausal but not postmenopausal breast cancer patients. Furthermore, elevated BCL6 or CK5 protein levels were associated with unfavorable clinical outcome. Collectively, we propose a model in which prolactin-Stat5 signaling inhibits Pg-induced expansion of the CK5-positive cell population and associated therapy-resistance through suppression of Pg-induced BCL6.
Results
Prolactin suppresses CK5 mRNA and protein levels in human breast cancer cells
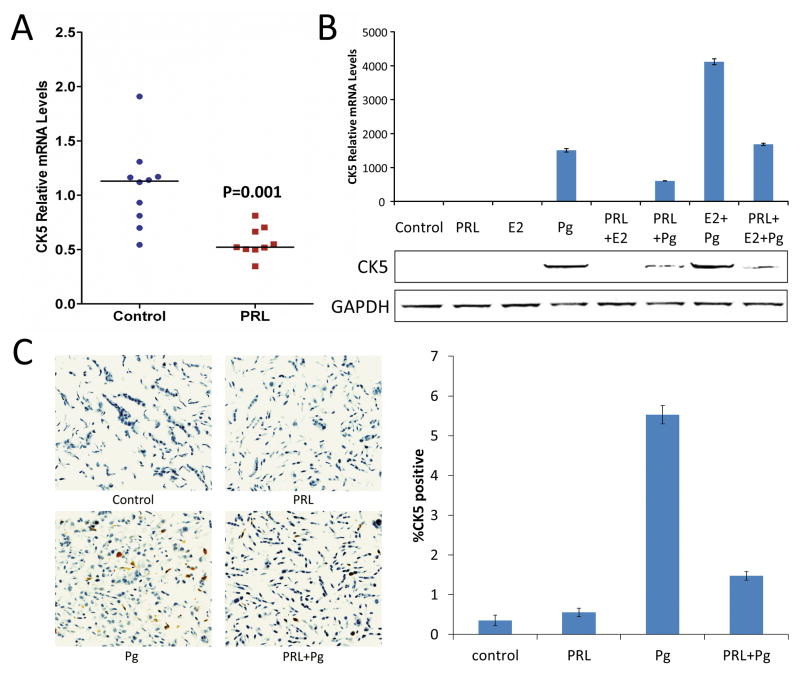
An initial observation indicating that prolactin may suppress expression of the basal cytokeratin, CK5, in luminal breast cancer originated from analysis of mRNA extracted from T47D xenograft tumors in nude mice that had been treated with either prolactin or saline for 48 h. Levels of CK5 mRNA as measured by qRT-PCR were significantly lower in tumors from mice treated with human prolactin than in tumors from untreated mice (Figure 1A; P=0.001). We then investigated whether prolactin could inhibit CK5 mRNA and protein levels in T47D cells in vitro, and incorporated progestin treatment to further elevate CK5 expression in T47D cells as reported previously (8). The synthetic progestin R5020 (Pg) alone or in combination with 17β-Estradiol (E2) increased CK5 mRNA levels in T47D cells exposed to the hormones for 48 h (Figure 1B top). Consistent with the in vivo data, prolactin markedly suppressed CK5 mRNA levels induced by Pg or Pg+E2 treatment (Figure 1B top). This suppressive effect of prolactin on Pg-induction of CK5 was also verified at the protein level (Figure 1B, bottom). Since E2 alone did not significantly stimulate CK5 protein levels, and the apparent interaction between Pg and E2 at the CK5 mRNA level did not translate into corresponding interaction at the CK5 protein level, subsequent T47D experiments focused on the effects of Pg.
Figure 1. Prolactin suppresses CK5 mRNA and protein levels in human breast cancer cells.
(A) qRT-PCR analysis of CK5 mRNA extracted from T47D xenograft tumors in mice treated with either vehicle or prolactin for 48 h. Individual values are plotted and horizontal markers indicate median levels (P=0.001 by Mann-Whitney U test). (B) qRT-PCR (top) and immunoblot (bottom) analysis of CK5 mRNA and protein levels, respectively, in extracts of cultured T47D treated with vehicle (Control), Prolactin (PRL), β-Estradiol (E2), or R5020 (Pg) for 48 h. (C) Immunocytochemistry using DAB chromogen (brown) for CK5 and hematoxylin counterstain of sections of formalin-fixed, paraffin-embedded pellets of T47D cells treated with hormones as indicated for 24 h. Representative images are shown (top) with quantification for cellular CK5-positivity plotted (bottom). In Pg-treated cells, the percentage of CK5-positive cells was 3.8 times higher (95% CI: 1.6, 8.8, P=0.005) than in prolactin+Pg-treated cells.
To determine whether Pg-induction of CK5 protein represented expansion of a CK5-positive cell population or a general up-regulation of CK5 in all cells, we performed immunocytochemistry (ICC) using DAB chromogen for CK5 detection using a mini-array of formalin-fixed, paraffin-embedded pellets of T47D cells that had been treated with vehicle, prolactin, Pg, or Pg plus prolactin. Immunostaining for CK5 protein in these specimens did in fact verify that progestin significantly induced a rare and distinctly CK5-positive cell population in T47D cells (Figure 1C). Vehicle (Control) or prolactin-treated cells included very few CK5-positive cells (0.35% and 0.55%, respectively). In Pg-treated cells, the percentage of CK5-positive cells (5.5%) was 3.8 times higher (95% CI: 1.6, 8.8, P=0.005) than in prolactin+Pg-treated cells (1.5%). These data collectively show that Pg induces a CK5-positive cell population in T47D cells and prolactin markedly suppresses Pg-induction of this cell population.
Progestin-induction of chemoresistant CK5-positive cells is suppressed by prolactin
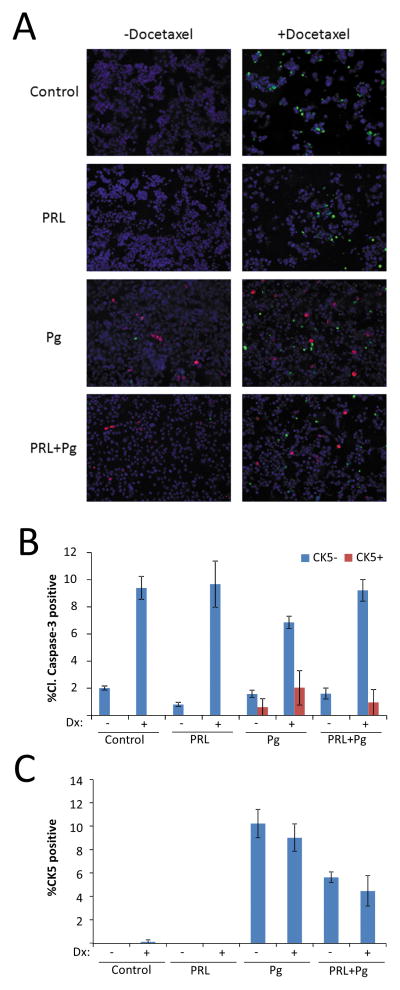
CK5-positive T47D cells were recently reported to be resistant to chemotherapy, a characteristic of tumor-initiating cells (9). We tested whether Pg-induced CK5-positive T47D cells were resistant to chemotherapy by co-staining cells for CK5 and cleaved caspase-3, a marker of apoptosis. For these studies, cells were pre-treated with Pg and/or prolactin for 24 h followed by treatment with the chemotherapeutic docetaxel for 48 h. Analysis of cultures co-stained for CK5 and cleaved caspase-3 revealed that CK5-positive cells only rarely were positive for the apoptosis marker (Figure 2A). Quantification revealed a four-fold reduced rate of cleaved caspase-3 staining in CK5-positive cells than in CK5-negative cells (odds ratio 0.25, 95% CI: 0.11, 0.56, P<0.001) (Figure 2B). Furthermore, the odds of CK5-positivity were 50% lower for cells treated with prolactin plus Pg (5.7%) than for cells exposed to Pg alone (10.3%) (odds ratio= 0.50, 95% CI: 0.39, 0.64, P<0.001), supporting the novel concept that prolactin may limit a chemoresistant cell population (Figure 2C).
