Abstract
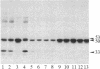
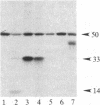
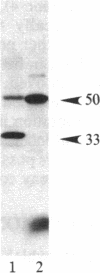
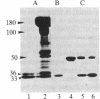
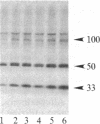
A protein kinase activity was observed in coated vesicles, prepared from bovine brain, that had clathrin-associated protein 2 (CAP2, also known as clathrin light chain 2) as its principal substrate. Coated vesicles were purified by sucrose density gradient centrifugation followed by Sephacryl S-1000 column chromatography, and all buffers utilized in these procedures contained a mixture of proteolysis inhibitors to maintain CAP2 kinase activity. Incubation of vesicles with [gamma-32P]ATP in the presence of 7 microM polylysine resulted in an overall increase in the incorporation of phosphate. NaDodSO4/PAGE revealed that the principal recipient of this additional phosphate was CAP2 (Mr 33,000), the faster-migrating component of the clathrin coat-associated proteins, whereas CAP1 (Mr 36,000) was not phosphorylated. A number of other proteins, in the Mr 140,000 and 100,000 regions, were phosphorylated to a lesser extent. Polyarginine and polyethylenimine also supported CAP2 phosphorylation, but arginine and lysine were ineffective. The phosphorylated protein was identified as CAP2 because addition of exogenous CAPs resulted in increased incorporation of label into Mr 33,000 polypeptides and because heat treatment of labeled vesicles followed by ultracentrifugation resulted in recovery of labeled Mr 33,000 protein in the supernatant. Phosphorylation of CAP2 may play a regulatory role in clathrin coat/coated vesicle functions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell C., Squicciarini J., Shia M., Pilch P. F., Fine R. E. Identification of a protein kinase as an intrinsic component of rat liver coated vesicles. Biochemistry. 1984 Sep 11;23(19):4420–4426. doi: 10.1021/bi00314a028. [DOI] [PubMed] [Google Scholar]
- DeLorenzo R. J., Freedman S. D., Yohe W. B., Maurer S. C. Stimulation of Ca2+-dependent neurotransmitter release and presynaptic nerve terminal protein phosphorylation by calmodulin and a calmodulin-like protein isolated from synaptic vesicles. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1838–1842. doi: 10.1073/pnas.76.4.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgac M., Cantley L., Wiedenmann B., Altstiel L., Branton D. Clathrin-coated vesicles contain an ATP-dependent proton pump. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1300–1303. doi: 10.1073/pnas.80.5.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway G. M., Traugh J. A. Interaction of polyamines and magnesium with casein kinase II. Arch Biochem Biophys. 1984 Aug 15;233(1):133–138. doi: 10.1016/0003-9861(84)90609-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lisanti M. P., Schook W., Moskowitz N., Beckenstein K., Bloom W. S., Ores C., Puszkin S. Brain clathrin: studies of its ultrastructural assemblies. Eur J Biochem. 1982 Jan;121(3):617–622. doi: 10.1111/j.1432-1033.1982.tb05830.x. [DOI] [PubMed] [Google Scholar]
- Lisanti M. P., Schook W., Moskowitz N., Ores C., Puszkin S. Brain clathrin and clathrin-associated proteins. Biochem J. 1982 Feb 1;201(2):297–304. doi: 10.1042/bj2010297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti M. P., Shapiro L. S., Moskowitz N., Hua E. L., Puszkin S., Schook W. Isolation and preliminary characterization of clathrin-associated proteins. Eur J Biochem. 1982 Jul;125(2):463–470. doi: 10.1111/j.1432-1033.1982.tb06706.x. [DOI] [PubMed] [Google Scholar]
- Moskowitz N., Glassman A., Ores C., Schook W., Puszkin S. Phosphorylation of brain synaptic and coated vesicle proteins by endogenous Ca2+/calmodulin- and cAMP-dependent protein kinases. J Neurochem. 1983 Mar;40(3):711–718. doi: 10.1111/j.1471-4159.1983.tb08037.x. [DOI] [PubMed] [Google Scholar]
