Abstract
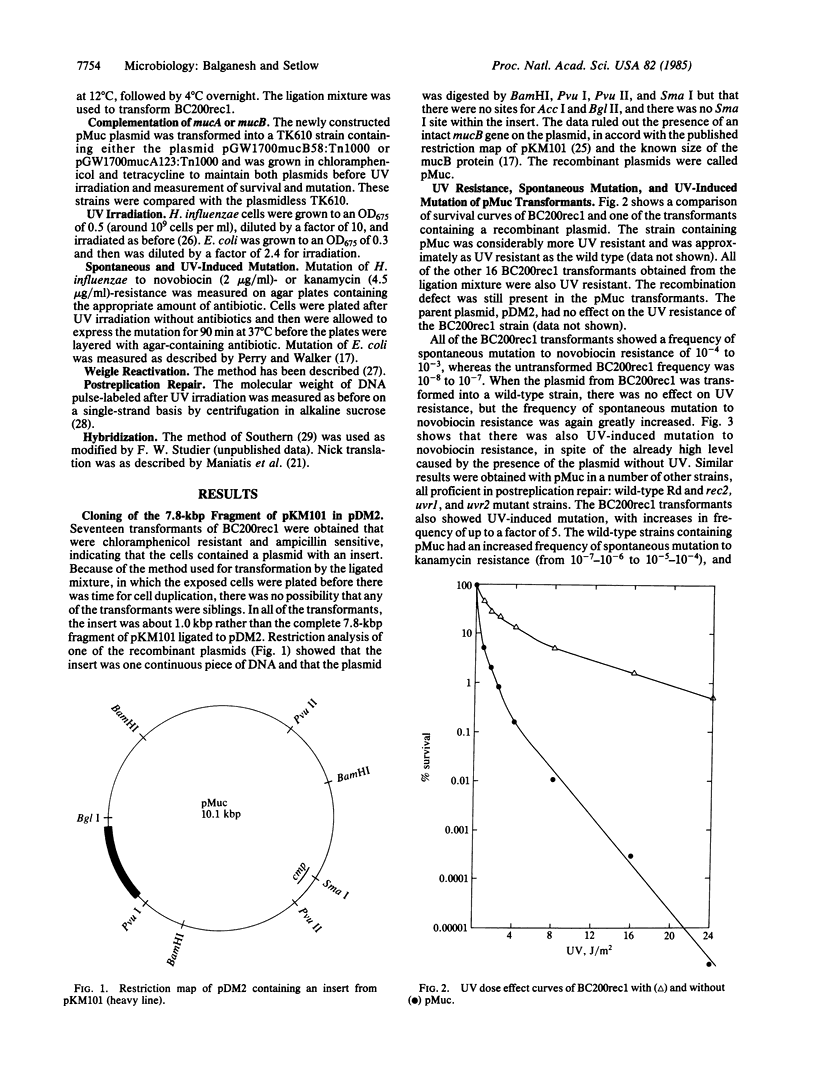
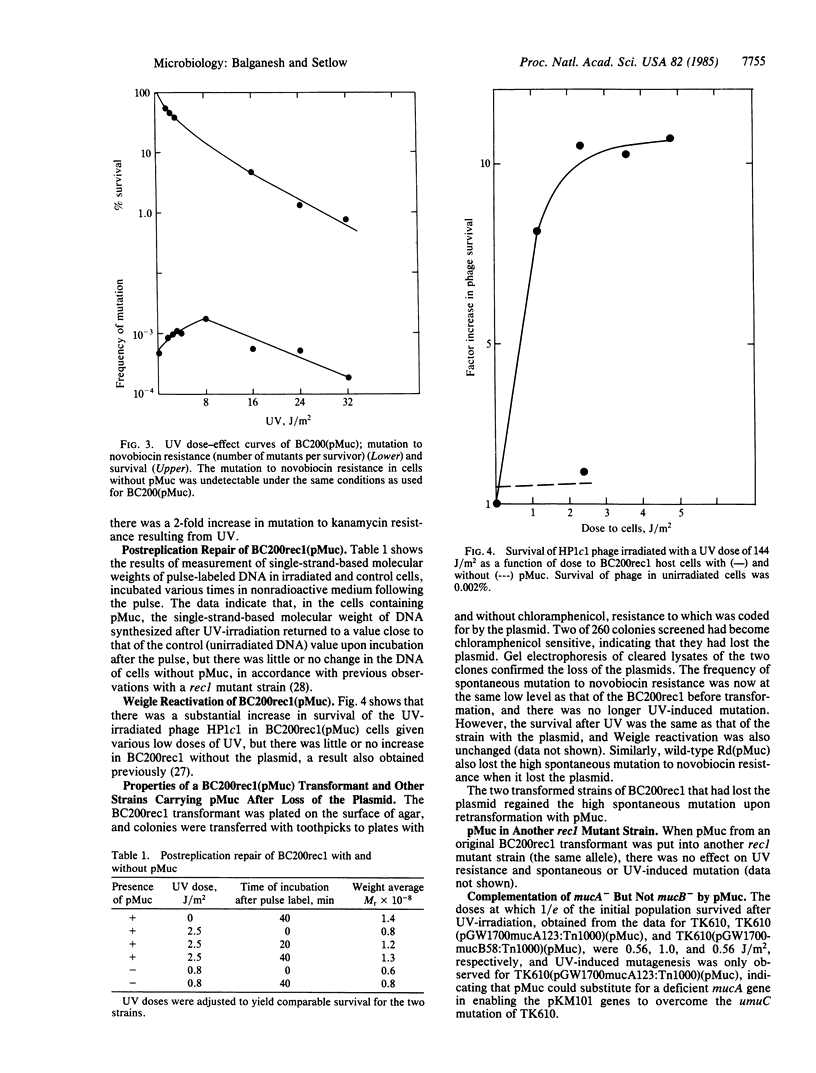
Haemophilus influenzae, normally not mutable by UV, became UV mutable with a recombinant plasmid insertion. A 7.8-kilobase-pair (kbp) fragment of the plasmid pKM101 containing the mucA and mucB genes was ligated to the shuttle vector pDM2, and a Rec- strain of H. influenzae was transformed with the ligated mixture. All of the transformants, unlike the parent Rec- strain, were resistant to UV, could carry out postreplication repair and Weigle reactivation, showed greatly increased spontaneous mutation, and contained a plasmid carrying an insert of only 1.2 rather than 7.8 kbp. This plasmid in a umuC mutant strain of Escherichia coli complemented a pKM101 derivative lacking mucA function but with an intact mucB gene, although there was no complementation with a mucA+ mucB- plasmid, suggesting that the newly constructed plasmid coded for the mucA protein; this is in accord with the restriction analysis and hybridization between the plasmid and a probe containing all of the mucA gene but only a small fraction of mucB. When one of the H. influenzae Rec- transformants lost the plasmid, the resistance to UV was retained but the high spontaneous mutation and UV mutability were not. The fact that there was hybridization between the chromosome of the "cured" strain and a probe containing both muc genes but none when almost no mucB was present suggested that at least part of the mucB gene had been integrated into the Rec- chromosome. Five different postreplication repair-proficient strains became UV mutable and had high spontaneous mutation rates caused by the putative mucA plasmid, indicating that these strains already possessed a chromosomal equivalent of the mucB gene.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balganesh M., Setlow J. K. Prophage induction in Haemophilus influenzae and its relationship to mutation by chemical and physical agents. Mutat Res. 1984 Jan;125(1):15–22. doi: 10.1016/0027-5107(84)90027-7. [DOI] [PubMed] [Google Scholar]
- Barnhart B. J., Cox S. H. Radiation-sensitive and radiation-resistant mutants of Haemophilus influenzae. J Bacteriol. 1968 Jul;96(1):280–282. doi: 10.1128/jb.96.1.280-282.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boling M. E., Kimball R. F. Mutation induction by MNNG in a bacteriophage of Haemophilus influenzae. Mutat Res. 1976 Oct;37(1):1–10. doi: 10.1016/0027-5107(76)90050-6. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Walker G. C. Proteins required for ultraviolet light and chemical mutagenesis. Identification of the products of the umuC locus of Escherichia coli. J Mol Biol. 1983 Feb 25;164(2):175–192. doi: 10.1016/0022-2836(83)90074-8. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Walker G. C. The muc genes of pKM101 are induced by DNA damage. J Bacteriol. 1983 Sep;155(3):1306–1315. doi: 10.1128/jb.155.3.1306-1315.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARM W., RUPERT C. S. INFECTION OF TRANSFORMABLE CELLS OF HAEMOPHILUS INFLUENZAE BY BACTERIOPHAGE AND BACTERIOPHAGE DNA. Z Vererbungsl. 1963 Dec 30;94:336–348. doi: 10.1007/BF00897593. [DOI] [PubMed] [Google Scholar]
