Abstract
Context:
Adipose tissue is a highly active endocrine organ that secretes many factors that affect other tissues and whole-body metabolism. Adipocytes are responsive to several glycoprotein 130 (gp130) cytokines, some of which have been targeted as potential antiobesity therapeutics.
Objective:
Oncostatin M (OSM) is a gp130 family member known to inhibit adipocyte differentiation in vitro, but its effects on other adipocyte properties are not characterized. The expression of OSM in white adipose tissue (WAT) has not been evaluated in the context of obesity. Thus, our objective was to examine the expression of adipose tissue OSM in obese animals and humans.
Design:
OSM expression was examined in adipose tissues from mice with diet-induced and genetic obesity and in obese humans as well as in fractionated adipose tissue from mice. Murine adipocytes were used to examine OSM receptor expression and the effects of OSM on adipocytes, including the secretion of factors such as plasminogen activator inhibitor 1 and IL-6, which are implicated in metabolic diseases.
Results:
OSM expression is increased in rodent and human obesity/type 2 diabetes mellitus. In humans, OSM levels correlate with body weight and insulin and are inversely correlated with glucose disposal rate as measured by hyperinsulinemic-euglycemic clamp. OSM is not produced from the adipocytes in WAT but derives from cells in the stromovascular fraction, including F4/80+ macrophages. The specific receptor of OSM, OSM receptor-β, is expressed in adipocytes and adipose tissue and increased in both rodent models of obesity examined. OSM acts on adipocytes to induce the expression and secretion of plasminogen activator inhibitor 1 and IL-6.
Conclusions:
These data indicate that WAT macrophages are a source of OSM and that OSM levels are significantly induced in murine and human obesity/type 2 diabetes mellitus. These studies suggest that OSM produced from immune cells in WAT acts in a paracrine manner on adipocytes to promote a proinflammatory phenotype in adipose tissue.
The IL-6 family is a group of functionally and structurally related cytokines that include IL-6, IL-11, IL-27, leukemia inhibitory factor (LIF), oncostatin M (OSM), ciliary neurotrophic factor, cardiotrophin-1 (CT-1), novel neurotrophin-1/B cell stimulating factor-3 or cardiotrophin-like cytokine, and neuropoietin (NP) (reviewed in Ref. 1). Because all members of this family use glycoprotein 130 (gp130) as a common signal transducer that is required within their receptor complexes, the IL-6 family members are referred to as the gp130 cytokines. The primary signal transduction pathway that mediates response to gp130 cytokines is the Janus kinase/signal transducer and activator of transcription (STAT) pathway, which primarily activates STAT3.
The gp130 cytokines regulate a variety of biological processes, including hematopoiesis, immune responses, inflammation, stem cell potency, cardiovascular action, and neuronal survival (reviewed in Ref. 2). Circulating levels of many gp130 cytokines, namely IL-6, CT-1, LIF, and OSM, have been observed in humans (3–9). Although the actions of this cytokine family in white adipose tissue (WAT) have not been fully elucidated, some gp130 cytokines, including ciliary neurotrophic factor and IL-6, have been targeted as potential therapeutic strategies in the treatment of obesity (10). Some gp130 cytokines can exert profound effects on WAT, body weight, and glucose and lipid metabolism in rodents and humans (11–20). WAT and its primary constituents, adipocytes, are highly responsive to gp130 cytokines (19, 21–25), but the effects of these cytokines in adipose tissue have not been fully elucidated.
An important function of adipose tissue includes the production and secretion of factors (adipokines) that mediate whole-body metabolism. Several WAT-derived factors have been investigated and shown to modulate physiological systems as well as insulin resistance, inflammation, and other pathological conditions (reviewed in Ref. 26). WAT is a source of at least 2 gp130 cytokines, IL-6 (27, 28) and CT-1 (7). In obesity, the expansion of adipose tissue can be accompanied by a dysregulation of adipocyte function and alterations in adipokine secretion that can contribute to the development of metabolic diseases (reviewed in Ref. 29). Obesity is often associated with a state of chronic low-grade inflammation, mediated in part by macrophage infiltration in adipose tissue (30), and increased expression and secretion of proinflammatory cytokines that contribute to insulin resistance and type 2 diabetes mellitus (T2DM) (reviewed in Ref. 31). To enhance our understanding of the potential role of cytokines in the pathogenesis of obesity and related disorders, we have focused on the expression and function of gp130 cytokines in adipose tissue.
OSM is a gp130 cytokine that shares substantial sequence identity with LIF (32, 33). Although originally identified for its ability to inhibit cancer growth in humans (34), OSM can modulate a variety of biological processes. However, unlike other gp130 cytokines, OSM has its own specific receptor that heterodimerizes with gp130 (35), and this receptor, OSM receptor-β (OSMRβ), mediates most OSM effects. OSM can regulate inflammatory responses and is produced by activated T cells and macrophages (34, 36, 37). OSM can suppress inflammation in murine models of chronic inflammatory disease such as rheumatoid arthritis and multiple sclerosis (reviewed in Ref. 38). Elevated OSM levels are found in inflammatory diseases in humans, including rheumatoid arthritis and atherosclerosis (39, 40). OSM can also regulate developmental and regenerative processes in the liver (41–43).
Previous studies have shown that murine adipocytes in vitro and WAT in vivo are responsive to OSM (44), and importantly, this cytokine can inhibit preadipocyte differentiation (25, 45). Cytokines that inhibit adipogenesis, such as TNFα and interferon-γ, tend to have metabolically unfavorable effects such as the induction of insulin resistance (reviewed in Ref. 46). One study suggests OSM can attenuate adiponectin expression in human adipocytes (47). Overall, the role of OSM in the pathogenesis of obesity and related metabolic diseases is not understood. Nevertheless, recent studies by Komori et al (48) suggest a potential metabolically protective role for the OSM receptor, because mice with a global depletion of OSMRβ exhibited obesity, insulin resistance, and adipose tissue inflammation. However, our studies suggest that OSM has negative effects on adipocytes.
