Lowering levels of peripheral amyloid-β has been proposed as a strategy to reduce plaques in patients with Alzheimer’s disease. Henderson et al. test a modified version of the amyloid-degrading enzyme neprilysin in rats, monkeys and Tg2576 mice. Levels of amyloid-β were reduced in the bloodstream, but not in the CNS.
Keywords: Alzheimer’s disease, peripheral sink hypothesis, amyloid-β, neprilysin
Abstract
Alzheimer’s disease is characterized by the accumulation of amyloid deposits in the brain and the progressive loss of cognitive functions. Although the precise role of amyloid-β in disease progression remains somewhat controversial, many efforts to halt or reverse disease progression have focussed on reducing its synthesis or enhancing its removal. It is believed that brain and peripheral soluble amyloid-β are in equilibrium and it has previously been hypothesized that a reduction in peripheral amyloid-β can lower brain amyloid-β, thereby reducing formation of plaques predominantly composed of insoluble amyloid-β; the so-called peripheral sink hypothesis. Here we describe the use of an amyloid-β degrading enzyme, the endogenous metallopeptidase neprilysin, which is fused to albumin to extend plasma half-life and has been engineered to confer increased amyloid-β degradation activity. We used this molecule to investigate the effect of degradation of peripheral amyloid-β on amyloid-β levels in the brain and cerebrospinal fluid after repeated intravenous dosing for up to 4 months in Tg2576 transgenic mice, and 1 month in rats and monkeys. This molecule proved highly effective at degradation of amyloid-β in the periphery but did not alter brain or cerebrospinal fluid amyloid-β levels, suggesting that the peripheral sink hypothesis is not valid and is the first time that this has been demonstrated in non-human primates.
Introduction
Alzheimer’s disease is a progressive, fatal and incurable neurodegenerative disorder affecting millions of people worldwide. Alzheimer’s disease is characterized by a shrinking of the brain mass and the presence of proteinacious plaques and tangles visible by silver staining. Much of the deposited protein in plaques consists of amyloid-β, a peptide fragment derived from the proteolytic cleavage of amyloid precursor protein (APP). The amyloid cascade hypothesis suggests that one of the main causes of the pathology of Alzheimer’s disease is the accumulation of amyloid-β within the brain leading to synaptic dysfunction and neuronal loss (Hardy and Higgins, 1992; Hardy and Selkoe, 2002; Karran et al., 2011; Schenk et al., 2012).
Recent data suggest that the accumulation of amyloid-β in the brain in sporadic cases of Alzheimer’s disease is due to a reduction in the brain’s ability to clear amyloid-β rather than an increase in the production of amyloid-β through cleavage of APP (Mawuenyega et al., 2010). Amyloid-β can be imported across the blood–brain barrier from the systemic circulation into the brain by the receptor for advanced glycation end-products (RAGE) (Deane et al., 2003) and exported from brain to plasma by Lipoprotein receptor related protein-1 (LRP1) (Shibata et al., 2000). This has contributed to the hypothesis that the central and peripheral pools are in equilibrium. A change in the balance of these two pathways during the ageing process may contribute significantly to the accumulation of amyloid-β (Deane et al., 2004). Amyloid-β is also subject to catabolism by proteolytic enzymes (Hersh, 2003; Nalivaeva et al., 2012) and studies have shown that inhibition of metallopeptidases can result in a substantial increase in the rate of amyloid-β accumulation in the brain (Iwata et al., 2000). The type II membrane metallopeptidase neprilysin (now known as MME, membrane metallo-endopeptidase) functions as an ectoenzyme which catalyses the degradation of a wide range of biologically active peptides including soluble amyloid-β (Roques et al., 1993; Takaki et al., 2000; Shirotani et al., 2001; Turner et al., 2001). Mice deficient in the gene for neprilysin show defects in their ability to degrade both endogenous and exogenously applied amyloid-β (Iwata et al., 2001) and over-expression of neprilysin in the brain reduces amyloid-β (Leissring et al., 2003a; Marr et al., 2003; El-Amouri et al., 2008; Spencer et al., 2011), which suggests an important role for neprilysin in the metabolism of amyloid-β.
Direct reduction of amyloid-β in the brain may not be the only way to modulate the levels of central amyloid-β. Intriguingly it has been shown that administration of agents that can sequester amyloid-β in the periphery can affect the levels of amyloid-β in the brain. Systemic administration of monoclonal antibodies (Bard et al., 2000; DeMattos et al., 2001; Wilcock et al., 2004; Levites et al., 2006; Karlnoski et al., 2009) and other amyloid-β sequestering agents, such as the extracellular domain of LRP1 (Sagare et al., 2007), can result in an apparent efflux of amyloid-β from the brain to the periphery. As most large proteins, including antibodies, are largely excluded from the brain, their observed effects on central amyloid-β are believed to be a result of sequestration of amyloid-β in the periphery thereby promoting efflux of amyloid-β from the brain; the so-called peripheral sink hypothesis (DeMattos et al., 2001; Zhang and Lee, 2011).
To test the peripheral sink hypothesis, we used neprilysin to enzymatically degrade amyloid-β in the periphery, rather than a sequestering agent such as an antibody, and measured the ability of neprilysin to reduce brain amyloid levels in rodents and non-human primates after systemic administration. Amyloid-β degradation was maximized by engineering neprilysin to enhance enzymatic activity against amyloid-β and by prolonging its plasma half-life by fusing it to albumin. Our data indicate that this fusion protein is able to efficiently reduce amyloid-β in the periphery but does not alter brain or CSF amyloid-β levels even after up to 4 months of dosing at levels resulting in sustained depletion of peripheral amyloid-β. Our data therefore extend the recent findings of Walker et al. (2013) using an engineered form of neprilysin in a different strain of transgenic mouse and also in rats and non-human primates.
Materials and methods
Animal studies
Animal studies were conducted at AstraZeneca R&D, Sweden, Ricerca Biosciences SAS, France and Maccine Pte Ltd, Singapore according to protocols reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the testing facility and in compliance with the Guide for the Care and Use of Laboratory Animals (NRC, 1996) and the AstraZeneca Animal Welfare and Bioethics policies.