- Kato T. Effects of chloramphenicol and caffeine on postreplication repair in uvr A- umuC- und uvrA- recF- strains of Escherichia coli K-12. Mol Gen Genet. 1977 Nov 14;156(2):115–120. doi: 10.1007/BF00283483. [DOI] [PubMed] [Google Scholar]
- Kato T., Shinoura Y. Isolation and characterization of mutants of Escherichia coli deficient in induction of mutations by ultraviolet light. Mol Gen Genet. 1977 Nov 14;156(2):121–131. doi: 10.1007/BF00283484. [DOI] [PubMed] [Google Scholar]
- Kimball R. F., Hirsch B. F. Tests for the mutagenic actions of a number of chemicals on Haemophilus influenzae with special emphasis on hydrazine. Mutat Res. 1975 Oct;30(1):9–20. [PubMed] [Google Scholar]
- Kooistra J., Setlow J. K. Similarity in properties and mapping of three Rec mutants of Haemophilus influenzae. J Bacteriol. 1976 Jul;127(1):327–333. doi: 10.1128/jb.127.1.327-333.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer P. J., Shanabruch W. G., Walker G. C. Functional organization of plasmid pKM101. J Bacteriol. 1981 Mar;145(3):1310–1316. doi: 10.1128/jb.145.3.1310-1316.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeClerc J. E., Setlow J. K. Effects of combining ultraviolet repair and recombination mutations in Haemophilus influenzae. Nat New Biol. 1973 Feb 7;241(110):172–174. doi: 10.1038/newbio241172a0. [DOI] [PubMed] [Google Scholar]
- Leclerc J. E., Setlow J. K. Postreplication repair of ultraviolet damage in Haemophilus influenzae. J Bacteriol. 1972 Jun;110(3):930–934. doi: 10.1128/jb.110.3.930-934.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann J., Spingarn N. E., Kobori J., Ames B. N. Detection of carcinogens as mutagens: bacterial tester strains with R factor plasmids. Proc Natl Acad Sci U S A. 1975 Mar;72(3):979–983. doi: 10.1073/pnas.72.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy D., Clayton N. L., Setlow J. K. A plasmid cloning vehicle for Haemophilus influenzae and Escherichia coli. J Bacteriol. 1982 Sep;151(3):1605–1607. doi: 10.1128/jb.151.3.1605-1607.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Mortelmans K. E., Stocker B. A. Segregation of the mutator property of plasmid R46 from its ultraviolet-protecting property. Mol Gen Genet. 1979 Jan 2;167(3):317–327. doi: 10.1007/BF00267425. [DOI] [PubMed] [Google Scholar]