Our results demonstrate for the first time that OSM mRNA and protein levels are elevated in obese/T2DM mice and humans. In addition, we observed a potential relationship between OSM expression and body mass index (BMI) in human WAT. Interestingly, OSM is present in nonadipocyte cells of human adipose tissue. In mice, OSM is present in both Cd11c+ and Cd11c− macrophages that are F4/80+ and found in adipose tissue of wild-type and ob/ob mice. OSMRβ is highly expressed in adipocytes, and its levels are increased in ob/ob mice and high-fat diet (HFD)-fed mice compared with appropriate controls. We also observed that OSM induces PAI-1 secretion. In addition to body weight, increased OSM levels correlate with insulin levels and have a negative association with glucose disposal rates in a small human study. Collectively, our data suggest that adipose tissue-derived OSM may be a factor in metabolic disease states.
Materials and Methods
Materials
DMEM was purchased from Sigma. Bovine and fetal bovine sera were purchased from Hyclone. Mouse recombinant OSM, CT-1, NP, and TNFα and mouse PAI-1, OSM, and OSMRβ antibodies were purchased from R&D Systems. Antibodies directed against human OSM, STAT5A, peroxisome proliferator-activated receptor-γ, F4/80, and the ERK antibodies were all purchased from Santa Cruz Biotechnology. Mouse recombinant LIF was purchased from BD Transduction. Adiponectin polyclonal antibody was from Affinity Bioreagents. Monocyte chemoattractant protein-1 (MCP-1) rabbit polyclonal antibody was from Cell Signaling. CT-1 antibody was from Calbiochem. Trizol was from Invitrogen. Nitrocellulose was purchased from Bio-Rad. The BCA kit and the enhanced chemiluminescence kit were from Pierce. Horseradish peroxidase-conjugated secondary antibodies were from Jackson ImmunoResearch Laboratories.
Mice
Animals used for this study included genetically obese male B6.V-Lepob/J (B6-ob/ob) mice and their lean littermates as well as C57BL/6J mice rendered obese by dietary intervention and their lean controls. All mice were housed by the supplier (The Jackson Laboratory) until shipment 1 to 2 weeks before tissue harvest. B6-ob/ob mice and lean littermates were purchased for experimentation at 6 weeks of age and given free access to standard laboratory chow. C57BL/6J mice subjected to diet-induced obesity were fed an HFD consisting of 60% kcal from fat (Research Diets Inc; D12492) from 6 weeks of age. Lean C57BL/6J control mice were fed a control diet consisting of 10% kcal from fat (Research Diet Inc; D12450B) from 6 weeks of age. Both diets contained 10% kcal from protein with the balance in caloric intake provided by differences in carbohydrate content. Mice receiving both diets were given free access to food and shipped for experimentation at 11 weeks of age. All animals were euthanized by CO2 gas asphyxiation, and tissues were collected for total RNA or whole-tissue extract isolation. Animal care and use was approved by the Institutional Animal Care and Use Committee at Pennington Biomedical Research Center (PBRC). All animal studies were conducted with 4 to 7 mice in each group.
Cell culture
Murine 3T3-L1 preadipocytes were grown to 2 days after confluence in DMEM with 10% bovine serum. Medium was changed every 48 hours. A cocktail containing 0.5 mmol/L 3-isobutyl-methylxanthine, 1 μmol/L dexamethasone, and 1.7 μmol/L insulin was used to induce preadipocytes differentiation in DMEM containing 10% fetal bovine serum. After 48 hours, the medium was replaced by DMEM with 10% fetal bovine serum.
Human subjects and adipose tissue biopsies
All procedures and protocols were reviewed and approved by PBRC or Indiana University Institutional Review Boards. Subcutaneous adipose tissues were obtained with informed written consent from healthy nondiabetic subjects undergoing elective liposuction surgery. For fractionation, lipoaspirates were washed in PBS, digested in PBS supplemented with 1% BSA, 0.1% collagenase type II (Worthington, Lakewood, NJ), and 2mM calcium chloride for 60 minutes with rocking at 37°C. The tissue digest was centrifuged at 300g for 5 minutes at room temperature to separate the floating mature adipocytes from the stromovascular fraction (SVF) cell pellet. For RNA analysis, sc adipose tissue was collected from the abdomen (5 cm to left/right of the umbilicus) using a Bergstrom needle in compliance with standard clinical practice at PBRC. Subjects undergoing bariatric surgery had approximately 20 g of visceral adipose tissue extracted laparoscopically while under anesthesia.
RNA isolation from cells and tissue
RNA from ∼100 mg of tissue was isolated by column purification (QIAGEN) and yield determined by spectrophotometry (NanoDrop Technologies). From each RNA sample, 200 ng was reverse transcribed to cDNA using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems). Relative quantification of mRNA expression was analyzed using ABI PRISM 7900 (Applied Biosystems). Mouse primers came from Integrated DNA Technologies, and the sequences were as follows: PAI-1 forward, CGGCACAACCCGACAGAGACA; PAI-1 reverse, TCCGAGGTCTGGGATGCTGGT; OSM forward, AGGCACGGGCCAGAGTACCA; OSM reverse, GGCGGATATAGGGCTCCAAGAGTG; OSMRβ forward, CGTTCCCCTGTGAGGCCGAG; OSMRβ reverse, TCCTCCAAGACTTCGCTTCGGG; MCP1 forward, GCAGAGAGCCAGACGGGAGGA; MCP1 reverse, TGGGGCGTTAACTGCATCTGG; IL6 forward, TCCTCTCTGCAAGAGACTTCCATCC; IL6 reverse, AAGCCTCCGACTTGTGAAGTGGT; ADPN forward, AAAAGGGCTCAGGATGCTACTG; ADPN reverse, TGGGCAGGATTAAGAGGAACA; PPIA (cyclophilin A) forward, CCACTGTCGCTTTTCGCCGC; PPIA (cyclophilin A) reverse, TGCAAACAGCTCGAAGGAGACGC; and GAPDH forward, TTCCAGGAGCGAGACCCCAC; GAPDH reverse, TTCAAGTGGGCCCCGGCCTT. For human RNA, custom TaqMan gene expression microfluidic cards for OSM (Hs00968300_g1) were used. Samples were run in triplicate, and expression levels were normalized to cyclophilin B (Hs00168719_m1).