Repeat dose study in Tg2576 mice
Seventy-two female Tg2576 mice, over-expressing the human APP695 (Swedish) mutation, on a B6; SJL mixed background (strain #1349) (Hsiao et al., 1996) aged 10–12 weeks were purchased from Taconic and acclimatized until 26 weeks of age. Female mice were used in this study because of ethical reasons: mature male mice cannot be group-housed owing to their aggressive nature. Brain amyloid-β content and plaque load were not expected to differ in male and female transgenic mice at the age of animals used (44-weeks-old at termination) (Callahan et al., 2001).
Animals were randomized into two groups (n = 36) and dosed with mouse serum albumin-mouse neprilysin variant fusion protein (MSA-mNEPv) in PBS pH 7.2, at twice weekly intervals for 4 months by slow intravenous bolus injection at 5 ml/kg bodyweight into a tail vein at dose levels of 0 (vehicle control) or 25 mg/kg. Animals were dosed by the intraperitoneal route towards the end of the study if it was difficult to locate a tail vein. Blood was taken from 12 randomized mice per treatment group at 25 and 73 h after the 10th, 18th, 24th and 34th doses such that each mouse was bled only once per month and at termination, 24 h after the final (35th) dose. Blood was collected into pre-chilled tubes containing EDTA and plasma was prepared by centrifugation at 4°C. CSF was aspirated from the cisterna magna under terminal anaesthesia into a pre-chilled tube and diluted 1 in 40 in cold amyloid β ELISA kit buffer (Invitrogen). Brains were removed after in situ perfusion with PBS (pH 7.4 at room temperature) at 1–2 ml/min for 4–5 min. CSF and brain samples were visually inspected for blood contamination and contaminated samples were excluded from exposure analysis and analysis of amyloid-β in CSF. All samples were immediately snap-frozen on dry ice and stored at −70°C. Samples for amyloid-β analysis were kept cold and processed without delay to prevent degradation of amyloid-β ex vivo.
Repeat dose studies in rat
Forty-one male and 41 female Sprague-Dawley rats (Charles River), aged ∼8–10 weeks, were dosed in the morning with human serum albumin-human neprilysin variant fusion protein (HSA-hNepv) in PBS (pH 7.2) at twice weekly intervals (nine doses in total) by slow intravenous bolus injection (over 2 min) at dose levels of 0 (vehicle control), 5, 50 and 143 mg/kg. The top dose level was the maximum possible at the supplied concentration at a dose volume of 3 ml/kg. Animals were acclimatized for a minimum of 2 weeks before dosing. Each treated group contained five male and five female main study animals for assessment of toxicity endpoints and sporadic exposure and plasma amyloid-β1–40 measurements and separate groups of six male and six female satellite animals were used to obtain full exposure and plasma amyloid-β1–40 profiles due to restrictions in the total volume of blood that can be taken from an individual rat. Blood samples were obtained from three male and three female satellite animals prior to and at 1, 6, 24 and 72 h (HSA-hNEPv-treated animals) or 24 and 72 h (vehicle controls) after the first and last doses. Blood samples were obtained from all 10 main study animals per group prior to and 1 h after the seventh dose on Day 21 and at termination, 4 days after the final dose (Day 32). Blood was collected into tubes containing EDTA. All animals were sacrificed 4 days after the last dose and CSF was collected from the cisterna magna of all animals under isoflurane anaesthesia immediately before termination.
Blood and CSF were collected in tubes stored on wet ice, centrifuged within 15 min (blood) or 5 min (CSF) of collection and the resultant plasma and CSF for amyloid-β1–40 analysis was frozen within 5 min of the end of centrifugation to prevent degradation of amyloid-β ex vivo. At termination of the study, brain hemispheres were taken from the main study animals only, immediately snap-frozen and stored at −70°C.
Repeat dose study in monkeys
The effect of HSA-hNepv on amyloid-β in plasma, CSF and brain of cynomolgus monkeys (Macaca fascicularis) was studied after repeated dosing as part of a toxicity study. Twelve male monkeys (bodyweight range 2.3–2.7 kg) were housed in groups of three and fed on a daily diet of monkey chow supplemented with fruit or vegetables. Animals were acclimatized for a minimum of 4 weeks in the primate unit before dosing.
Animals were randomized into four groups of three and dosed with HSA-hNepv in PBS (pH 7.2) in the morning at twice weekly intervals for a month (nine doses in total) by slow intravenous bolus injection (over 2 min) at dose levels of 0 (vehicle control), 5, 50 and 143 mg/kg and a dose volume of 3 ml/kg bodyweight. Blood samples were collected into tubes containing EDTA before and at 5 min, 1, 6, 24, 48 and 72 h after the first dose; immediately before the fourth dose; at 1 h after the fourth and sixth dose; immediately before the seventh dose; and immediately before and at 5 min, 1, 6, 24, 48 and 72 h after the final (ninth) dose. The final dose was administered to all animals by intravenous infusion over 10 min to avoid a repeat of an anti-drug antibody-mediated acute anaphylactic reaction, which occurred in one animal after the eighth dose.
All animals were sacrificed 3 days after the last dose and CSF was collected from the cisterna magna under isoflurane anaesthesia immediately before termination. Red cells and free haemoglobin were measured in a portion of each CSF sample to ensure minimal contamination with blood. At termination, a portion of prefrontal cortex, temporal cortex and hippocampus was immediately snap-frozen and stored at −70°C.
Blood and CSF samples were collected and processed as described above.