Figure 2. Prolactin suppresses the fraction of chemoresistant Pg-induced CK5-positive cells.
(A) Representative images of multiplexed immunocytochemistry of T47D cell cultures treated with vehicle (Control), Prolactin (PRL), β-Estradiol (E2), or R5020 (Pg) for 24 h before addition of docetaxel (Dx) or vehicle for 48 h and stained for CK5 (red), cleaved caspase-3 (green), and DAPI (blue). (B) Bar graph of percent of CK5-negative (blue bars) or CK5-positive (red bars) cells that were positive for cleaved caspase-3. Quantification revealed a four-fold reduced rate of cleaved caspase-3 staining in CK5-positive cells than in CK5-negative cells (odds ratio 0.25, 95% CI: 0.11, 0.56, P<0.001) (C) Overall percentage of cells positive for CK5 in the cultures shown in panel A. Odds of CK5 positivity were 50% lower for cells treated with prolactin plus Pg (5.7% CK5+) than for cells exposed to Pg alone (10.3% CK5+) (odds ratio= 0.50, 95% CI: 0.39, 0.64, P<0.001).
Progestin-induced CK5 expression is preceded by BCL6 expression and prolactin suppresses progestin-induced BCL6
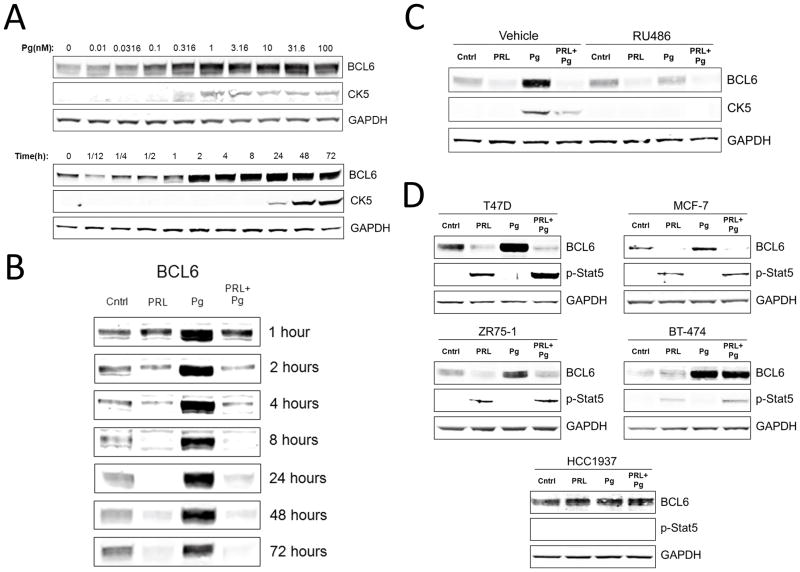
Since CK5-positive T47D cells represent a tumor-initiating population induced by Pg and suppressed by prolactin (Figures 1 and 2), we determined whether Pg could induce BCL6, an oncogene and transcriptional regulator reported to maintain tumor-initiating cells (22) and to be suppressed by prolactin (23). An initial genome-wide transcript analysis of T47D cells listed BCL6 mRNA as up-regulated two-fold by progestin but this was not verified by qRT-PCR or at the protein level (24). We treated T47D cells for various times and with various concentrations of Pg. Marked BCL6 protein induction by Pg was detectable at doses as low as 100 pM. The effect was rapidly detectable within 2h and prolonged for at least 72h, demonstrating that Pg is a potent inducer of BCL6 protein (Figure 3A). Importantly, induction of BCL6 preceded induction of CK5 protein, which was detectably induced only after 24 h (Figure 3A).
Figure 3. Progestin induces BCL6 expression and prolactin suppresses progestin-induced BCL6.
(A) Immunoblots of BCL6, CK5, and GAPDH protein levels of T47D cells treated with the synthetic progestin R5020 (Pg) at the indicated doses for 24 h (top), or the indicated times with 20nM R5020 (Pg) (bottom). (B) Immunoblots of BCL6 protein in extracts of T47D cells treated with vehicle (Cntrl), Prolactin (PRL), β-Estradiol (E2), or R5020 (Pg) for the indicated times. (C) Immunoblots of BCL6, CK5, and GAPDH protein levels in extracts of T47D cells treated with hormones in the presence or absence of the progesterone receptor antagonist RU486 for 48 h. (D) Immunoblots of BCL6, phosphorylated-Stat5 (p-Stat5), and GAPDH protein levels in extracts of T47D, MCF-7, ZR75-1, BT-474, and HCC1937 cells treated with hormones for 72 h.
We and others have previously demonstrated that prolactin is capable of repressing basal levels of BCL6 protein (23, 25), but it is unknown whether prolactin also could suppress the highly elevated Pg-induced BCL6 protein levels. Simultaneous exposure of T47D cells to prolactin and Pg robustly decreased the levels of Pg-induced BCL6 protein at every time point from 1 to 72h (Figure 3B). In fact, from 2h on, prolactin suppressed Pg-induced BCL6 below the basal levels. Next, we investigated whether the Pg induction of BCL6 was through the progesterone receptor (PR) by utilizing the PR antagonist RU486. T47D cells treated with the PR antagonist RU486 blocked the ability of Pg to induce BCL6 levels, indicating that Pg induction of BCL6 is mediated by PR (Figure 3D).
We then extended our studies to other PR-positive breast cancer cell lines including MCF-7, ZR75-1, and BT-474. Since PR levels in these cell lines are lower than in T47D cells, we treated the cells with E2 during prolactin and Pg treatment in order to elevate PR expression. Immunoblot analysis revealed that Pg induced BCL6 in all four cell lines, although the degree of PR responsiveness varied (Figure 3E). Prolactin effectively repressed Pg-induction of BCL6 in MCF-7 and ZR75-1, but not in BT-474 cells. BT-474 had the lowest phosphorylated-Stat5 response which most likely explains the lack of suppression by prolactin (Figure 3E). As expected, the prolactin receptor/PR-negative basal cell line HCC1937 expressed BCL6 and CK5 but was unaffected by both Pg and prolactin (Figure 3E).