Adipose tissue macrophage isolation
SVF isolated from mouse adipose tissue samples were cooled on ice, centrifuged at 500g for 5 minutes, and resuspended in fluorescence-activated cell sorting (FACS) buffer at a concentration of 7 × 106 cells/mL. Cells were incubated in the dark at 4°C on a bidirectional shaker for 30 minutes in FcBlock (20 μg/mL) (BD Pharmingen) and then for an additional 50 minutes with fluorophore-conjugated primary antibodies or isotype control antibodies. After incubation with primary antibodies, 1 mL FACS buffer was added to the cells. Cells were centrifuged at 500g for 5 minutes and resuspended in 1 mL FACS buffer. The wash was repeated twice. Cells were analyzed on a FACSCalibur and analysis was performed using CellQuest software (Becton, Dickinson and Co).
Gel electrophoresis and immunoblotting
Proteins were separated in 7.5% or 10% polyacrylamide gels containing sodium dodecyl sulfate and transferred to nitrocellulose membrane (Bio-Rad) in 25 mmol/L Tris, 192 mmol/L glycine, and 20% methanol. After transfer, the membrane was blocked in 4% milk for 1 hour at room temperature and incubated with primary antibody overnight at 4°C. Results were visualized with horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratory) and enhanced chemiluminescence (Pierce).
Statistical analysis
Results are expressed as mean ± SEM. Differences between specified groups were analyzed using the Student's t test (two-tailed) for comparing 2 groups with P < .05 considered statistically significant.
Results
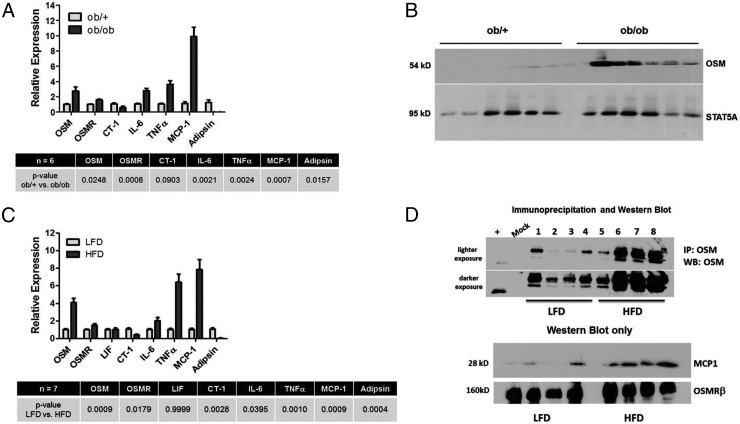
Epididymal adipose tissue from 6-week-old ob/+ and ob/ob mice was used to isolate total RNA or to obtain whole-tissue extracts for Western blot analysis. As shown in Figure 1A, the levels of OSM and OSMRβ mRNA were increased in adipose tissue from ob/ob mice compared with lean littermates. We also observed a modest decrease in CT-1 mRNA in the obese mice. As expected, we observed statistically significant increases in IL-6, TNFα, and MCP-1 and a decline in adipsin mRNA in ob/ob mice. An analysis of OSM protein levels revealed a notable increase in adipose tissue from ob/ob mice compared with lean littermates. The expression of STAT5A is shown as a protein loading control. Similar data were observed in epididymal adipose tissue obtained from 18-week-old mice fed a HFD for 12 weeks (Figure 1C). In this study, C57BL/6J mice fed a HFD had significant increases in OSM and OSMRβ mRNA levels compared with mice fed a low-fat diet over the same period. There was no change in LIF mRNA levels and a modest decline in CT-1 mRNA levels. As expected, we observed statistically significant increases in mRNA of IL-6, TNFα, and MCP-1 and a decline in adipsin mRNA in samples from the HFD mice. Because we observed some nonspecific bands when performing Western blot analysis on adipose tissue extracts from the feeding study, we performed an immunoprecipitation with an OSM antibody and examined the immunoprecipitated extract with a different OSM antibody. As shown in both lighter and darker exposures in Figure 1D, we observed a substantial increase in OSM protein levels in HFD. Western blot analysis of these extracts also revealed an expected increase in MCP-1 protein levels.
Figure 1.
OSM mRNA and protein levels are increased in adipose tissue of leptin-deficient mice and HFD-fed mice. A, Relative mRNA abundance was assessed in epididymal fat pads of male 6-week-old lean (ob/+) and leptin-deficient (ob/ob) mice. Data were normalized to 18S, and differences between lean and obese animals were determined via Student's t test where a P value of < .05 was considered significant. B, Whole-tissue extracts were prepared from epididymal adipose tissue, and 100 μg of protein was used for Western blot analysis to examine OSM protein levels. C, Relative mRNA abundance was assessed in epididymal fat pads of male mice fed a low-fat diet (LFD) or HFD for 12 weeks. Data were normalized to 18S, and differences between lean and obese animals were determined via Student's t test where a P value of <.05 was considered significant. D, Whole-tissue extracts were prepared from epididymal adipose tissue, and 100 μg of protein was used for Western blot analysis to examine OSM protein levels. Abbreviations: IP, immunoprecipitation; WB, Western blot.