Repeat dose study with in-life cerebrospinal fluid sampling in monkeys
The effect of HSA-hNepv on amyloid-β levels was measured in plasma and CSF taken longitudinally over the course of a 1 month repeat dose study. Eighteen male cynomolgus monkeys (bodyweight range 2.5–3.7 kg) were fed on a diet of monkey chow and fruits offered twice daily. Animals were acclimatized for a minimum of 7 days in the animal room, sedated with ketamine and intubated under isoflurane anaesthesia while an indwelling catheter was surgically implanted into the cisterna magna and connected to a subcutaneous access port to facilitate in-life collection of CSF samples. Animals were allowed a minimum 14-day recovery period after surgery before commencement of dosing. Animals were randomized into three groups of six and dosed in the morning with HSA-hNEPv in PBS (pH 7.2) at twice weekly intervals for 1 month (eight doses in total) by slow intravenous infusion (over 15 min) at dose levels of 0 (vehicle control), 5 and 50 mg/kg and a dose volume of 5 ml/kg bodyweight.
Blood samples were collected into tubes containing EDTA before and at 5 min, 1, 12 and 24 h after the first dose; immediately before and at 1 h after the third and sixth dose; and immediately before and at 1 and 24 h, 2, 6, 10 and 14 days after the final dose.
CSF samples were taken from the indwelling catheter at the same times as blood sampling except that a CSF sample was not taken 5 min after the first dose. A volume of CSF equivalent to the dead volume (∼0.15 ml) was removed and discarded before collection of each sample, which was visually inspected for blood contamination. Animals were sacrificed 14 days after the last dose. Blood and CSF samples were collected and processed as described above. One control animal and one low dose (5 mg/kg) animal tested positive for Mycobacterium tuberculosis at the end of the study so plasma and CSF amyloid-β samples were not analysed from these animals.
Extraction of amyloid-β from brain
Soluble amyloid-β was extracted from frozen brain hemisphere (rats) or hippocampus (monkeys) by homogenization in 0.2% diethylamine (DEA) and 50 mM NaCl before centrifugation at 4°C for 1 h. Supernatants were neutralized to pH 8.0 with 2 M Tris-HCl, snap-frozen and stored at −80°C.
Insoluble amyloid-β from mouse brain was extracted from the pellet remaining after extraction of soluble amyloid-β by sonication in 70% formic acid (2 × 10 s) before centrifugation at 4°C for 1 h. Supernatants were neutralized to pH 7.5 with 1 M Tris-HCl, snap-frozen and stored at −80°C.
Measurement of amyloid-β
Amyloid-β1–40 and amyloid-β1–42 in mouse and rat matrices were measured using qualified ELISA kits for mouse amyloid-β1–40 or amyloid-β1–42 (Invitrogen) with demonstrated cross-reactivity to rat amyloid-β. Amyloid-β1–42 is undetectable in rat plasma therefore levels were only measured in brain.
Amyloid-β1–40 and amyloid-β1–42 in monkey matrices were measured using qualified ELISA kits for human amyloid-β1–40 (Invitrogen hAβ40 ELISA) or amyloid-β1–42 [Innotest βamyloid (1-42) ELISA; Innogenetics] with demonstrated cross-reactivity to cynomolgus monkey. Amyloid β1–42 was measured in monkey plasma, CSF and brain but amyloid-β1–40 was only measured in monkey CSF and brain.
In all cases, the lower limit of quantification was determined for each immunoassay plate based on the lowest standard point having a coefficient of variation <20% and an accuracy (back-calculated concentration) of 80–120%.
Drug measurements
Mouse serum albumin-mouse neprilysin variant fusion protein (MSA-mNEPv) in mouse plasma was measured by ELISA. A microtitre plate was coated with anti-mNEP antibody (R&D Systems) and incubated overnight at 4°C. Plates were blocked before sample application and washed before addition of biotinylated anti-mNEP detection antibody (R&D Systems). Plates were then washed, incubated with streptavidin-horseradish peroxidase (R&D Systems), washed again and TMB (3,3′,5,5′-tetramethylbenzidine) substrate (Invitrogen) added. The reaction was stopped and absorbance read at 450 nm.
MSA-mNEPv in mouse brain was extracted as follows: one hemisphere from each mouse was transferred to homogenizing tubes containing 1.4 mm zirconium oxide beads (CK14, Bertin Technologies) and PBS containing protease inhibitor cocktail (Complete™, Roche diagnostics) at a ratio of 3:1 (3 µl of PBS: 1 mg brain). Samples were homogenized and centrifuged for 1 h at 4°C. The supernatant, containing the parenchymal fraction, was then removed and stored at −80°C for subsequent analysis.
MSA-mNEPv in mouse CSF and brain homogenate, and HSA-hNEPv in rat plasma and CSF or monkey plasma was measured using qualified sandwich ELISA’s using a GyroLab assay platform as follows: biotinylated anti-mouse or anti-human neprilysin monoclonal antibody (anti-mNEP or anti-hNEP, respectively) was captured on streptavidin-coated columns and incubated with pre-diluted test samples, standards and controls. MSA-mNEPv/HSA-hNEPv was detected by an antibody labelled with Alexa Fluor® 647 and quantified by fluorescence. The amount of fluorescence was proportional to the amount of drug in the sample.
Amyloid precursor protein analysis
Cynomolgus prefrontal cortex homogenates were prepared as described previously (Tamayev et al., 2011). Briefly, brain sections from monkeys dosed at 0, 5, 50 and 143 mg/kg in the repeat dose study were homogenized in a 1:2 (w/v) ratio with 20 mM HEPES (pH 7.4), 1 mM EDTA, 1 mM EGTA and 250 mM sucrose, supplemented with protease and phosphatase inhibitors. Supernatants containing 150 µg total protein were resolved by SDS-PAGE, transferred to polyvinylidene difluoride membrane and proteins detected using anti-APP (Abcam ab15272) and anti-actin (Abcam 3280) primary antibodies and appropriately labelled secondary antibodies.
Statistical analysis
All results are expressed as means ± SEM, unless otherwise indicated. To compare dose groups, we used an unpaired, two tailed, t-test with a 95% confidence interval (GraphPad Prism, GraphPad Software). The results were considered statistically significant at P < 0.05.