Progestin induction of CK5 is dependent on BCL6 expression
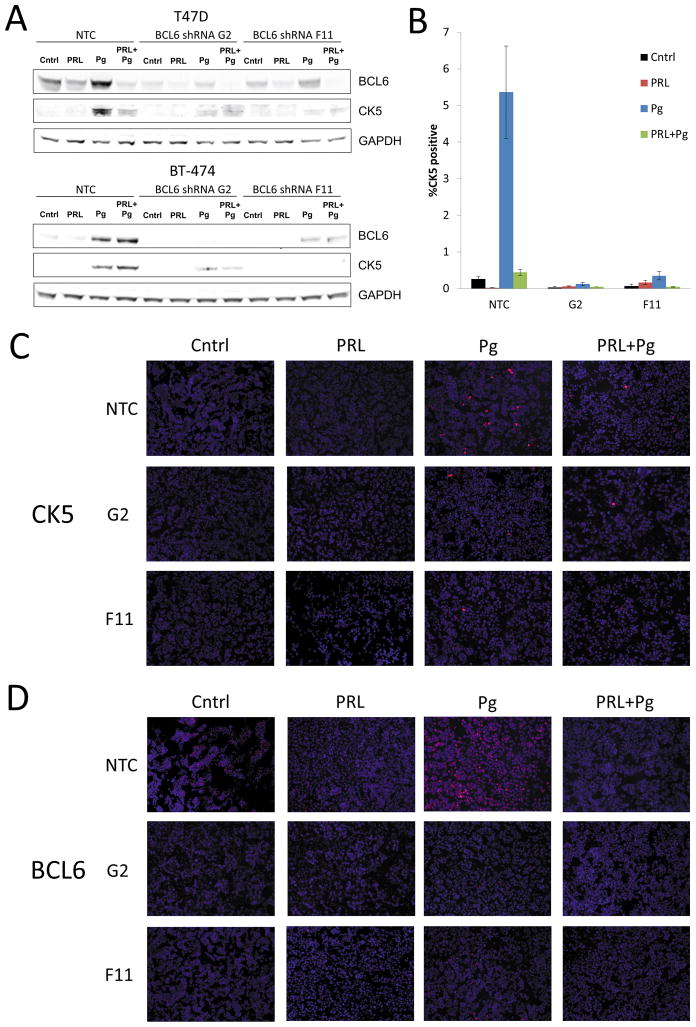
We next determined whether Pg-induction of CK5 expression required BCL6. BCL6 levels were knocked down by two independent lentiviral shRNAs (G2 and F11) in T47D and BT-474 cells, the two cell lines that had the most robust CK5 protein increase in response to Pg. Both shRNAs successfully reduced BCL6 protein levels under basal as well as Pg-treated conditions (Figure 4A). BCL6 knock-down led to marked reduction of Pg-induced CK5 protein levels in both cell lines (Figure 4A), indicating CK5 induction by Pg requires induction of BCL6. To extend these studies, T47D cells were treated under the same experimental conditions as above and ICC was performed. Again, both shRNAs were able to inhibit the increase of the CK5 population in response to Pg, and the BCL6 shRNA G2 (0.13%) and BCL6 shRNA F11 (0.35%) conditions displayed a much lower CK5-positive population in response to Pg compared to the NTC condition (5.36%) (Figure 4B). Representative images of the slides stained for CK5 and BCL6 display potent BCL6 knockdown and suppression of the Pg-induced CK5 population in the presence of BCL6 shRNA (Figure 4C and D). We conclude that Pg induces the CK5-positive cell population through a mechanism that requires BCL6, and prolactin impedes Pg-induction of the CK5-positive population through down-regulation of BCL6.
Figure 4. Progestin induction of CK5 requires induction of BCL6.
(A) Immunoblot of BCL6, CK5 and GAPDH in extracts of T47D and BT-474 cultures treated with non-target shRNA (NTC) or one of two independent BCL6 shRNAs (G2 and F11) for 48 h, followed by addition of vehicle (Cntrl), Prolactin (PRL), β-Estradiol (E2), or R5020 (Pg) for 48 h. (B) Quantification of CK5 immunocytochemistry (ICC) of T47D cultures exposed to either NTC shRNA or BCL6 shRNAs G2 or F11. Percent CK5 positive cells are indicated. In Pg treated cells, the percentage of CK5+ cells was 21.1 times higher (95% CI: 5.2, 31.1, P<0.001) under NTC condition as compared to G2 condition, and the percentage of CK5+ cells was 12.7 times higher (95% CI: 5.2, 31.1, P<0.001) under NTC condition as compared to F11 condition. (C) Representative images of ICC stained for CK5 (red) and DAPI (blue). (D) Representative images of ICC stained for BCL6 (red) and DAPI (blue).
Prolactin suppression of BCL6 and subsequent CK5 is mediated by the Jak2-Stat5 pathway
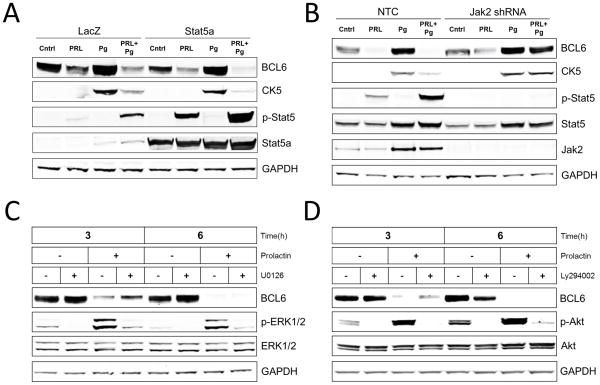
Prolactin activates multiple signaling pathways including Jak2-Stat5, ERK1/2, and Akt in T47D and other prolactin receptor-positive human breast cancer cell lines (26), and prolactin required Stat5 signaling to down-regulate basal levels of BCL6 (23). Therefore, we hypothesized that Stat5 signaling is important for prolactin-suppression of Pg-induced BCL6 and CK5. Consistent with this hypothesis, immunoblot analysis revealed that cells with enhanced expression of Stat5a displayed a complete prolactin-induced suppression of both Pg-induced BCL6 and CK5 when compared to partial suppression in control cells (Figure 5A). Furthermore, knock-down of Jak2 by lentiviral shRNA delivery abrogated prolonged PRL-Stat5 signaling and inhibited PRL-suppression of both Pg-induced BCL6 and CK5 (Figure 5B). In contrast, pharmacologic inhibition of ERK1/2 or Akt pathways did not block prolactin-suppression of BCL6. To avoid toxicity from long-term drug exposure, cells were first pretreated with Pg for 24 h to induce BCL6 and then treated with or without Mek-inhibitor U0126 or PI3K inhibitor LY294002 in the presence or absence of prolactin for 3 and 6 h. Despite effective inhibition of prolactin-induced ERK1/2 activation or prolactin-induced Akt activation by U0126 and LY294002, respectively, prolactin remained fully capable of suppressing Pg-induction of BCL6 protein levels at both 3 and 6 h (Figure 5C, D). Collectively, these studies support the conclusion that prolactin-suppression of BCL6 and subsequently CK5 is mediated by the Stat5 pathway.
Figure 5. Prolactin suppression of BCL6 and subsequently CK5 is mediated by the Stat5 pathway.