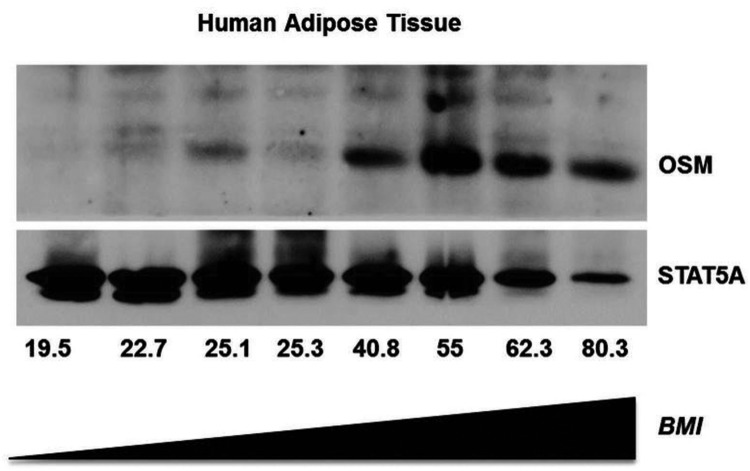
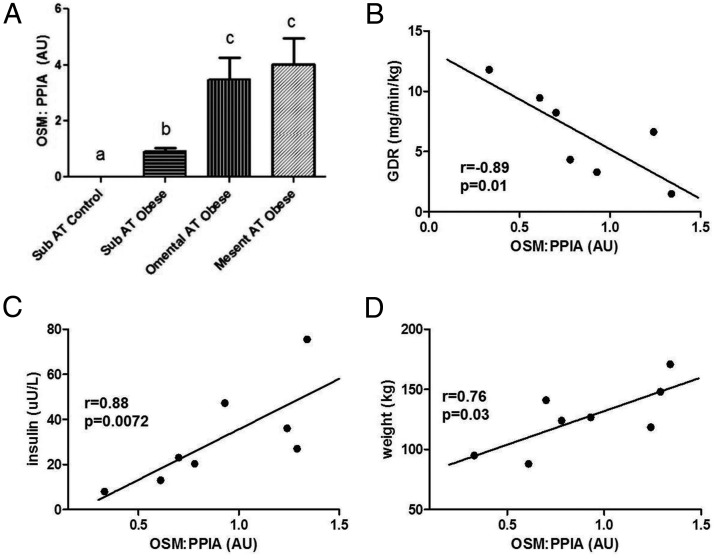
To determine whether the induction in OSM expression also occurred in human fat tissue in conditions of obesity, we examined the expression of OSM protein from subjects with BMIs ranging from 19.5 to 80.3. As shown in Figure 2, there were very low levels of OSM protein in BMIs ranging from 19.5 to 25.3. However, over a BMI range of 40.8 to 80.3, there was a significant increase in the expression of OSM protein. To follow up on these intriguing results, we examined OSM expression in adipose tissue samples from morbidly obese individuals that were candidates for gastric bypass surgery. We had access only to samples taken at the time of surgery. As shown in Figure 3A, we detected elevated OSM mRNA levels in omental and mesenteric fat. We also observed an increase in OSM mRNA levels from sc adipose tissue in the obese group when compared with lean. Clinical data from these patients were used to search for potential correlations, and in our limited study of 8 female patients, we observed that sc OSM mRNA levels correlated with insulin levels (Figure 3C) and body weight (Figure 3D). The results in Figure 3B also indicate OSM mRNA was inversely correlated with glucose disposal rate. We did not observe any significant correlations with OSMRβ expression and body weight, insulin levels, or GDR in these subjects (data not shown).
Figure 2.
OSM levels are increased with higher BMI in sc human adipose tissue. Frozen human adipose tissue samples were obtained and used to prepare whole-tissue extracts. These were subjected to Western blot analysis for human OSM. The expression of STAT5 was examined to demonstrate equivalent protein loading for each sample. The results were visualized with horseradish peroxidase-conjugated secondary antibodies and chemiluminescence.
Figure 3.
OSM mRNA in adipose tissue depots and correlations with body weight, insulin levels, and glucose disposal rate (GDR) in humans. A, Different depots of human adipose tissue were obtained before gastric bypass surgery, and RNA was extracted and processed for OSM mRNA analysis. The results are expressed as mean values ± SEM. Lowercase letters represent statistical differences of P < .05. B–D, Subcutaneous adipose tissue from different patients was processed for mRNA analysis. Euglycemic clamp was used for assessment of GDR in these patients. Glucose disposal rate (milligrams per minute per kilogram) was calculated from the high dose of a 5-hour 2-step euglycemic (120 mg/dL), hyperinsulinemic (last 30 minutes of 2.5 hours of 400 mU/m2·min insulin infusion after 2.5 hours at 50 mU/m2·min). Body weight and blood insulin levels were also measured. Abbreviations: AT, adipose tissue; AU, arbitrary units; Mesent, mesenteric; Sub, sc; PPIA, cyclophilin A.
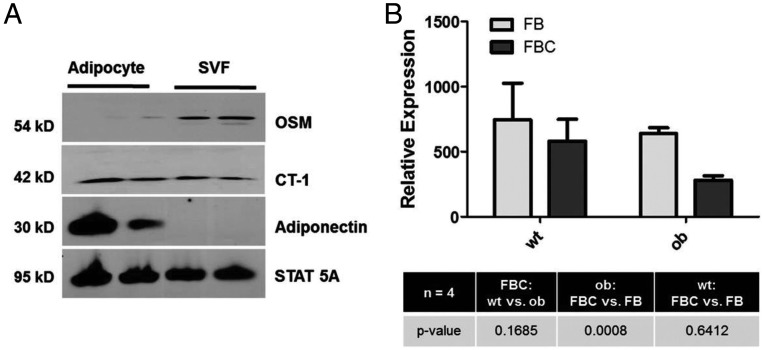
Adipose tissue is composed of a variety of cell types, and many cytokines produced in adipose tissue are made in immune cells and not in adipocytes. We fractionated human adipose tissue from 2 obese subjects into adipocytes and the other cells present, commonly referred to as the SVF. As shown in Figure 4A, we observed that OSM was primarily expressed in the SVF. In contrast, CT-1, another gp130 cytokine, was expressed in both adipocytes and SVF cells. Adiponectin was examined as a positive control restricted solely to adipocyte expression, and STAT5A was examined to demonstrate protein loading in both the adipocytes and SVF cells. We also looked for the presence of OSM in cultured adipocytes and were unable to detect OSM secretion from adipocytes (Supplemental Figure 1, published on The Endocrine Society's Journals Online website at http://jcem.endojournals.org). OSM is known to be expressed in hematopoietic organs and is found in various immune cell types, including macrophages (49, 50). Hence, we examined the expression of OSM mRNA in adipose tissue macrophages (ATMs) from 4 independent cohorts of male C57BL/6 lean and ob/ob mice at 14 weeks of age. FBC represents ATMs that are F4/80+, CD11b+, and CD11c+. FB represents ATMs that are F4/80+, CD11b+, and CD11c−. These results clearly demonstrate the expression of OSM in both types of ATMs. Also, there was a modest decrease in OSM mRNA in FBC compared with FB in ob/ob mice (Figure 4B).
Figure 4.
OSM is present in nonadipocyte cells of human WAT. A, Human adipose tissue was obtained and processed to separate adipocytes from the SVF as described in Materials and Methods. From each sample 100 μg was separated by SDS-PAGE, transferred to nitrocellulose, and subjected to Western blot analysis. B, ATMs from 4 independent cohorts of male C57/BL6 lean and ob/ob mice at 14 weeks of age were isolated as indicated in Materials and Methods. FBC represents ATMs that are F4/80+, CD11b+, and CD11c+. FB represents ATMs that are F4/80+, CD11b+, and CD11c−.