Results
Depletion of amyloid-β in plasma, cerebrospinal fluid and brain of Tg2576 mice
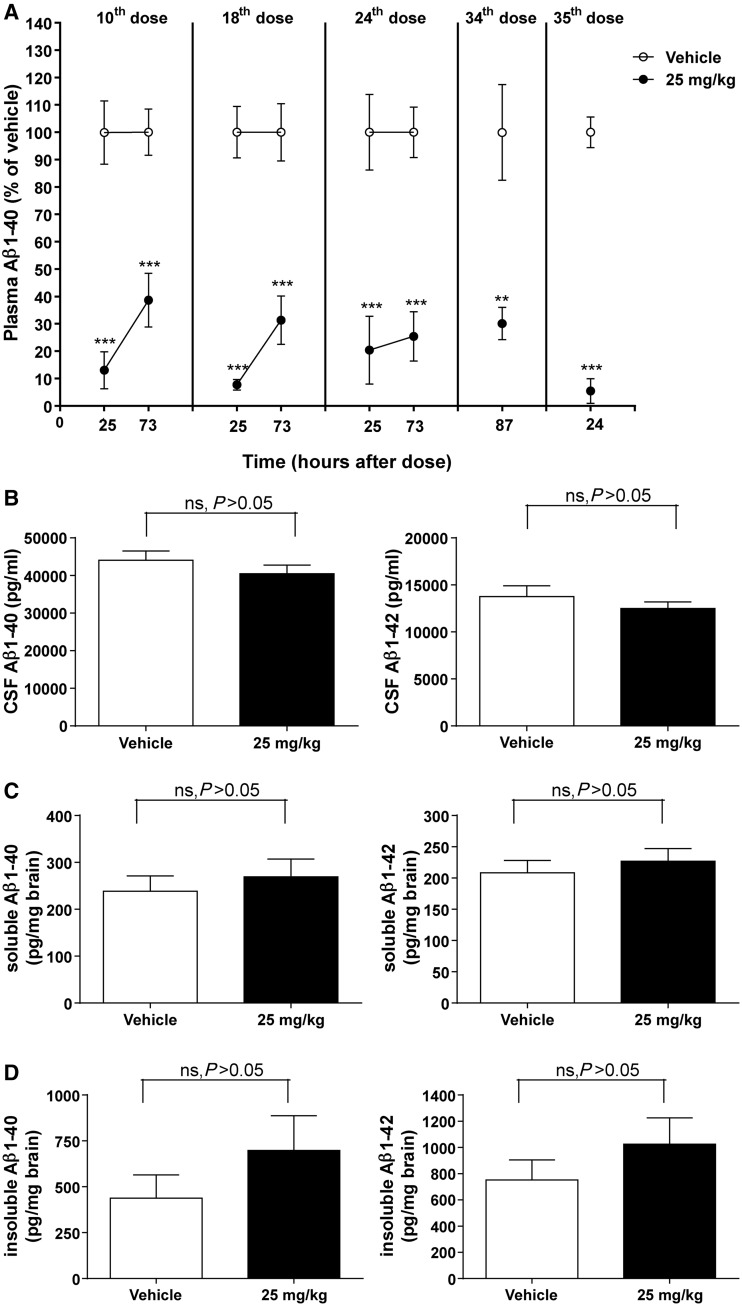
It was not possible to study the effects of long-term administration of HSA-hNEPv in mice because of a profound immunogenic response that manifested 1–2 weeks after repeated dosing. As a consequence, a mouse surrogate protein, MSA-mNEPv, was used. The increase in soluble and insoluble forms of amyloid-β peptides in the brain of APPswe Tg2576 mice is exponential from the age of 6 months, therefore, we performed repeated intravenous (slow bolus) dosing twice a week with 25 mg/kg MSA-mNEPv for 4 months in female mice that were 6 months old at the start of the study. This resulted in exposures similar to that predicted on the basis of a single dose (Supplementary material). The treatment decreased amyloid-β1–40 in plasma by 61–95% throughout the duration of the study (Fig. 1A). Amyloid-β1–42 levels were only measured at termination (24 h after the last dose) because of blood volume limitations but had decreased by 93% compared with vehicle (data not shown). This is similar to the reduction in amyloid-β1–42 (96% compared with vehicle) observed at this time.
Figure 1.
Amyloid-β1–40 (Aβ1–40) and/or amyloid-β1–42 (Aβ1–42) concentrations in plasma, CSF and brain of female Tg2576 mice after twice-weekly intravenous administration of MSA-mNEPv (0 and 25 mg/kg) for 4 months. (A) Mean (± SEM) amyloid-β1–40 concentrations in plasma (expressed as per cent of mean vehicle concentration at the equivalent time) after 5 weeks (10th dose), 9 weeks (18th dose), 12 weeks (24th dose) or 18 weeks (34th and 35th dose) of treatment (open circle = vehicle, filled circle = MSA-NEPv 25 mg/kg). Amyloid-β1–40 concentrations in vehicle-treated animals ranged from 520 to 9400 pg/ml. Samples were taken from 10–12 of 34–36 mice per treatment group at each time point in a rolling scheme such that each mouse was bled only once a month except at termination (24 h after the 35th dose) where all animals were sampled. ***P < 0.001; **P < 0.01. (B) Mean (± SEM) amyloid-β1–40 (left) and amyloid-β1–42 (right) concentrations in CSF. Samples were taken 24 h after the final dose. Data only presented for samples without blood contamination (n = 14–19 per group). ns = not statistically significant. (C) Mean (± SEM) soluble amyloid-β1–40 (left) and amyloid-β1–42 (right) concentrations in brain at 24 h after the final dose (n = 33–34 per group). (D) Mean (± SEM) insoluble amyloid-β1–40 (left) and amyloid-β1–42 (right) concentrations in brain at 24 h after the final dose (n = 33–34 per group).
After 4 months of treatment, and despite the consistent reduction of amyloid-β in plasma, no decrease in levels of CSF amyloid-β (Fig. 1B) or soluble and insoluble amyloid-β in the brain (Fig. 1C and D) was observed. The concentration of MSA-mNEPv in CSF was determined at the end of the 4-month treatment and found to be 0.2 ± 0.2 µg/ml (mean ± SD, n = 13), whereas brain exposure was 58 ± 40 ng/ml (mean ± SD, n = 5). The mean levels of MSA-mNepv in CSF and brain were, 0.2% and <0.1% of the mean concentrations measured in plasma at termination indicating minimal penetration of the blood–brain barrier.