(A) Immunoblots of BCL6, CK5, phosphorylated-Stat5 (p-Stat5), Stat5a, and GAPDH protein levels in extracts of T47D cultures exposed to adenovirus carrying either Stat5a or LacZ control for 24 h and subsequently treated with vehicle (Cntrl), Prolactin (PRL), β-Estradiol (E2), or R5020 (Pg) for 48 h. (B) Immunoblots of BCL6, CK5, phosphorylated-Stat5 (p-Stat5), Stat5, Jak2, and GAPDH protein levels in extracts of T47D cultures treated with non-target shRNA (NTC) or Jak2 shRNA for 48h, followed by addition of vehicle (Cntrl), Prolactin (PRL), β-Estradiol (E2), or R5020 (Pg) for 48 h. (C) Immunoblots of BCL6, phosphorylated-ERK1/2 (p-ERK1/2), ERK1/2, and GAPDH protein levels in extracts of T47D cultures pre-treated with Pg for 24 h followed by incubation with or without prolactin (PRL) in the presence or absence of Mek inhibitor (U0126) for 3 and 6 h. (D) Immunoblots of BCL6, phosphorylated-Akt (p-Akt), Akt, and GAPDH protein levels in extracts of T47D cultures pre-treated with Pg for 24 h followed by incubation with or without prolactin (PRL) in the presence or absence of PI3K inhibitor (Ly294002) for 3 and 6 h.
BCL6 and CK5 protein levels in human breast cancer tissues and their association with clinical outcome
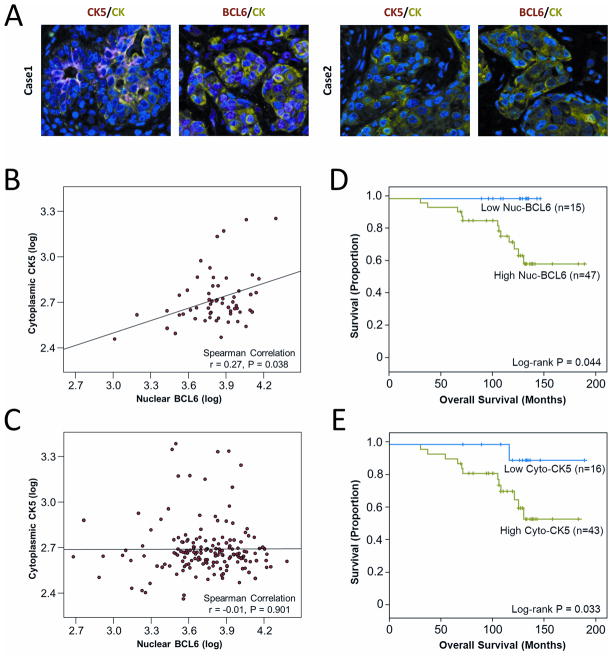
To evaluate the clinical relevance of our experimental observations, we quantified levels of BCL6 and CK5 proteins in situ using fluorescence-based immunohistochemistry in 276 breast cancer specimens. Representative immunostained images of cases that were either high or low in BCL6 and CK5 are shown (Figure 6A). Indeed, protein levels of CK5 correlated positively with BCL6 protein levels in ER-positive tumors from premenopausal women (Figure 6B; N=58, r=0.27, P=0.038) but not in tumors from postmenopausal women (Figure 6C; N=164, r=−0.01, P=0.901), or in patients with ER-negative breast cancer (data not shown; N=57, r=−0.14 P=0.303). A positive correlation between CK5 and BCL6 protein levels in premenopausal patients but not in postmenopausal patients is consistent with the experimentally observed mechanistic role of BCL6 in mediating progesterone-induction of CK5, since progesterone is virtually absent in postmenopausal women. Furthermore, survival analysis of the ER-positive, premenopausal patients revealed less favorable clinical outcome in patients whose tumors expressed high nuclear BCL6 protein compared to patients whose tumors expressed low nuclear BCL6 (P=0.044) (Figure 6D). Likewise, patients whose tumors expressed high cytoplasmic CK5 levels also were associated with unfavorable clinical outcome compared to patients whose tumors expressed low cytoplasmic CK5 (P=0.033) (Figure 6E).
Figure 6. CK5 positively correlates with BCL6 in ER-positive breast cancer tissues from premenopausal patients and high BCL6 or CK5 levels are associated with decreased patient survival.
(A) Representative immunofluorescence images of breast cancer tissue sections of ER-positive, premenopausal patients stained for either BCL6 (red) or CK5 (red) and cytokeratin (green) and DAPI (blue). Case 1 represents a tumor with high CK5 and high BCL6, while Case 2 represents a patient with low CK5 and low BCL6. (B) Scatter plots and correlation of levels of nuclear BCL6 and cytoplasmic CK5 proteins in ER-positive tumors of patients younger than 50 years old at diagnosis (premenopausal; Spearman N=58, r=0.27, P=0.038). (C) Scatter plots and correlation of levels of nuclear BCL6 and cytoplasmic CK5 proteins in ER-positive tumors of patients older than 49 years at diagnosis (postmenopausal; Spearman N=164, r=−0.01, P=0.901). (D) Kaplan-Meier analysis of nuclear BCL6 in ER-positive tumors of patients younger than 50 years of age (Logrank P=0.044). (E) Kaplan-Meir analysis of cytoplasmic CK5 in ER-positive tumors of patients younger than 50 years of age (Logrank P=0.033).
Discussion
The present study provides evidence for a novel role of prolactin in luminal breast cancer as a suppressor of a progestin-induced, chemoresistant, CK5-positive cell population. This CK5-positive cell population in T47D cells was four-fold less likely to undergo apoptosis in response to docetaxel treatment than CK5-negative cells, indicating that prolactin signaling limits the number of chemoresistant CK5-positive cells in luminal breast cancer. Prolactin inhibited Pg-induction of CK5-positive cells through a mechanism that involves suppression of BCL6 protein, which we discovered was potently induced by Pg and was required for Pg-induction of CK5. The study also suggested that prolactin suppression of Pg-induced BCL6 and subsequent CK5 was mediated by Jak2-Stat5, but not Erk or Akt pathways. Clinical relevance for a PR-BCLmK5-regulatory axis in luminal breast cancer was provided by a positive correlation between BCL6 and CK5 protein levels in ER-positive tumors in premenopausal but not in postmenopausal patients. Furthermore, elevated BCL6 or CK5 protein levels in ER-positive tumors of premenopausal patients were associated with unfavorable clinical outcome. We propose that loss of prolactin-Stat5 signaling in PR-driven luminal breast cancer may lead to a differentiation blockade that involves emergence of undifferentiated tumor-initiating CK5-positive cells. In fact, loss of Stat5 signaling in breast cancer correlated with poor prognosis (16) and resistance to anti-estrogen therapy (14, 18), which may in part be a result of up-regulation of BCL6 and the CK5-positive cell population.