Because adipocytes are highly responsive to OSM and this cytokine is known to primarily use the OSMRβ, we examined the expression of this highly specific OSM receptor over a time course of adipocyte differentiation in 3T3-L1 cells. As shown in Supplemental Figure 2A, OSMRβ mRNA and protein levels are nearly equivalent in both preadipocytes and mature adipocytes. The efficacy of adipogenesis is shown in Supplemental Figure 2B by examining peroxisome proliferator-activated receptor-γ expression. Of note, we did observe an increase in OSMRβ levels during the clonal expansion of adipogenesis. We also examined expression of OSMRβ in a variety of murine tissues. OSMRβ is highly expressed in brown and white adipose tissue (Supplemental Figure 2C). These results clearly indicate that expression of OSMRβ is present in adipocytes in vitro and adipose tissue in vivo.
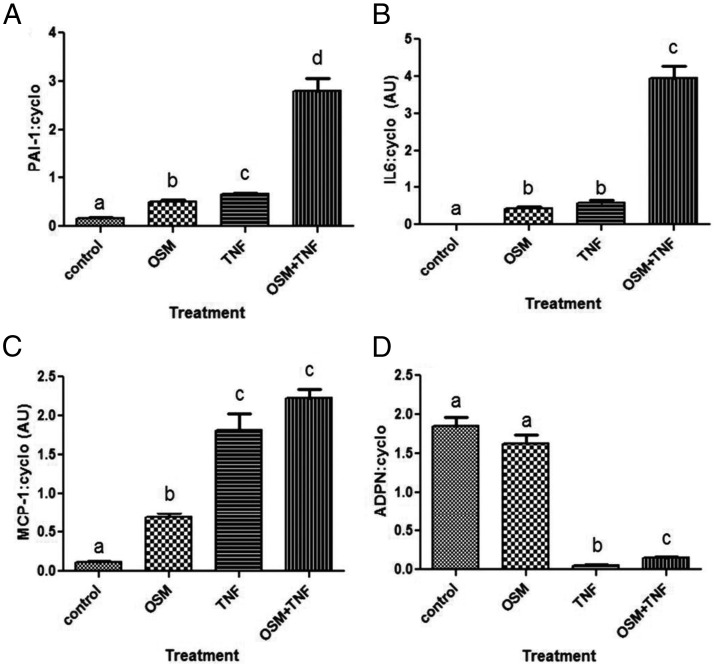
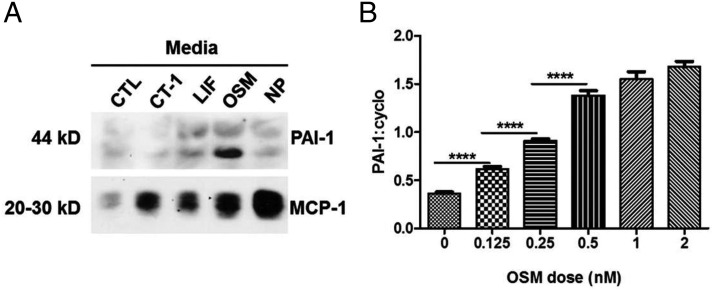
To date, few studies have focused on the actions of OSM on adipocytes. Because many cell types are present in adipose tissue, we examined the effects of OSM on fully differentiated murine 3T3-L1 adipocytes. As shown in Figure 5, OSM and TNFα induce PAI-1 and IL-6 mRNA in adipocytes. However, the effects of both of these cytokines results in an effect that is at least additive, suggesting these cytokines mediate their effect via independent mechanisms. OSM also induces MCP-1 mRNA levels in mature adipocytes, but the induction is stronger with TNFα. TNFα also inhibits adiponectin mRNA expression, but we did not observe any effects of OSM on adiponectin levels in the presence or absence of TNFα. Next, we examined the effects of several gp130 cytokines on the secretion of 2 known adipokines, MCP-1 and PAI-1, from murine adipocytes. As shown in Figure 6A, all of the gp130 cytokines examined (CT-1, LIF, OSM, and NP) resulted in an induction of MCP-1 secretion. However, only OSM resulted in a robust increase in PAI-1 secretion. To assess whether this response was directly regulated by OSM, we treated mature adipocytes for 90 minutes with various doses of OSM. Acute OSM treatment resulted in a dose-dependent increase in PAI-1 mRNA levels (Figure 6B) and in the phosphorylation of OSMRβ, gp130, and STAT3 (data not shown).
Figure 5.
OSM and TNFα induce PAI-1, MCP-1, and IL-6 mRNA in murine adipocytes. Fully differentiated 3T3-L1 adipocytes were treated with 1nM TNFα, 1nM OSM, or both cytokines for 72 hours before harvesting. RNA was extracted and processed for PAI-1 (A), IL-6 (B), MCP-1 (C), and ADPN (D) mRNA measurement. Each figure represents an experiment independently performed 3 times. The results are expressed as mean values ± SEM. Lowercase letters represent statistical differences (P < .05).
Figure 6.
OSM induces PAI-1 secretion from murine adipocytes. A, Fully differentiated 3T3-L1 adipocytes were treated with 1nM of the various cytokines indicated in the figure. CTL represents untreated control cells. The mature adipocytes were treated daily, and after 96 hours, the medium was collected and used for Western blot analysis. B, Fully differentiated 3T3-L1 adipocytes were treated with various doses of OSM for 90 minutes. RNA was extracted and processed for PAI-1 and cyclophilin mRNA measurement. Each figure represents an experiment independently performed 3 times. The ratio of PAI-1 to cyclophilin was calculated. Results are expressed as mean ± SEM. ****, P < .0001 between groups.
Discussion
Adipose tissue is highly sensitive to several gp130 cytokines, including OSM (44). Also, OSM inhibits adipocyte differentiation in vitro (25, 45). In these studies, we observed that OSM mRNA and protein levels are increased in 2 rodent models of obesity/T2DM. In human studies, OSM protein was detectable in human adipose tissue and OSM expression was elevated in WAT obtained from individuals with BMI >40. We also analyzed fat tissue obtained from patients before gastric bypass surgery and observed that OSM mRNA levels are higher in omental and mesenteric fat when compared with sc fat tissue. In addition, in the restricted number of samples we were able to analyze, OSM levels in sc fat in women correlated with weight and insulin levels and had an inverse correlation with glucose disposal rates. Although this analysis was limited to 8 subjects, the overall modulation of OSM in human fat samples correlates with the increased expression of OSM mRNA and protein levels we observed in both ob/ob and HFD mice. Although further validation will be needed to identify human phenotypes associated with OSM expression, our observations suggest that OSM levels are increased in adipose tissue in conditions of obesity. Future investigations will be needed to determine whether OSM and its receptor are affected by gender, race, and/or age.