Depletion of amyloid-β in plasma, cerebrospinal fluid and brain of rats
Studies were conducted in healthy rats with normal physiological levels of amyloid-β to understand how neprilysin-induced reduction of peripheral amyloid-β impacted levels in the brain and CSF compartments of a non-transgenic rodent. HSA-hNEPv exhibited dose-proportional pharmacokinetics in the rat when administered by the intravenous route at dose levels of 5, 50 and 143 mg/kg twice weekly for 1 month (data not shown).
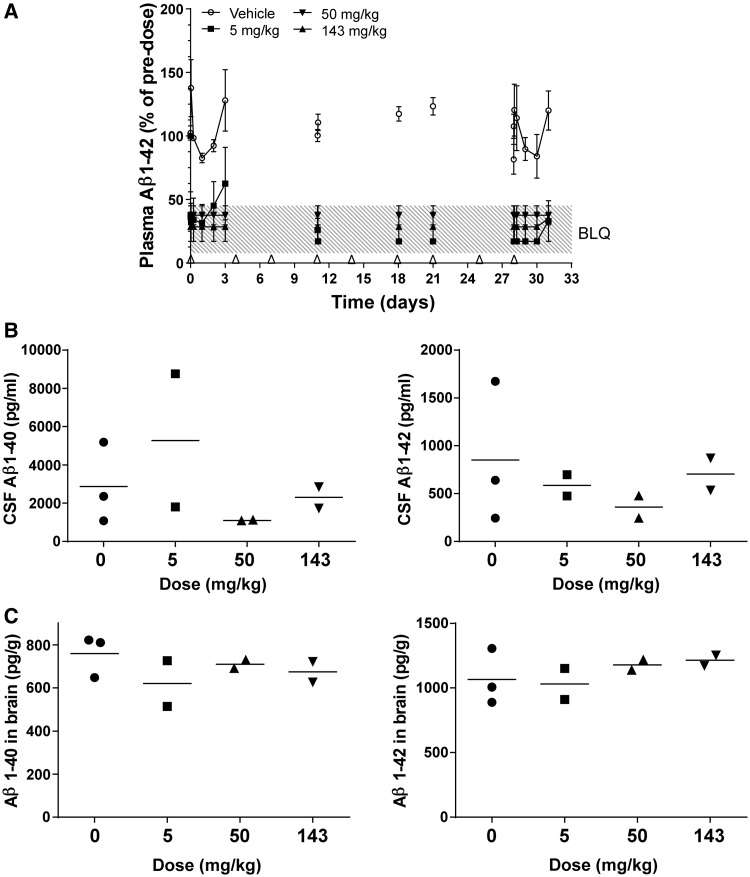
Dose-dependent depletion of plasma amyloid-β1–40 was observed after intravenous (slow bolus) administration of HSA-hNEPv to male and female rats at dose levels of 5, 50 and 143 mg/kg twice weekly for a month (Fig. 2A and B). After the first dose at 5 mg/kg, mean plasma amyloid-β1–40 levels decreased to below the limit of accurate quantification (<16 pg/ml) for up to 24 h before a return towards baseline after 3 days. At this dose level, plasma amyloid-β1–40 was also at baseline levels before dosing on Day 21, before the final dose and in all animals at the time of termination, 4 days after the last dose.
Figure 2.
Soluble amyloid-β1–40 (Aβ1–40) and/or amyloid-β1–42 (Aβ1–42) concentrations in plasma, CSF and brain of male and female rats after twice-weekly intravenous administration of HSA-hNEPv (0, 5, 50 and 143 mg/kg) for 1 month. All plots include animals that were positive for anti-drug antibodies at the end of the study. ns = not statistically significant. (A) Mean (± SEM) amyloid-β1–40 concentrations in plasma (expressed as per cent of pre-dose). Arrows denote times of dosing prior to collection of samples for analysis. Amyloid-β levels on Days 21 and 28 are shown pre- and post-dose to illustrate the degree of suppression during the study. Pre-dose plasma amyloid-β1–40 concentrations ranged from 30–100 pg/ml [n = 6–10, 10–12, 10–11 and 10–11 animals/time point for 0 (open circle), 5 (filled square), 50 (filled triangle) and 143 (open triangle) mg/kg, respectively]. ***P < 0.001, **P < 0.01, *P < 0.05. (B) Amyloid-β 1–40 concentrations in plasma of individual animals (expressed as pg/ml) at termination (Day 32; n = 10 per group, bar represents mean). **P < 0.01. (C) Amyloid-β1–40 concentrations in CSF of individual animals at termination (Day 32). Filled squares refer to animals that tested negative for anti-drug antibody and open squares refer to animals that were positive for anti-drug antibody (n = 15, 20, 18 and 18 for 0, 5, 50 and 143 mg/kg, respectively; bar represents mean). (D) Soluble amyloid-β1–40 (left) and soluble amyloid-β1–42 (right) concentrations in brain of individual main study animals at termination (Day 32). Filled squares refer to animals that tested negative for anti-drug antibody and open squares refer to animals that were positive for anti-drug antibody (n = 10 per group, bar represents mean). BLQ = below the limit of accurate quantification.
After the first dose at 50 mg/kg, plasma amyloid-β1–40 remained below the limit of accurate quantification in the majority of animals for the first 3 days, and in about half the animals before dosing on Day 21 and before the final dose. After the final dose in the 50 mg/kg group, the mean plasma amyloid-β1–40 concentration was initially reduced to below the limit of accurate quantification, but then returned towards the baseline after 3 days and had normalized in 8 of 10 animals in which it was measured at termination. Hence, amyloid-β was not fully depleted for the entire duration of the study in any animal at the lowest dose level and in the majority of animals at the intermediate dose level.
