Abstract
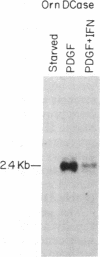
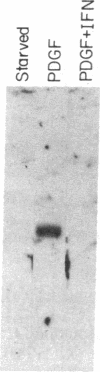
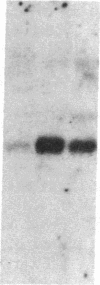
The G0/G1 to S transition in quiescent BALB/c 3T3 cells stimulated by serum growth factors can be specifically blocked by the administration of interferon (IFN) to the system. In the present communication, we studied whether IFN inhibits the early events in the G0/G1 phase that are initiated by the platelet-derived growth factor (PDGF). The results show that IFN inhibits most of the PDGF-mediated increase of c-myc, ornithine decarboxylase, and beta-actin mRNAs measured 3 hr after stimulation. c-fos mRNA levels are reduced by IFN as early as 20 min after exposure of the quiescent cells to PDGF. The expression of several genes that belong to the competence gene family is, therefore, inhibited by IFN and this could account for the failure of the IFN-treated cells to enter into the S phase when growth factors present in the platelet-poor plasma are added. We also report that the PDGF-mediated increase in the uptake of deoxyglucose is not impaired by IFN, thus suggesting that the early effects of IFN on gene expression do not result from inhibition of binding of PDGF to its cell-surface receptors. Unlike the direct stimulatory effect of PDGF, which is not sensitive to cycloheximide, the inhibitory effect of IFN on c-myc mRNA levels depends in part on protein synthesis. We propose that a putative product of one of the IFN-induced genes could mediate the decrease in expression of the PDGF-regulated gene family.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N., Scher C. D., Stiles C. D. Purification of human platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1809–1813. doi: 10.1073/pnas.76.4.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armelin H. A., Armelin M. C., Kelly K., Stewart T., Leder P., Cochran B. H., Stiles C. D. Functional role for c-myc in mitogenic response to platelet-derived growth factor. Nature. 1984 Aug 23;310(5979):655–660. doi: 10.1038/310655a0. [DOI] [PubMed] [Google Scholar]
- Balkwill F., Taylor-Papadimitriou J. Interferon affects both G1 and S+G2 in cells stimulated from quiescence to growth. Nature. 1978 Aug 24;274(5673):798–800. doi: 10.1038/274798a0. [DOI] [PubMed] [Google Scholar]
- Bernard O., Cory S., Gerondakis S., Webb E., Adams J. M. Sequence of the murine and human cellular myc oncogenes and two modes of myc transcription resulting from chromosome translocation in B lymphoid tumours. EMBO J. 1983;2(12):2375–2383. doi: 10.1002/j.1460-2075.1983.tb01749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Curran T., Peters G., Van Beveren C., Teich N. M., Verma I. M. FBJ murine osteosarcoma virus: identification and molecular cloning of biologically active proviral DNA. J Virol. 1982 Nov;44(2):674–682. doi: 10.1128/jvi.44.2.674-682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einat M., Resnitzky D., Kimchi A. Close link between reduction of c-myc expression by interferon and, G0/G1 arrest. Nature. 1985 Feb 14;313(6003):597–600. doi: 10.1038/313597a0. [DOI] [PubMed] [Google Scholar]
- Friedman R. L., Manly S. P., McMahon M., Kerr I. M., Stark G. R. Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells. Cell. 1984 Oct;38(3):745–755. doi: 10.1016/0092-8674(84)90270-8. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Inglot A. D., Oleszak E., Kisielow B. Antagonism in action between mouse or human interferon and platelet growth factor. Arch Virol. 1980;63(3-4):291–296. doi: 10.1007/BF01315035. [DOI] [PubMed] [Google Scholar]
- Jonak G. J., Knight E., Jr Selective reduction of c-myc mRNA in Daudi cells by human beta interferon. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1747–1750. doi: 10.1073/pnas.81.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana C., Nathans D. Isolation of cloned cDNA encoding mammalian ornithine decarboxylase. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3645–3649. doi: 10.1073/pnas.81.12.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Kimchi A., Shure H., Lapidot Y., Rapoport S., Panet A., Revel M. Antimitogenic effects of interferon and (2'-5')-oligoadenylate in synchronized 3T3 fibroblasts. FEBS Lett. 1981 Nov 16;134(2):212–216. doi: 10.1016/0014-5793(81)80604-7. [DOI] [PubMed] [Google Scholar]