Our in vitro studies in cultured 3T3-L1 adipocytes and in vivo analyses in rodent and human adipose tissue support a working model whereby OSM is produced in adipose tissue in conditions of excess adiposity. Our results suggest that OSM is not produced by or secreted from adipocytes but is expressed in 2 F4/80+ populations of macrophages that are CD11c+ and CD11c−. Although we observed a significant increase in OSM mRNA and protein in mouse and human adipose tissue with obesity, we did not observe this same pattern of OSM mRNA regulation in either population of F4/80+ adipose tissue macrophages (Figure 4B). These results suggest that OSM is likely produced in additional nonadipocyte cells present in adipose tissue, and further studies are necessary to identify all of the cellular sources of OSM in adipose tissue. We hypothesize that nonadipocyte-derived OSM acts in a paracrine manner on adipocytes to promote metabolic dysfunction. Our data showing that OSM induces IL-6 and PAI-1 in a manner similar to TNFα support this hypothesis. OSM also induces MCP-1, but not to the same level as TNFα exposure. Although there is some evidence that OSM can inhibit adiponectin expression (47), we were unable to observe this effect. The discrepancy in our results and the published study (47) can likely be attributed to cells that were not fully differentiated adipocytes. We and others have shown that OSM inhibits adiponectin and other proteins that are highly expressed during adipogenesis in vitro. Conversely, all of our studies were performed in fully differentiated mature adipocytes.
Our results also indicate that OSM is distinct from other gp130 cytokines that use the Janus kinase/STAT signaling pathway in adipocytes. As shown in Figure 6, exposure to OSM, but not other gp130 cytokines, increased secretion of PAI-1 from cultured adipocytes. PAI-1 is known to be secreted from adipocytes and upregulated in conditions of obesity/T2DM (reviewed in Ref. 51). Previous studies have also shown that PAI-1 expression is induced by OSM in human preadipocytes and adipocytes isolated from sc and visceral adipose tissue (52) and in human coronary artery and aortic cells (53). PAI-1 is also known to have profound effects on tissue fibrosis (reviewed in Ref. 54). Elevated OSM in adipose tissue may contribute to fibrosis, in part by modulation of PAI-1. Overall, our observations suggest that nonadipocyte-derived OSM acts in a paracrine manner on adipocytes to promote the induction of IL-6, MCP-1, and PAI-1.
A very recent study examined the phenotype of mice that lack the specific OSM receptor, OSMRβ. These studies showed that OSMRβ-null mice had mature-onset obesity and insulin resistance (48). Moreover, administration of OSM increased insulin sensitivity and polarized macrophages to an M2 phenotype. The conclusion from these studies was that OSM suppresses the development of insulin resistance. This study contrasts with our current observations. One difference between our studies is that they showed OSM mRNA was present at equivalent levels in both the SVF and adipocyte fractions of adipose tissue. Our studies failed to detect OSM in cultured murine adipocytes, and fractionation of adipose tissue revealed that most OSM protein was present in the SVF (Figure 4A). The other study did not observe OSMRβ in adipocytes, although we have substantial data documenting the presence of OSMRβ in mature adipocytes (Supplemental Figure 2B). Moreover, rodent adipose tissue in vivo is highly responsive to OSM exposure (44), indicating that the OSMRβ is present in adipocytes. We also observed an increase in OSMRβ in the 2 rodent models of obesity we examined (Figure 1), raising the possibility that adipocytes might be more sensitive to OSM under these conditions. Our model supports a role of OSM in inducing the expression of genes associated with inflammation and insulin resistance. Therefore, we hypothesize that adipose tissue-derived OSM promotes a metabolically unfavorable phenotype. We predict that OSMRβ signaling in adipocytes is likely involved in the pathogenesis of T2DM but does not serve in a protective capacity as recently suggested in mice that have a global knockout of the OSM receptor (48). Studies in liver indicate that OSM, produced in Kuppfer cells, can promote hepatic insulin resistance by inhibiting insulin-induced Akt phosphorylation (55). There is little question that many additional studies will be necessary to identify all the adipose tissue sources of OSM and to determine the overall function of this cytokine in T2DM. Nonetheless, our results clearly demonstrate that OSM levels are induced in murine and human obesity, and OSM can modulate adipocyte gene expression in a manner that is similar, but distinct, from TNFα.
Acknowledgments
We thank Anik Boudreau for cell culture support and Ryan Grant for comments regarding manuscript preparation.
This work was supported by Grant R01DK052968-15 from the National Institutes of Health (to J.M.S.) and a Sara Borrell contract from Instituto de Salud Carlos III (Madrid, Spain) (to D.S.-I.).
D.S.-I. and U.A.W. conducted most of the experiments. D.S.-I., U.A.W., and C.M.E. wrote and J.M.S. edited the manuscript to produce the final version. C.M.E. performed studies on receptor expression. A.W.F. provided the data on OSM expression in macrophages. R.V.C. and J.G. provided human tissue or tissue fractions for Western blot analysis that was conducted by D.S.-I., R.F.M. performed RNA analysis on rodent tissues prepared by J.M.S., E.R. provided human tissue that was used by D.S.-I. for RNA analysis. E.R. also provided additional information on study participants. All of the authors contributed to editing the manuscript before submission.
Disclosure Summary: D.S.-I., U.A.W., C.M.E., R.F.M., E.R., and J.M.S. have nothing to disclose. R.V.C. consults for Merck Research Laboratories and receives grant funding from Merck Research Laboratories and Eli Lilly and Company. A.W.F. receives grant support from AstraZeneca Plc. J.M.G. is the cofounder and Chief Scientific Officer of LaCell LLC, a for-profit biotechnology company focusing on the use of adipose stromal/stem cells for research and regenerative medical applications.