Previous studies have provided evidence that PR activation may promote breast cancer progression. PR target gene expression signatures in human breast cancers predicted early metastasis and poor outcome (27). Epidemiological studies have linked the use of progestin in hormonal replacement therapy to increased risk of being diagnosed with invasive breast cancer (28, 29), presumably the result of progression of precursor lesions (30). Furthermore, female mice implanted with progestin pellets displayed elevated mammary tumor incidence following DMBA treatment (31). One mechanism of progestin-promotion of breast cancer development and progression may involve the ability of progestins to expand the de-differentiated ER-negative/PR-negative/CK5-positive cell population (7–9). These Pg-induced CK5-positive cells co-expressed CD44, another marker of tumor-initiating cells, frequently lost ER/PR expression, and displayed increased tumorigenic potential in mice (8), as well as resistance to apoptosis from chemotherapy and endocrine therapy (9, 10). Recent progress has also identified KLF5 and the Notch pathway as positive regulators of the CK5-positive breast cancer cell population, but these pathways appear not be the sole regulators, since knockdown of neither KLF5 nor Notch completely abrogated induction of CK5 (10, 32).
The transcriptional repressor and proto-oncogene BCL6 is critical for blocking terminal differentiation of germinal center B cells (33, 34). Chromosomal rearrangements of BCL6 that up-regulate BCL6 expression have been detected in ~40% of diffuse large B-cell lymphoma (DLBCL), and mouse models carrying a rearranged BCL6 locus develop lymphomas resembling DLBCL (35). Inhibitors targeting the corepressor binding groove of the BCL6 BTB binding domain display promising capacity to suppress growth as well as induce killing of BCL6-positive DLBCL cells (36, 37). Intriguingly, recent studies have revealed that BCL6 is critical in the maintenance of leukemia-initiating cells, and the BCL6 inhibitor RI-BPI led to elimination of the leukemia-initiating cells and the associated drug resistance (22, 38). If BCL6 is critical for induction of drug-resistant CK5-positive cell populations in a subgroup of breast cancers, BCL6 inhibitors may be explored therapeutically for select breast cancer patients. This notion is further supported by the novel observation that high BCL6 or CK5 protein levels are associated with poor clinical outcome in premenopausal patients with ER-positive breast cancer, although this association needs to be confirmed in an independent cohort with a larger number of patients.
One intriguing observation is that progestin-induced BCL6 in T47D cells occurs in the majority of cells, while CK5 induction is limited to a small cell population. One possibility is that a rare population of cells may be programmed epigenetically to respond to an increase in BCL6 protein by increasing CK5 expression and decreasing ER/PR expression. In fact, a recent study demonstrated that inhibiting DNA methylation in hematological and epithelial malignancies reduced the tumor-initiating cell population, consistent with a model where tumor-initiating cells are maintained through epigenetic and not genetic mechanisms (39). Future work will explore if cells that become CK5-positive in response to progestins are epigenetically distinct from CK5-negative cells.
Does the observed negative regulation by prolactin of progesterone receptor-mediated expansion of the CK5-positive cell population in breast cancer recapitulate a regulatory mechanism operating in normal breast epithelia? In the mammary gland, BCL6 is expressed during early pregnancy when progesterone levels and proliferative expansion of undifferentiated secretory cells are high (40). BCL6 blocks differentiation of mouse mammary EpH4 cells and BCL6 expression is increased in de-differentiated aggressive breast cancers (23, 40). Intriguingly, recent studies have demonstrated progesterone-induced up-regulation of mammary epithelial stem cells in mice (41, 42), raising the possibility that some luminal breast cancers exploit progesterone receptor signaling to up-regulate stem cell populations. Such a mechanism may be further promoted by loss of Stat5 signaling which is associated with increased risk of antiestrogen therapy-failure in luminal breast cancer (14, 18).
The present progress is supported by a previous study which reported BCL6 mRNA as one among 94 transcripts modulated by Pg in a genome-wide analysis of T47D cells (24). However, Pg-up-regulation of BCL6 mRNA was modest (two-fold) and BCL6 protein expression was not analyzed. The present study demonstrated robust up-regulation of BCL6 protein by Pg, and further documented that up-regulation of BCL6 is critical for Pg-induction of CK5-positive cells. Repression of basal BCL6 levels by prolactin-Stat5 appeared to be at the mRNA level (23, 25), but the mechanisms underlying downregulation of Pg-induced BCL6 protein by prolactin is likely to be much more complex. While an initial prolactin-repression of Pg-induced BCL6 mRNA was observed, this effect was temporary and BCL6 mRNA levels were restored by 24 h despite continued complete suppression of BCL6 protein (data not shown). Furthermore, the proteasomal inhibitor, MG132, prevented a decrease in Pg-induced BCL6 protein in prolactin-treated cells, indicating involvement of a proteasomal degradation pathway (data not shown). Future studies will elucidate the exact mechanisms of how prolactin controls Pg-induced BCL6 transcript and protein expression in breast cancer. Luminal MCF-7 and ZR-75-1 breast cancer cells express relatively low PR levels which may explain the inability of Pg to effectively induce CK5 in these cells (data not shown). Nonetheless, key relevance of the cell line data was provided by our observation of a positive correlation between BCL6 and CK5 protein levels in ER-positive tumors of premenopausal but not postmenopausal patients, or in ER-negative tumors regardless of menopausal status. However, some of the PR-positive tumors from postmenopausal patients nonetheless co-expressed both BCL6 and CK5. Given the existence of ligand-independent activation mechanisms for PR (43–45), future work will also explore the possibility of PR-regulation of BCL6 and CK5 in postmenopausal breast cancer.
Luminal breast cancers generally confer a favorable prognosis, but many patients experience long-term recurrence. In fact, more than half of luminal breast cancer recurrences happen six or more years after diagnosis (2), indicating the presence of residual dormant, therapy-resistant populations of cancer cells. These cell populations may very well include the CK5-positive cells with tumor-initiating features as supported by the present study and others (8, 9). BCL6 may represent a potential therapeutic target to eliminate CK5-positive residual cells and prevent recurrence in patients of luminal breast cancer with active progesterone receptor signaling. Furthermore, the present progress identifies a novel pro-differentiation effect of the prolactin-Stat5 pathway expected to be relevant for a subset of luminal breast cancers.
Materials and Methods
Cell culture
T47D, BT-474, ZR75-1, HCC1937(ATCC, Manassas, VA, USA) were cultured in RPMI (Cellgro, Manassas, VA, USA) containing 10% fetal bovine serum (FBS) and 1 mM sodium pyruvate. MCF-7 (ATCC) and HEK-293FT (Invitrogen, Carlsbad, CA, USA) cells were cultured in DMEM (Cellgro) with 10% FBS and 1mM sodium pyruvate. Cells were treated with 20nM recombinant human prolactin (AFP795; provided by Dr. A.F. Parlow; National Hormone and Pituitary Program, Torrence, CA), 1nM 17β-estradiol (Sigma, St Louis, MO, USA), 20nM R5020 (PerkinElmer, Waltham, MA, USA), and/or 2μM RU486 (Sigma) unless indicated otherwise. For chemotherapy experiments, cells were treated with hormones for 24 h before treatment with 10nM docetaxel (Sigma) for 48 h. For MEK/PI3K inhibitor experiments, cells were pretreated with 20nM R5020 for 24 h prior to incubation with 20nM prolactin in the presence or absence of 10μM U0126 (Cell Signaling Technology, Danvers, MA, USA) or 10μM LY294002 (Cell Signaling Technology). Culture media were changed to RPMI containing 5% charcoal-stripped serum (Thermo Scientific, Logan, UT, USA) 24 h prior to experiments involving hormone treatments.