- Knight E., Jr, Anton E. D., Fahey D., Friedland B. K., Jonak G. J. Interferon regulates c-myc gene expression in Daudi cells at the post-transcriptional level. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1151–1154. doi: 10.1073/pnas.82.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- Larner A. C., Jonak G., Cheng Y. S., Korant B., Knight E., Darnell J. E., Jr Transcriptional induction of two genes in human cells by beta interferon. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6733–6737. doi: 10.1073/pnas.81.21.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Battey J., Lenoir G., Moulding C., Murphy W., Potter H., Stewart T., Taub R. Translocations among antibody genes in human cancer. Science. 1983 Nov 18;222(4625):765–771. doi: 10.1126/science.6356357. [DOI] [PubMed] [Google Scholar]
- Merlin G., Chebath J., Benech P., Metz R., Revel M. Molecular cloning and sequence of partial cDNA for interferon-induced (2'-5')oligo(A) synthetase mRNA from human cells. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4904–4908. doi: 10.1073/pnas.80.16.4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Curran T., Verma I. M. c-fos protein can induce cellular transformation: a novel mechanism of activation of a cellular oncogene. Cell. 1984 Jan;36(1):51–60. doi: 10.1016/0092-8674(84)90073-4. [DOI] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. Induction of DNA synthesis in BALB/c 3T3 cells by serum components: reevaluation of the commitment process. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4481–4485. doi: 10.1073/pnas.74.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel M. F., Kimchi A. Initial characterization of a spontaneous interferon secreted during growth and differentiation of Friend erythroleukemia cells. Mol Cell Biol. 1982 Dec;2(12):1472–1480. doi: 10.1128/mcb.2.12.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher C. D., Shepard R. C., Antoniades H. N., Stiles C. D. Platelet-derived growth factor and the regulation of the mammalian fibroblast cell cycle. Biochim Biophys Acta. 1979 Aug 10;560(2):217–241. doi: 10.1016/0304-419x(79)90020-9. [DOI] [PubMed] [Google Scholar]
- Shani M., Nudel U., Zevin-Sonkin D., Zakut R., Givol D., Katcoff D., Carmon Y., Reiter J., Frischauf A. M., Yaffe D. Skeletal muscle actin mRNA. Characterization of the 3' untranslated region. Nucleic Acids Res. 1981 Feb 11;9(3):579–589. doi: 10.1093/nar/9.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreevalsan T., Rozengurt E., Taylor-Papadimitriou J., Burchell J. Differential effect of interferon on DNA synthesis, 2-deoxyglucose uptake and ornithine decarboxylase activity in 3T3 cells stimulated by polypeptide growth factors and tumor promotors. J Cell Physiol. 1980 Jul;104(1):1–9. doi: 10.1002/jcp.1041040102. [DOI] [PubMed] [Google Scholar]
- Sreevalsan T., Taylor-Papadimitriou J., Rozengurt E. Selective inhibition by interferon of serum-stimulated biochemical events in 3T3 cells. Biochem Biophys Res Commun. 1979 Apr 13;87(3):679–685. doi: 10.1016/0006-291x(79)92012-6. [DOI] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Shearer M., Rozengurt E. Inhibitory effect of interferon on cellular DNA synthesis: modulation by pure mitogenic factors. J Interferon Res. 1981;1(3):401–409. doi: 10.1089/jir.1981.1.401. [DOI] [PubMed] [Google Scholar]
- Tominaga S., Lengyel P. beta-Interferon alters the pattern of proteins secreted from quiescent and platelet-derived growth factor-treated BALB/c-3T3 cells. J Biol Chem. 1985 Feb 25;260(4):1975–1978. [PubMed] [Google Scholar]
- Yarden A., Shure-Gottlieb H., Chebath J., Revel M., Kimchi A. Autogenous production of interferon-beta switches on HLA genes during differentiation of histiocytic lymphoma U937 cells. EMBO J. 1984 May;3(5):969–973. doi: 10.1002/j.1460-2075.1984.tb01915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]