Footnotes
- ATM
- adipose tissue macrophage
- BMI
- body mass index
- CT-1
- cardiotrophin-1
- FACS
- fluorescence-activated cell sorting
- gp130
- glycoprotein 130
- HFD
- high-fat diet
- LIF
- leukemia inhibitory factor
- MCP-1
- monocyte chemoattractant protein-1
- NP
- neuropoietin
- OSM
- oncostatin M
- OSMRβ
- OSM receptor-β
- PBRC
- Pennington Biomedical Research Center
- STAT
- signal transducer and activator of transcription
- SVF
- stromovascular fraction
- T2DM
- type 2 diabetes mellitus
- WAT
- white adipose tissue.
References
- 1. Fasnacht N, Müller W. Conditional gp130 deficient mouse mutants. Semin Cell Dev Biol. 2008;19:379–384 [DOI] [PubMed] [Google Scholar]
- 2. Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hansen D, Dendale P, Beelen M, et al. Plasma adipokine and inflammatory marker concentrations are altered in obese, as opposed to non-obese, type 2 diabetes patients. Eur J Appl Physiol. 2010;109:397–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–E751 [DOI] [PubMed] [Google Scholar]
- 5. Celik A, Sahin S, Koc F, et al. Cardiotrophin-1 plasma levels are increased in patients with diastolic heart failure. Med Sci Monit. 2012;18:CR25–CR31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. López B, Gonzalez A, Querejeta R, Barba J, Diez J. Association of plasma cardiotrophin-1 with stage C heart failure in hypertensive patients: potential diagnostic implications. J Hypertens. 2009;27:418–424 [DOI] [PubMed] [Google Scholar]
- 7. Natal C, Fortuño MA, Restituto P, et al. Cardiotrophin-1 is expressed in adipose tissue and upregulated in the metabolic syndrome. Am J Physiol Endocrinol Metab. 2008;294:E52–E60 [DOI] [PubMed] [Google Scholar]
- 8. Slevin M, Krupinski J, Mitsios N, et al. Leukaemia inhibitory factor is over-expressed by ischaemic brain tissue concomitant with reduced plasma expression following acute stroke. Eur J Neurol. 2008;15:29–37 [DOI] [PubMed] [Google Scholar]
- 9. Pradeep AR, S TM, Garima G, Raju A. Serum levels of oncostatin M (a gp 130 cytokine): an inflammatory biomarker in periodontal disease. Biomarkers. 2010;15:277–282 [DOI] [PubMed] [Google Scholar]
- 10. Febbraio MA. gp130 receptor ligands as potential therapeutic targets for obesity. J Clin Invest. 2007;117:841–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wallenius V, Wallenius K, Ahrén B, et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8:75–79 [DOI] [PubMed] [Google Scholar]
- 12. Di Gregorio GB, Hensley L, Lu T, Ranganathan G, Kern PA. Lipid and carbohydrate metabolism in mice with a targeted mutation in the IL-6 gene: absence of development of age-related obesity. Am J Physiol Endocrinol Metab. 2004;287:E182–E187 [DOI] [PubMed] [Google Scholar]
- 13. Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem. 2003;278:45777–45784 [DOI] [PubMed] [Google Scholar]
- 14. Gloaguen I, Costa P, Demartis A, et al. Ciliary neurotrophic factor corrects obesity and diabetes associated with leptin deficiency and resistance. Proc Natl Acad Sci U S A. 1997;94:6456–6461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lambert PD, Anderson KD, Sleeman MW, et al. Ciliary neurotrophic factor activates leptin-like pathways and reduces body fat, without cachexia or rebound weight gain, even in leptin-resistant obesity. Proc Natl Acad Sci U S A. 2001;98:4652–4657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sleeman MW, Garcia K, Liu R, et al. Ciliary neurotrophic factor improves diabetic parameters and hepatic steatosis and increases basal metabolic rate in db/db mice. Proc Natl Acad Sci U S A. 2003;100:14297–14302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ettinger MP, Littlejohn TW, Schwartz SL, et al. Recombinant variant of ciliary neurotrophic factor for weight loss in obese adults: a randomized, dose-ranging study. JAMA. 2003;289:1826–1832 [DOI] [PubMed] [Google Scholar]
- 18. Blüher S, Moschos S, Bullen J, Jr, et al. Ciliary neurotrophic factorAx15 alters energy homeostasis, decreases body weight, and improves metabolic control in diet-induced obese and UCP1-DTA mice. Diabetes. 2004;53:2787–2796 [DOI] [PubMed] [Google Scholar]
- 19. Zvonic S, Cornelius P, Stewart WC, Mynatt RL, Stephens JM. The regulation and activation of ciliary neurotrophic factor signaling proteins in adipocytes. J Biol Chem. 2003;278:2228–2235 [DOI] [PubMed] [Google Scholar]
- 20. Crowe S, Turpin SM, Ke F, Kemp BE, Watt MJ. Metabolic remodeling in adipocytes promotes ciliary neurotrophic factor-mediated fat loss in obesity. Endocrinology. 2008;149:2546–2556 [DOI] [PubMed] [Google Scholar]
- 21. Balhoff JP, Stephens JM. Highly specific and quantitative activation of STATs in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 1998;247:894–900 [DOI] [PubMed] [Google Scholar]
- 22. Tenney R, Stansfield K, Pekala PH. Interleukin 11 signaling in 3T3-L1 adipocytes. J Cell Physiol. 2005;202:160–166 [DOI] [PubMed] [Google Scholar]
- 23. Stephens JM, Lumpkin SJ, Fishman JB. Activation of signal transducers and activators of transcription 1 and 3 by leukemia inhibitory factor, oncostatin-M, and interferon-gamma in adipocytes. J Biol Chem. 1998;273:31408–31416 [DOI] [PubMed] [Google Scholar]
- 24. Zvonic S, Hogan JC, Arbour-Reily P, Mynatt RL, Stephens JM. Effects of cardiotrophin on adipocytes. J Biol Chem. 2004;279:47572–47579 [DOI] [PubMed] [Google Scholar]
- 25. White UA, Stewart WC, Mynatt RL, Stephens JM. Neuropoietin attenuates adipogenesis and induces insulin resistance in adipocytes. J Biol Chem. 2008;283:22505–22512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lehr S, Hartwig S, Sell H. Adipokines: a treasure trove for the discovery of biomarkers for metabolic disorders. Proteomics Clin Appl. 2012;6:91–101 [DOI] [PubMed] [Google Scholar]