Adenoviral and Lentiviral Production and Infection
Lentiviral particle production was performed as described previously (23). shRNA lentiviral vectors (Open Biosystems, Lafayette, CO, USA) were obtained for non-target control (SC002), BCL6 [TRCN0000013606 (G2), TRCN0000013603(F11)], and Jak2 [TRCN0000003180]. The cells were infected with lentivirus overnight and allowed to grow for 48 h before hormone induction for an additional 48 h. Adenovirus for gene delivery of Stat5a or control (LacZ) was prepared using double cesium chloride centrifugation (46) and incubated with T47D cells (1×106 cells/well in 6 well dish; multiplicity of infection = 10) for 24 h before addition of hormones for another 48 h and subsequent extraction for immunoblot analysis.
T47D xenograft tumors
T47D xenotransplants were performed as described previously (23). Briefly, nude mice implanted with 17β-estradiol pellets (0.72 mg; Innovative Research of America) were injected s.c. with 5 × 106 T47D cells suspended in Matrigel into two dorsolateral sites. Once tumors averaging 0.5 cm had formed, mice were injected s.c. with either vehicle control (n = 10) or 5 μg/g body mass of human prolactin (n = 10) every 12 h for 48 h. Tumors were harvested and processed for immunohistochemistry and qRT-PCR.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
qRT-PCR assays were performed as described previously (23). Primers used as followed: GAPDH (forward-AATCCATCACCATCTTCCA, reverse-TGGACTCCACGACGTACTCA), CISH (forward-CTGCTGTGCATAGCCAAGAC, reverse-GTGCCTTCTGGCATCTTCTG), BCL6 (forward-CTGCAGATGGAGCATGTTGT, reverse-TCTTCACGAGGAGGCTTGAT) and CK5 (forward-CACTTACCGCAAGCTGCTG, reverse- AAACACTGCTTGTGACAACAGAG).
Immunoblotting
T47D, BT-474, MCF-7 and ZR-75-1 were lysed as described previously (47). Proteins were resolved by SDS-PAGE and immunoblotted with antibodies to phospho-Stat5 (BD #611964, San Jose, CA, USA), total Stat5 (BD #610192), Stat5a (Advantex BioReagents, El Paso, TX, USA), BCL6 (Dako PG-B6P, Carpinteria, CA, USA), CK5 (Thermo Scientific XM26), GAPDH (Santa Cruz Biotechnology FL-335, Santa Cruz, CA, USA), phospho-ERK1/2 (Cell Signaling Technology #9106), ERK1/2 (Cell Signaling Technology #4695), phospho-Akt (Cell Signaling Technology #4051), Akt (Cell Signaling Technology #4691) followed by secondary antibodies Alexa Fluor 680-conjugated goat anti-mouse IgG (Invitrogen) or IRDye 800 CW-conjugated goat anti-rabbit IgG (Licor, Lincoln, NE, USA) depending on primary antibodies. Immunoblots were scanned using the Odyssey Infrared Imaging System (Licor).
Immunohistochemistry
A breast cancer tissue microarray representing an unselected cohort of patients collected from the Thomas Jefferson University Hospital pathology archives under IRB approved protocols. Evaluable staining for both BCL6 and CK5 was achieved on 276 cases using immunofluorescent staining on a Dako Autostainer (Dako, Carpinteria, CA) and high-resolution digital images were captured at 20X using an Aperio® ScanScope FL slide scanner (Aperio, Vista, CA). BCL6 was detected using antigen retrieval with Tris/EDTA buffer (pH 9, Dako) and incubation with anti-BCL6 (1:50, (Dako PG-B6P) for 1 h, and CK5 detection was with antigen retrieval using citric acid buffer (pH 6, Dako) and incubation with anti CK5 (1:200, Thermo Scientific XM26) for 30 min. Pan-cytokeratin staining, secondary antibody staining, and slide coverslipping were performed as previously described (14). Quantitative analyses were performed using Definiens Tissue Studio® (Definiens AG, Munich, Germany) digital pathology image analysis software. Average intensity of staining for BCL6 in the nucleus of malignant epithelial cells and the average intensity of CK5 in the cytoplasm of epithelial cells were derived for each tumor. ER-positive tumors were defined by the top 80% of ER staining intensity in the unselected breast cancer cohort, and premenopausal patients were defined by age of diagnosis <50 years.
Immunocytochemistry
T47D cells that were grown on coverslips were fixed using 10% formalin and permeabilized with ice-cold acetone. The coverslips were then blocked with PBS+1% BSA before incubation with primary antibodies to BCL6, CK5, or Cleaved Caspase-3 (Cell Signaling Technology #9661), followed by secondary antibodies Alexa Fluor 555-conjugated goat anti-mouse antibody (Invitrogen) and Alexa Fluor 488-conjugated goat anti-rabbit antibody (Invitrogen). Coverslips were mounted onto slides using Prolong Gold antifade reagent with DAPI (Invitrogen). High-resolution digital images were captured at 20X using Aperio® ScanScope FL slide scanner (Aperio). Analysis was performed using the ImageScope Viewer Software (Aperio). CK5-positive pixels (red) were divided by the DAPI-positive pixels (blue) to approximate the percentage of cells positive for CK5. In order to quantify CK5-positive and CK5-negative cells that were positive for cleaved caspase-3, five independent images from each condition were manually scored for CK5-positive cells (red), cleaved caspase-3 positive cells (green), and dual positive cells (yellow).
Cell Pellets Arrays
T47D cells were treated with hormones for 24 h then fixed in 10% formalin. Cell pellets were obtained by centrifuging the fixed cells for 10 minutes at 1000 rpm. Heated Histogel (Thermo Scientific) was added to the cell pellet at a 1:1 volume ratio, solidified on ice, and further formalin-fixed overnight before processing and embedding into paraffin. Multiple cell pellets were arranged on one block during embedding to create a cell pellet array before subsequent analysis by immunocytochemistry.
Statistical Methods
Levels of CK5 mRNA in T47D xenotransplants from mice treated with or without prolactin were compared using Mann-Whitney test. Spearman rank correlation was used to evaluate association between nuclear BCL6 and cytoplasmic CK5. Analyses of overall survival were conducted using Kaplan-Meier plots and log-rank test based on optimal cutpoints to define high or low levels of nuclear BCL6 or cytoplasmic CK5. Proportions of cells undergoing apoptosis and CK5-positive cells were analyzed using generalized linear mixed effect (GLME) model with binomial distribution. Figures 1C and Figure 4B measured log-transformed percentages of CK5 positive cells and were analyzed using ANOVA models. Statistical analyses were performed in SAS 9.3 (SAS Institute Inc., Cary, NC, USA) and SPSS (ver15.0; SPCC Inc, Chicago, IL).