- Nandi P. K., Irace G., Van Jaarsveld P. P., Lippoldt R. E., Edelhoch H. Instability of coated vesicles in concentrated sucrose solutions. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5881–5885. doi: 10.1073/pnas.79.19.5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauloin A., Bernier I., eJollès P. Presence of cyclic nucleotide-Ca2+ independent protein kinase in bovine brain coated vesicles. Nature. 1982 Aug 5;298(5874):574–576. doi: 10.1038/298574a0. [DOI] [PubMed] [Google Scholar]
- Pauloin A., Jollès P. Internal control of the coated vesicle pp50-specific kinase complex. Nature. 1984 Sep 20;311(5983):265–267. doi: 10.1038/311265a0. [DOI] [PubMed] [Google Scholar]
- Pauloin A., Loeb J., Jollés P. Protein kinase(s) in bovine brain coated vesicles. Biochim Biophys Acta. 1984 Jun 29;799(3):238–245. doi: 10.1016/0304-4165(84)90266-6. [DOI] [PubMed] [Google Scholar]
- Pfeffer S. R., Drubin D. G., Kelly R. B. Identification of three coated vesicle components as alpha- and beta-tubulin linked to a phosphorylated 50,000-dalton polypeptide. J Cell Biol. 1983 Jul;97(1):40–47. doi: 10.1083/jcb.97.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S. R., Kelly R. B. Identification of minor components of coated vesicles by use of permeation chromatography. J Cell Biol. 1981 Nov;91(2 Pt 1):385–391. doi: 10.1083/jcb.91.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi D. F., Schatzman R. C., Mazzei G. J., Turner R. S., Raynor R. L., Liao S., Kuo J. F. Polyamines inhibit phospholipid-sensitive and calmodulin-sensitive Ca2+-dependent protein kinases. Biochem J. 1983 Aug 1;213(2):281–288. doi: 10.1042/bj2130281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury J. L., Condeelis J. S., Maihle N. J., Satir P. Receptor-mediated endocytosis by clathrin-coated vesicles: evidence for a dynamic pathway. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):733–741. doi: 10.1101/sqb.1982.046.01.070. [DOI] [PubMed] [Google Scholar]
- Schlossman D. M., Schmid S. L., Braell W. A., Rothman J. E. An enzyme that removes clathrin coats: purification of an uncoating ATPase. J Cell Biol. 1984 Aug;99(2):723–733. doi: 10.1083/jcb.99.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S. L., Braell W. A., Schlossman D. M., Rothman J. E. A role for clathrin light chains in the recognition of clathrin cages by 'uncoating ATPase'. Nature. 1984 Sep 20;311(5983):228–231. doi: 10.1038/311228a0. [DOI] [PubMed] [Google Scholar]
- Schmid S. L., Matsumoto A. K., Rothman J. E. A domain of clathrin that forms coats. Proc Natl Acad Sci U S A. 1982 Jan;79(1):91–95. doi: 10.1073/pnas.79.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone D. K., Xie X. S., Racker E. An ATP-driven proton pump in clathrin-coated vesicles. J Biol Chem. 1983 Apr 10;258(7):4059–4062. [PubMed] [Google Scholar]
- Usami M., Takahashi A., Kadota K. Protein kinase and its endogenous substrates in coated vesicles. Biochim Biophys Acta. 1984 Apr 24;798(3):306–312. doi: 10.1016/0304-4165(84)90103-x. [DOI] [PubMed] [Google Scholar]
- Usami M., Takahashi A., Kadota T., Katoda K. Phosphorylation of a clathrin light chain of coated vesicles in the presence of histones. J Biochem. 1985 Jun;97(6):1819–1822. doi: 10.1093/oxfordjournals.jbchem.a135243. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B., Lawley K., Grund C., Branton D. Solubilization of proteins from bovine brain coated vesicles by protein perturbants and Triton X-100. J Cell Biol. 1985 Jul;101(1):12–18. doi: 10.1083/jcb.101.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaremba S., Keen J. H. Assembly polypeptides from coated vesicles mediate reassembly of unique clathrin coats. J Cell Biol. 1983 Nov;97(5 Pt 1):1339–1347. doi: 10.1083/jcb.97.5.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]