- 27. Mohamed-Ali V, Goodrick S, Rawesh A, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-α, in vivo. J Clin Endocrinol Metab. 1997;82:4196–4200 [DOI] [PubMed] [Google Scholar]
- 28. Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–850 [DOI] [PubMed] [Google Scholar]
- 29. Blüher M. Clinical relevance of adipokines. Diabetes Metab J. 2012;36:317–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the metabolic syndrome–an allostatic perspective. Biochim Biophys Acta. 2010;1801:338–349 [DOI] [PubMed] [Google Scholar]
- 32. Rose TM, Lagrou MJ, Fransson I, et al. The genes for oncostatin M (OSM) and leukemia inhibitory factor (LIF) are tightly linked on human chromosome 22. Genomics. 1993;17:136–140 [DOI] [PubMed] [Google Scholar]
- 33. Rose TM, Bruce AG. Oncostatin M is a member of a cytokine family that includes leukemia-inhibitory factor, granulocyte colony-stimulating factor, and interleukin 6. Proc Natl Acad Sci U S A. 1991;88:8641–8645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zarling JM, Shoyab M, Marquardt H, Hanson MB, Lioubin MN, Todaro GJ. Oncostatin M: a growth regulator produced by differentiated histiocytic lymphoma cells. Proc Natl Acad Sci U S A. 1986;83:9739–9743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mosley B, De Imus C, Friend D, et al. Dual oncostatin M (OSM) receptors. Cloning and characterization of an alternative signaling subunit conferring OSM-specific receptor activation. J Biol Chem. 1996;271:32635–32643 [DOI] [PubMed] [Google Scholar]
- 36. Brown TJ, Lioubin MN, Marquardt H. Purification and characterization of cytostatic lymphokines produced by activated human T lymphocytes. Synergistic antiproliferative activity of transforming growth factor β1, interferon-γ, and oncostatin M for human melanoma cells. J Immunol. 1987;139:2977–2983 [PubMed] [Google Scholar]
- 37. Suda T, Chida K, Todate A, et al. Oncostatin M production by human dendritic cells in response to bacterial products. Cytokine. 2002;17:335–340 [DOI] [PubMed] [Google Scholar]
- 38. Wahl AF, Wallace PM. Oncostatin M in the anti-inflammatory response. Ann Rheum Dis. 2001;60(Suppl 3):iii75–iii80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hui W, Bell M, Carroll G. Detection of oncostatin M in synovial fluid from patients with rheumatoid arthritis. Ann Rheum Dis. 1997;56:184–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Albasanz-Puig A, Murray J, Preusch M, et al. Oncostatin M is expressed in atherosclerotic lesions: a role for Oncostatin M in the pathogenesis of atherosclerosis. Atherosclerosis. 2011;216:292–298 [DOI] [PubMed] [Google Scholar]
- 41. Kamiya A, Kinoshita T, Ito Y, et al. Fetal liver development requires a paracrine action of oncostatin M through the gp130 signal transducer. EMBO J. 1999;18:2127–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Okaya A, Kitanaka J, Kitanaka N, et al. Oncostatin M inhibits proliferation of rat oval cells, OC15–5, inducing differentiation into hepatocytes. Am J Pathol. 2005;166:709–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nakamura K, Nonaka H, Saito H, Tanaka M, Miyajima A. Hepatocyte proliferation and tissue remodeling is impaired after liver injury in oncostatin M receptor knockout mice. Hepatology. 2004;39:635–644 [DOI] [PubMed] [Google Scholar]
- 44. White UA, Stewart WC, Stephens JM. Gp130 cytokines exert differential patterns of crosstalk in adipocytes both in vitro and in vivo. Obesity (Silver Spring). 2011;19:903–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miyaoka Y, Tanaka M, Naiki T, Miyajima A. Oncostatin M inhibits adipogenesis through the RAS/ERK and STAT5 signaling pathways. J Biol Chem. 2006;281:37913–37920 [DOI] [PubMed] [Google Scholar]
- 46. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Song HY, Kim MR, Lee MJ, et al. Oncostatin M decreases adiponectin expression and induces dedifferentiation of adipocytes by JAK3- and MEK-dependent pathways. Int J Biochem Cell Biol. 2007;39:439–449 [DOI] [PubMed] [Google Scholar]
- 48. Komori T, Tanaka M, Senba E, Miyajima A, Morikawa Y. 2013 Lack of oncostatin M receptor beta leads to adipose tissue inflammation and insulin resistance by switching macrophage phenotype. J Biol Chem. 2013;288:21861–21875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wallace PM, MacMaster JF, Rouleau KA, et al. Regulation of inflammatory responses by oncostatin M. J Immunol. 1999;162:5547–5555 [PubMed] [Google Scholar]
- 50. Tamura S, Morikawa Y, Miyajima A, Senba E. Expression of oncostatin M in hematopoietic organs. Dev Dynam. 2002;225:327–331 [DOI] [PubMed] [Google Scholar]
- 51. Alessi MC, Poggi M, Juhan-Vague I. Plasminogen activator inhibitor-1, adipose tissue and insulin resistance. Curr Opin Lipidol. 2007;18:240–245 [DOI] [PubMed] [Google Scholar]
- 52. Rega G, Kaun C, Weiss TW, et al. Inflammatory cytokines interleukin-6 and oncostatin m induce plasminogen activator inhibitor-1 in human adipose tissue. Circulation. 2005;111:1938–1945 [DOI] [PubMed] [Google Scholar]
- 53. Demyanets S, Kaun C, Rychli K, et al. The inflammatory cytokine oncostatin M induces PAI-1 in human vascular smooth muscle cells in vitro via PI 3-kinase and ERK1/2-dependent pathways. Am J Physiol Heart Circ Physiol. 2007;293:H1962–H1968 [DOI] [PubMed] [Google Scholar]
- 54. Ghosh AK, Vaughan DE. PAI-1 in tissue fibrosis. J Cell Physiol. 2012;227:493–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Henkel J, Gärtner D, Dorn C, et al. Oncostatin M produced in Kupffer cells in response to PGE2: possible contributor to hepatic insulin resistance and steatosis. Lab Invest. 2011;91:1107–1117 [DOI] [PubMed] [Google Scholar]