Acknowledgments
This work was supported by Komen for the Cure Promise Grant KG091116 (H.R., T.H., J.A.H., A.J.K., C.D.S., T.S., A.R.P., M.A.G., C.L., B.F., and I.C.), NIH grants CA101841 (H.R.), CA118740 (H.R.), CA092900 (S.Y.F.), and NCI Support Grant 1P30CA56036 to the Kimmel Cancer Center. The Project is funded, in part, under a Commonwealth University Research Enhancement Program grant with the Pennsylvania Department of Health (H.R.). The Department specifically disclaims responsibility for any analyses, interpretations or conclusions. The views expressed in this article are those of the authors and do not reflect the official policy of the Department of the Army (DOA), Department of Defense (DOD), or US Government.
Footnotes
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Keen JC, Davidson NE. The biology of breast carcinoma. Cancer. 2003;97(3 Suppl):825–33. doi: 10.1002/cncr.11126. Epub 2003/01/28. [DOI] [PubMed] [Google Scholar]
- 2.Lim E, Metzger-Filho O, Winer EP. The natural history of hormone receptor-positive breast cancer. Oncology (Williston Park) 2012;26(8):688–94. 96. Epub 2012/09/11. [PubMed] [Google Scholar]
- 3.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–11. doi: 10.1038/35102167. Epub 2001/11/02. [DOI] [PubMed] [Google Scholar]
- 4.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5(4):275–84. doi: 10.1038/nrc1590. Epub 2005/04/02. [DOI] [PubMed] [Google Scholar]
- 5.Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008;10(2):R25. doi: 10.1186/bcr1982. Epub 2008/03/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100(9):672–9. doi: 10.1093/jnci/djn123. Epub 2008/05/01. [DOI] [PubMed] [Google Scholar]
- 7.Sartorius CA, Harvell DM, Shen T, Horwitz KB. Progestins initiate a luminal to myoepithelial switch in estrogen-dependent human breast tumors without altering growth. Cancer Res. 2005;65(21):9779–88. doi: 10.1158/0008-5472.CAN-05-0505. Epub 2005/11/04. [DOI] [PubMed] [Google Scholar]
- 8.Horwitz KB, Dye WW, Harrell JC, Kabos P, Sartorius CA. Rare steroid receptor-negative basal-like tumorigenic cells in luminal subtype human breast cancer xenografts. Proc Natl Acad Sci U S A. 2008;105(15):5774–9. doi: 10.1073/pnas.0706216105. Epub 2008/04/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kabos P, Haughian JM, Wang X, Dye WW, Finlayson C, Elias A, et al. Cytokeratin 5 positive cells represent a steroid receptor negative and therapy resistant subpopulation in luminal breast cancers. Breast Cancer Res Treat. 2011;128(1):45–55. doi: 10.1007/s10549-010-1078-6. Epub 2010/07/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haughian JM, Pinto MP, Harrell JC, Bliesner BS, Joensuu KM, Dye WW, et al. Maintenance of hormone responsiveness in luminal breast cancers by suppression of Notch. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1106509108. Epub 2011/10/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HJ, Ormandy CJ. Interplay between progesterone and prolactin in mammary development and implications for breast cancer. Mol Cell Endocrinol. 2012;357(1–2):101–7. doi: 10.1016/j.mce.2011.09.020. Epub 2011/09/29. [DOI] [PubMed] [Google Scholar]
- 12.Gouilleux F, Wakao H, Mundt M, Groner B. Prolactin induces phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. Embo J. 1994;13(18):4361–9. doi: 10.1002/j.1460-2075.1994.tb06756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Robinson GW, Gouilleux F, Groner B, Hennighausen L. Cloning and expression of Stat5 and an additional homologue (Stat5b) involved in prolactin signal transduction in mouse mammary tissue. Proc Natl Acad Sci U S A. 1995;92(19):8831–5. doi: 10.1073/pnas.92.19.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peck AR, Witkiewicz AK, Liu C, Stringer GA, Klimowicz AC, Pequignot E, et al. Loss of nuclear localized and tyrosine phosphorylated Stat5 in breast cancer predicts poor clinical outcome and increased risk of antiestrogen therapy failure. J Clin Oncol. 2011;29(18):2448–58. doi: 10.1200/JCO.2010.30.3552. Epub 2011/05/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamashita H, Nishio M, Ando Y, Zhang Z, Hamaguchi M, Mita K, et al. Stat5 expression predicts response to endocrine therapy and improves survival in estrogen receptor-positive breast cancer. Endocr Relat Cancer. 2006;13(3):885–93. doi: 10.1677/erc.1.01095. [DOI] [PubMed] [Google Scholar]
- 16.Nevalainen MT, Xie J, Torhorst J, Bubendorf L, Haas P, Kononen J, et al. Signal transducer and activator of transcription-5 activation and breast cancer prognosis. J Clin Oncol. 2004;22(11):2053–60. doi: 10.1200/JCO.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 17.Cotarla I, Ren S, Zhang Y, Gehan E, Singh B, Furth PA. Stat5a is tyrosine phosphorylated and nuclear localized in a high proportion of human breast cancers. Int J Cancer. 2004;108(5):665–71. doi: 10.1002/ijc.11619. [DOI] [PubMed] [Google Scholar]
- 18.Peck AR, Witkiewicz AK, Liu C, Klimowicz AC, Stringer GA, Pequignot E, et al. Low levels of Stat5a protein in breast cancer are associated with tumor progression and unfavorable clinical outcomes. Breast Cancer Res. 2012;14(5):R130. doi: 10.1186/bcr3328. Epub 2012/10/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sultan AS, Xie J, LeBaron MJ, Ealley EL, Nevalainen MT, Rui H. Stat5 promotes homotypic adhesion and inhibits invasive characteristics of human breast cancer cells. Oncogene. 2005;24(5):746–60. doi: 10.1038/sj.onc.1208203. [DOI] [PubMed] [Google Scholar]
- 20.Nouhi Z, Chughtai N, Hartley S, Cocolakis E, Lebrun JJ, Ali S. Defining the role of prolactin as an invasion suppressor hormone in breast cancer cells. Cancer Res. 2006;66(3):1824–32. doi: 10.1158/0008-5472.CAN-05-2292. [DOI] [PubMed] [Google Scholar]
- 21.Sultan AS, Brim H, Sherif ZA. Co-overexpression of Janus kinase 2 and signal transducer and activator of transcription 5a promotes differentiation of mammary cancer cells through reversal of epithelial-mesenchymal transition. Cancer Sci. 2008;99(2):272–9. doi: 10.1111/j.1349-7006.2007.00685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duy C, Hurtz C, Shojaee S, Cerchietti L, Geng H, Swaminathan S, et al. BCL6 enables Ph+ acute lymphoblastic leukaemia cells to survive BCR-ABL1 kinase inhibition. Nature. 2011;473(7347):384–8. doi: 10.1038/nature09883. Epub 2011/05/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tran TH, Utama FE, Lin J, Yang N, Sjolund AB, Ryder A, et al. Prolactin inhibits BCL6 expression in breast cancer through a Stat5a-dependent mechanism. Cancer Res. 2010;70(4):1711–21. doi: 10.1158/0008-5472.CAN-09-2314. Epub 2010/02/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002;277(7):5209–18. doi: 10.1074/jbc.M110090200. Epub 2001/11/22. [DOI] [PubMed] [Google Scholar]