- Notani N. K., Setlow J. K. Inducible repair system in Haemophilus influenzae unaccompanied by mutation. J Bacteriol. 1980 Jul;143(1):516–519. doi: 10.1128/jb.143.1.516-519.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notani N. K., Setlow J. K., McCarthy D., Clayton N. L. Transformation of Haemophilus influenzae by plasmid RSF0885. J Bacteriol. 1981 Dec;148(3):812–816. doi: 10.1128/jb.148.3.812-816.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry K. L., Walker G. C. Identification of plasmid (pKM101)-coded proteins involved in mutagenesis and UV resistance. Nature. 1982 Nov 18;300(5889):278–281. doi: 10.1038/300278a0. [DOI] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E., Beattie K. L., Kimball R. F. A complex of recombination and repair genes in Haemophilus influenzae. J Mol Biol. 1972 Jul 21;68(2):361–378. doi: 10.1016/0022-2836(72)90218-5. [DOI] [PubMed] [Google Scholar]
- Setlow J. K., Brown D. C., Boling M. E., Mattingly A., Gordon M. P. Repair of deoxyribonucleic acid in Haemophilus influenzae. I. X-ray sensitivity of ultraviolet-sensitive mutants and their behavior as hosts to ultraviolet-irradiated bacteriophage and transforming deoxyribonucleic acid. J Bacteriol. 1968 Feb;95(2):546–558. doi: 10.1128/jb.95.2.546-558.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa H., Kato T., Ise T., Makino K., Nakata A. Cloning and characterization of the umu operon responsible for inducible mutagenesis in Escherichia coli. Gene. 1983 Aug;23(2):167–174. doi: 10.1016/0378-1119(83)90048-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinhart W. L., Herriott R. M. Fate of recipient deoxyribonucleic acid during transformation in Haemophilus influenzae. J Bacteriol. 1968 Nov;96(5):1718–1724. doi: 10.1128/jb.96.5.1718-1724.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waleh N. S., Stocker B. A. Effect of host lex, recA, recF, and uvrD genotypes on the ultraviolet light-protecting and related properties of plasmid R46 in Escherichia coli. J Bacteriol. 1979 Feb;137(2):830–838. doi: 10.1128/jb.137.2.830-838.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C., Dobson P. P. Mutagenesis and repair deficiencies of Escherichia coli umuC mutants are suppressed by the plasmid pKM101. Mol Gen Genet. 1979 Apr 17;172(1):17–24. doi: 10.1007/BF00276210. [DOI] [PubMed] [Google Scholar]
- Walker G. C., Elledge S. J., Kenyon C. J., Krueger J. H., Perry K. L. Mutagenesis and other responses induced by DNA damage in Escherichia coli. Biochimie. 1982 Aug-Sep;64(8-9):607–610. doi: 10.1016/s0300-9084(82)80097-7. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Plasmid (pKM101)-mediated enhancement of repair and mutagenesis: dependence on chromosomal genes in Escherichia coli K-12. Mol Gen Genet. 1977 Mar 28;152(1):93–103. doi: 10.1007/BF00264945. [DOI] [PubMed] [Google Scholar]
- Winans S. C., Walker G. C. Conjugal transfer system of the IncN plasmid pKM101. J Bacteriol. 1985 Jan;161(1):402–410. doi: 10.1128/jb.161.1.402-410.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