However, after dosing at 143 mg/kg, the plasma amyloid-β1–40 concentrations were below the limit of accurate quantification for the first 3 days after the first dose, in 8 of the 10 main study animals before Day 21, in five out of six satellite animals sampled before the last dose, and in 6 of the 10 main study animals at termination, 4 days after the last dose. This indicates that plasma amyloid-β1–40 levels were fully depleted in the majority of animals at the high dose level for the entire duration of the study. In those animals with incomplete amyloid-β1–40 depletion there was a correlation with low plasma concentrations of HSA-hNEPv, probably due to the presence of anti-drug antibodies that were detected at the end of the study in most of the animals dosed at 5 and 50 mg/kg and about half of the animals dosed at 143 mg/kg (data not shown). Figure 2A illustrates the mean concentrations for all animals, including those that developed anti-drug antibodies. Two satellite animals (one dosed at 50 mg/kg and one dosed at 143 mg/kg) had anomalously high plasma amyloid-β1–40 concentrations (up to 300 pg/ml) and/or were unaffected by treatment so data from these animals have been omitted from the analysis.
Despite substantial depletion of plasma amyloid-β1–40 for a month in the majority of animals dosed at 143 mg/kg, no significant reduction in amyloid-β1–40 in CSF or amyloid-β1–40 and amyloid-β1–42 in brain was observed at termination, 4 days after the last dose (Fig. 2C and D). Similarly, no dose-response was observed in CSF or brain amyloid-β at dose levels of 5, 50 or 143 mg/kg, even in animals that did not generate anti-drug antibodies during the course of the study. The concentrations of HSA-hNEPv in CSF at the end of the study were <0.5% of plasma exposure.
Depletion of amyloid-β in plasma, cerebrospinal fluid and brain of monkeys
Pharmacokinetic parameters observed in repeat-dose studies with HSA-hNEPv in monkeys were in agreement of those in single dose studies (Supplementary material). Depletion of plasma amyloid-β1–42 was observed after twice-weekly intravenous administration of HSA-hNEPv to male cynomolgus monkeys at dose levels ranging from 5 to 143 mg/kg for a month (Fig. 3A). Reduced plasma exposures to HSA-hNEPv were observed in one of the three animals in each treated group by the end of the study. This correlated with increases in circulating amyloid-β, and as amyloid-β in CSF and brain was only measured at termination in this study, only data from animals that maintained exposures for the entire month of treatment are presented. After the first dose at 5 mg/kg, the mean plasma amyloid-β1–42 concentration decreased to below the limit of accurate quantification (≤8 or 16 pg/ml) immediately after dosing but recovered to baseline by 72 h post-dose. Complete plasma amyloid-β1–42 depletion was, however, observed after subsequent repeated dosing at 5 mg/kg. Plasma amyloid-β1–42 levels were below the limit of accurate quantification throughout the entire duration of the study for all animals dosed at 50 and 143 mg/kg except one high dose animal where amyloid-β1–42 recovered to 30% of its pre-dose value by 3 days after the final dose. However, despite complete depletion of plasma amyloid-β1–42 throughout the study until the time of termination in three animals, no reduction of amyloid-β1–40 or amyloid-β1–42 in CSF or brain was observed at termination (Fig. 3B and C).
Figure 3.
Amyloid-β1–40 (Aβ1–40) and/or amyloid-β1–42 (Aβ1–42) concentrations in plasma, CSF and brain of male cynomolgus monkeys after twice-weekly intravenous administration of HSA-hNEPv (0, 5, 50 and 143 mg/kg) for 1 month. Only animals that maintained exposure to HSA-hNEPv to the end of the study (i.e. that did not develop clearing antibodies) are included (n = 3 animals/time point at 0 mg/kg and n = 2 at 5, 50 and 143 mg/kg). (A) Mean (± SEM) amyloid-β1–42 concentrations in plasma (expressed as per cent of pre-dose) at 0 (open circle), 5 (filled square), 50 (filled inverted triangle), and 143 (filled triangle) mg/kg. Arrows denote times of dosing. Amyloid-β levels on Day 11 are shown pre- and post-dose to illustrate the degree of suppression during the study. Pre-dose plasma amyloid-β1–42 concentrations ranged from 20–100 pg/ml. (B) Amyloid-β 1–40 (left) and amyloid-β1–42 (right) concentrations in CSF of individual animals at termination (Day 31, bar represents mean). (C) Soluble Aβ1–40 (left) and soluble amyloid-β1–42 (right) concentrations in brain of individual animals at termination (Day 31, bar represents mean). BLQ = below the limit of accurate quantification.
Depletion of amyloid-β in monkeys with in-life cerebrospinal fluid sampling
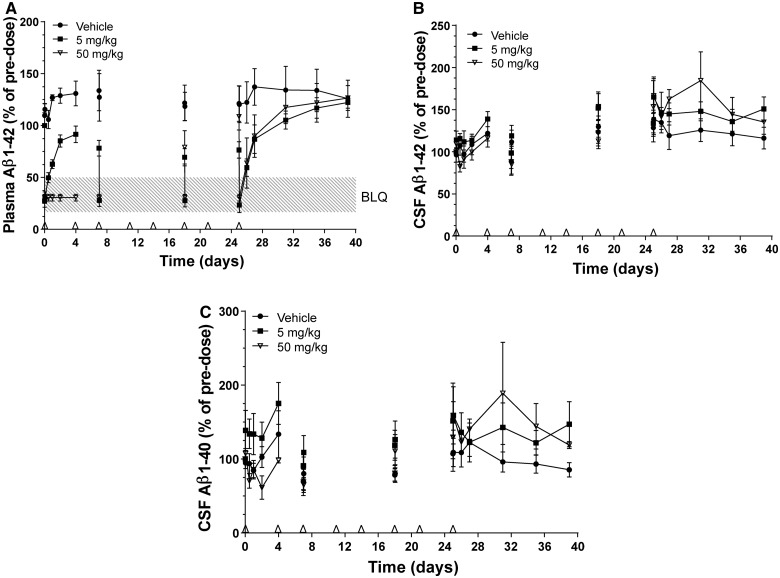
Dose-dependent depletion of plasma amyloid-β1–42 was observed after twice-weekly intravenous infusion (over 15 min) of HSA-hNEPv to male cynomolgus monkeys at dose levels of 5 and 50 mg/kg for 1 month (Fig. 4A). Plasma exposures to HSA-hNEPv were maintained in all animals for the first week but by 18 days after the first dose, two of the six animals dosed at 5 mg/kg and five of the six animals dosed at 50 mg/kg exhibited markedly reduced exposures presumably due to anti-drug antibody formation. One animal had plasma amyloid-β1–42 concentrations (∼100–160 pg/ml) that were ∼5-fold greater than all other animals and HSA-hNEPv at 50 mg/kg failed to reduce circulating amyloid-β. As a consequence, data from this animal have been omitted from this analysis.