- 25.Walker SR, Nelson EA, Zou L, Chaudhury M, Signoretti S, Richardson A, et al. Reciprocal effects of STAT5 and STAT3 in breast cancer. Mol Cancer Res. 2009;7(6):966–76. doi: 10.1158/1541-7786.MCR-08-0238. [DOI] [PubMed] [Google Scholar]
- 26.Neilson LM, Zhu J, Xie J, Malabarba MG, Sakamoto K, Wagner KU, et al. Coactivation of Jak1 Positively Modulates Prolactin-Jak2 Signaling in Breast Cancer: Recruitment of ERK and Stat3 and Enhancement of Akt and Stat5a/b Pathways. Mol Endocrinol. 2007 doi: 10.1210/me.2007-0173. [DOI] [PubMed] [Google Scholar]
- 27.Knutson TP, Daniel AR, Fan D, Silverstein KA, Covington KR, Fuqua SA, et al. Phosphorylated and sumoylation-deficient progesterone receptors drive proliferative gene signatures during breast cancer progression. Breast Cancer Res. 2012;14(3):R95. doi: 10.1186/bcr3211. Epub 2012/06/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. Jama. 2002;288(3):321–33. doi: 10.1001/jama.288.3.321. Epub 2002/07/19. [DOI] [PubMed] [Google Scholar]
- 29.Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362(9382):419–27. doi: 10.1016/s0140-6736(03)14065-2. Epub 2003/08/21. [DOI] [PubMed] [Google Scholar]
- 30.Horwitz KB, Sartorius CA. Progestins in hormone replacement therapies reactivate cancer stem cells in women with preexisting breast cancers: a hypothesis. J Clin Endocrinol Metab. 2008;93(9):3295–8. doi: 10.1210/jc.2008-0938. Epub 2008/07/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aldaz CM, Liao QY, LaBate M, Johnston DA. Medroxyprogesterone acetate accelerates the development and increases the incidence of mouse mammary tumors induced by dimethylbenzanthracene. Carcinogenesis. 1996;17(9):2069–72. doi: 10.1093/carcin/17.9.2069. Epub 1996/09/01. [DOI] [PubMed] [Google Scholar]
- 32.Liu R, Zhou Z, Zhao D, Chen C. The induction of KLF5 transcription factor by progesterone contributes to progesterone-induced breast cancer cell proliferation and dedifferentiation. Mol Endocrinol. 2011;25(7):1137–44. doi: 10.1210/me.2010-0497. Epub 2011/05/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tunyaplin C, Shaffer AL, Angelin-Duclos CD, Yu X, Staudt LM, Calame KL. Direct repression of prdm1 by Bcl-6 inhibits plasmacytic differentiation. J Immunol. 2004;173(2):1158–65. doi: 10.4049/jimmunol.173.2.1158. Epub 2004/07/09. [DOI] [PubMed] [Google Scholar]
- 34.Shaffer AL, Yu X, He Y, Boldrick J, Chan EP, Staudt LM. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 2000;13(2):199–212. doi: 10.1016/s1074-7613(00)00020-0. Epub 2000/09/12. [DOI] [PubMed] [Google Scholar]
- 35.Cattoretti G, Pasqualucci L, Ballon G, Tam W, Nandula SV, Shen Q, et al. Deregulated BCL6 expression recapitulates the pathogenesis of human diffuse large B cell lymphomas in mice. Cancer Cell. 2005;7(5):445–55. doi: 10.1016/j.ccr.2005.03.037. Epub 2005/05/17. [DOI] [PubMed] [Google Scholar]
- 36.Cerchietti LC, Yang SN, Shaknovich R, Hatzi K, Polo JM, Chadburn A, et al. A peptomimetic inhibitor of BCL6 with potent antilymphoma effects in vitro and in vivo. Blood. 2009;113(15):3397–405. doi: 10.1182/blood-2008-07-168773. Epub 2008/10/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cerchietti LC, Ghetu AF, Zhu X, Da Silva GF, Zhong S, Matthews M, et al. A small-molecule inhibitor of BCL6 kills DLBCL cells in vitro and in vivo. Cancer Cell. 2010;17(4):400–11. doi: 10.1016/j.ccr.2009.12.050. Epub 2010/04/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hurtz C, Hatzi K, Cerchietti L, Braig M, Park E, Kim YM, et al. BCL6-mediated repression of p53 is critical for leukemia stem cell survival in chronic myeloid leukemia. J Exp Med. 2011;208(11):2163–74. doi: 10.1084/jem.20110304. Epub 2011/09/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsai HC, Li H, Van Neste L, Cai Y, Robert C, Rassool FV, et al. Transient low doses of DNA-demethylating agents exert durable antitumor effects on hematological and epithelial tumor cells. Cancer Cell. 2012;21(3):430–46. doi: 10.1016/j.ccr.2011.12.029. Epub 2012/03/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Logarajah S, Hunter P, Kraman M, Steele D, Lakhani S, Bobrow L, et al. BCL-6 is expressed in breast cancer and prevents mammary epithelial differentiation. Oncogene. 2003;22(36):5572–8. doi: 10.1038/sj.onc.1206689. Epub 2003/08/29. [DOI] [PubMed] [Google Scholar]
- 41.Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, et al. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465(7299):803–7. doi: 10.1038/nature09091. Epub 2010/05/07. [DOI] [PubMed] [Google Scholar]
- 42.Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, et al. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465(7299):798–802. doi: 10.1038/nature09027. Epub 2010/04/13. [DOI] [PubMed] [Google Scholar]
- 43.Pierson-Mullany LK, Lange CA. Phosphorylation of progesterone receptor serine 400 mediates ligand-independent transcriptional activity in response to activation of cyclin-dependent protein kinase 2. Mol Cell Biol. 2004;24(24):10542–57. doi: 10.1128/MCB.24.24.10542-10557.2004. Epub 2004/12/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jacobsen BM, Schittone SA, Richer JK, Horwitz KB. Progesterone-independent effects of human progesterone receptors (PRs) in estrogen receptor-positive breast cancer: PR isoform-specific gene regulation and tumor biology. Mol Endocrinol. 2005;19(3):574–87. doi: 10.1210/me.2004-0287. Epub 2004/11/26. [DOI] [PubMed] [Google Scholar]
- 45.Daniel AR, Lange CA. Protein kinases mediate ligand-independent derepression of sumoylated progesterone receptors in breast cancer cells. Proc Natl Acad Sci U S A. 2009;106(34):14287–92. doi: 10.1073/pnas.0905118106. Epub 2009/08/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dagvadorj A, Kirken RA, Leiby B, Karras J, Nevalainen MT. Transcription factor signal transducer and activator of transcription 5 promotes growth of human prostate cancer cells in vivo. Clin Cancer Res. 2008;14(5):1317–24. doi: 10.1158/1078-0432.CCR-07-2024. Epub 2008/03/05. [DOI] [PubMed] [Google Scholar]
- 47.Johnson KJ, Peck AR, Liu C, Tran TH, Utama FE, Sjolund AB, et al. PTP1B suppresses prolactin activation of Stat5 in breast cancer cells. Am J Pathol. 2010;177(6):2971–83. doi: 10.2353/ajpath.2010.090399. Epub 2010/10/19. [DOI] [PMC free article] [PubMed] [Google Scholar]