Figure 4.
Amyloid-β1–40 (Aβ1–40) and/or amyloid-β1–42 (Aβ1–42) concentrations in plasma and CSF of male cynomolgus monkeys with implanted CSF catheters after twice-weekly intravenous administration of HSA-hNEPv (0, 5 and 50 mg/kg) for 1 month. Arrows denote times of dosing. Amyloid-β levels on Days 7, 18 and 25 are shown pre- and post-dose to illustrate the degree of suppression during the study. All plots include animals where exposure was reduced presumably as a consequence of development of anti-drug antibodies. (A) Mean (± SEM) amyloid-β1–42 concentrations (expressed as per cent of pre-dose) in plasma after dosing of HSA-NEPv at 0 (filled circle), 5 (filled square) and 50 (open inverted triangle) mg/kg. Pre-dose plasma amyloid-β1–42 concentrations ranged from 20–50 pg/ml. (B) Mean (± SEM) amyloid-β1–42 concentrations in CSF after dosing HSA-NEPv at 0 (filled circle), 5 (filled square) and 50 (open inverted triangle) mg/kg. Pre-dose CSF Aβ1–42 concentrations ranged from 300–1000 pg/ml. (C) Mean (± SEM) amyloid-β1–40 concentrations in CSF after dosing at 0 (filled circle), 5 (filled square), and 50 (open inverted triangle) mg/kg. Pre-dose CSF amyloid-β1–40 concentrations ranged from 400–3500 pg/ml.
Plasma amyloid-β1–42 concentrations in animals dosed at 5 mg/kg decreased to below the limit of accurate quantification within 1 h of dosing and then recovered to pre-dose levels within 4 days in almost all animals. The magnitude of amyloid-β1–42 depletion increased with repeated doses yielding concentrations below the limit of accurate quantification within 1 h after the third, sixth and eighth dose although incomplete depletion (∼30%) was evident immediately before these doses. After the final dose, amyloid-β1–42 levels recovered to pre-dose values within ∼6 days.
At 50 mg/kg, plasma Aβ1–42 levels were depleted to below the limit of accurate quantification within 1 h of administration and remained below the limit throughout the first dosing interval of 4 days in all animals and immediately before the third dose (Day 7). However, before the sixth dose (Day 18), incomplete amyloid-β1–42 depletion was observed in four of five animals. This correlated with reduced exposure in these animals (data not shown), probably because of immunogenicity, and suggests that amyloid-β1–42 was only fully depleted for 1–3 weeks in most animals. Complete depletion of plasma amyloid-β1–42 was, however, observed in one animal treated at 50 mg/kg throughout the entire duration of the study and levels did not return to pre-dose values until 6 days after the final dose.
The concentrations of amyloid-β1–40 and amyloid-β1–42 in CSF throughout the study are illustrated in Fig. 4B and C. Despite variability in CSF levels in all dose groups, no depletion occurred, even during the first week at which time plasma amyloid-β1–42 levels were fully depleted in all animals dosed at 50 mg/kg. Furthermore, no depletion of amyloid-β1–40 or amyloid-β1–42 was observed in CSF from the animal dosed at 50 mg/kg which exhibited complete depletion of plasma amyloid-β1–42 for a month.
Amyloid precursor protein expression in response to neprilysin treatment
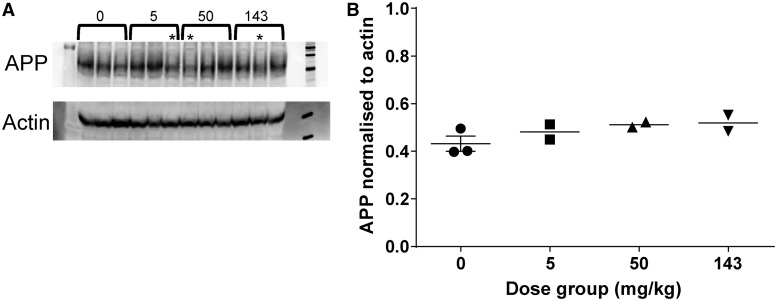
Western blot analysis of protein extracts from the prefrontal cortex of the cynomolgus monkeys dosed twice weekly with HSA-NEPv at 0, 5, 50, and 143 mg/kg for 1 month demonstrated no change that could be attributed to treatment (Fig. 5).
Figure 5.
Expression of APP in male cynomolgus monkeys after twice-weekly intravenous administration of HSA-hNEPv (0, 5, 50 and 143 mg/kg) for 1 month. (A) Protein extract from frontal cortex samples taken 3 days after the last dose (Day 31) analysed by western blot probed with anti-APP and anti-actin. Dose level (mg/kg) is indicated, asterisk indicates animals that lost exposure to the drug because of immunogenicity. (B) APP expression levels normalized to the signal from anti-actin. Signal intensity for APP and actin in the western blot were quantified and the APP signal divided by the actin signal. Only animals which maintained exposure to HSA-hNEPv to the end of the study (i.e. which did not develop clearing antibodies) are included (n = 3 at 0 mg/kg and n = 2 at 5, 50 and 143 mg/kg). Bar represents mean.
Discussion
The peripheral sink hypothesis postulates that peripheral reduction of amyloid-β levels should lead to reduced brain or CSF amyloid-β levels (DeMattos et al., 2001; Zhang and Lee, 2011). In this context we have undertaken a series of studies to investigate the potential of the metallopeptidase neprilysin to lower peripheral amyloid-β and monitored its impact on amyloid-β levels in brain and CSF. Neprilysin has received attention as a potential therapeutic for Alzheimer’s disease because of its ability to degrade amyloid peptides (Howell, 1995; Iwata et al., 2000; Takaki et al., 2000) and several studies have shown that amyloid-β levels in the circulation and brains can be lowered in animals where neprilysin expression is increased (Leissring et al., 2003a; Marr et al., 2003; Iwata et al., 2004; Hong et al., 2006; Hemming et al., 2007). Despite these positive results, neprilysin actually has a relatively poor catalytic activity for degradation of amyloid-β (Leissring et al., 2003b). We therefore engineered human neprilysin to increase its activity resulting in a variant, hNEPv, with a ∼10-fold improvement in its ability to degrade amyloid-β and the concentration of enzyme required to achieve significant depletion of amyloid-β in vitro and in vivo (Supplementary material). The plasma half-life of soluble neprilysin is short, therefore, to evaluate the potential of NEPv to lower peripheral amyloid-β for a prolonged period without frequent dosing, we extended serum half-life while retaining its catalytic activity by fusing it to serum albumin. We have used this fusion protein to investigate its ability to lower amyloid in the peripheral and central compartments in Tg2576 transgenic mice, wild-type rats and cynomolgus monkeys.
In a preliminary repeat dose study with twice weekly intraperitoneal dosing in Tg2576 mice, the human fusion protein, HSA-hNEPv, elicited a profound immunogenic response in a large number of animals and it was not possible to continue the study (data not shown). We therefore engineered an alternative molecule with similar enzymatic properties to HSA-hNEPv but with lower immunogenic potential. As mouse and human neprilysin share a high sequence homology (94%) we postulated that the same two mutations that improved the activity of human neprilysin for amyloid-β would have a similar effect on the mouse molecule. We therefore generated mouse neprilysin with the same mutations as the human variant and characterized its ability to degrade amyloid-β. This variant exhibited increased degradation activity compared with the wild-type mouse enzyme (Supplementary material) and was suitable for chronic dosing in mice.
We found that twice weekly intravenous administration of MSA-mNEPv (25 mg/kg) to Tg2576 mice resulted in a sustained reduction of amyloid-β in the plasma for 4 months, but did not result in statistically significant changes in the amyloid-β levels in CSF or soluble and insoluble amyloid-β in the brain. This is in agreement with the recent study by Walker et al. (2013) where treatment of wild-type mice for up to 35 days with a peripherally administered neprilysin-Fc fusion protein resulted in efficient degradation of plasma amyloid-β but no changes in the levels of soluble amyloid-β in brain, or insoluble amyloid-β in brains of APP23 transgenic mice treated for 3 months.
The Tg2576 mice used for our study have significantly elevated levels of amyloid-β compared with healthy wild-type mice and other mammals and given the slightly lower amyloid-β degrading activity of the mNEPv molecule compared with hNEPv, there was still the possibility that mNEPv was not able to reduce peripheral amyloid-β sufficiently to cause a statistically significant reduction in brain amyloid-β levels. We therefore initiated studies with HSA-hNEPv to investigate whether these findings translated to wild-type rats and non-human primates that have peripheral amyloid-β levels more similar to those found in patients with Alzheimer’s disease. We found that in both these species, there was evidence of anti-drug antibody formation in many, but not all, animals treated with HSA-hNEPv after repeated dosing, but that these rarely caused the severe anaphylactic responses seen in mice. Despite the immunogenicity, there was a substantial reduction of mean plasma amyloid-β1–40 in rats dosed twice weekly by the intravenous route at 50 and 143 mg/kg for the entire duration of the 1 month study and amyloid-β1–40 remained fully depleted in most of the animals at the high dose level even at 4 days after the last dose. In cynomolgus monkeys dosed intravenously with HSA-NEPv at 50 and 143 mg/kg twice weekly for 1 month, three animals had plasma amyloid-β levels below the limit of quantification throughout the study. However, in both rats and cynomolgus monkeys, amyloid-β levels in CSF and brain were found to be unaltered. To rule out the possibility that degradation of amyloid-β stimulates a compensatory increase in amyloid-β production by the brain, we measured APP in the prefrontal cortex of the monkeys dosed with HSA-hNEPv twice weekly at 0, 5, 50 and 143 mg/kg for 1 month. No treatment-related changes in APP were detected, thereby ruling out an increase in APP expression as a reason for the lack of effect on brain and CSF amyloid-β levels.
Amyloid-β levels in the CSF of cynomolgus monkeys are particularly variable (Jeppsson et al., 2012) and because of the small numbers of animals used in this study it is difficult to draw any conclusions about the effect of HSA-NEPv on CSF levels of amyloid-β. It is also possible that in these rat and monkey studies, the delay in taking CSF and brain samples until several days after the last dose resulted in recovery of central levels of amyloid-β, thereby masking any effect the drug may have had during its peak effect shortly after dosing. To investigate this possibility we undertook a further study in cynomolgus monkeys where the animals were fitted with a catheter into the cisterna magna facilitating CSF sampling at times consistent with peak drug effect. Monkeys were dosed twice-weekly by intravenous infusion of HSA-hNEPv at dose levels of 5 and 50 mg/kg for 1 month and blood and CSF were taken at the same time points to enable a direct comparison to be made between peripheral and central effects. The amyloid-β1–42 levels in plasma remained below the level of quantification for at least 7 days after the first dose for five animals dosed at 50 mg/kg but thereafter, most animals developed anti-drug antibodies such that even with repeated dosing, peripheral amyloid-β reduction was transient and only remained fully depleted for the entire month of treatment in one animal. However, there was no parallel reduction in CSF levels of amyloid-β1–40 or amyloid-β1–42 in any animal during the first week, or in the single animal in which full depletion of amyloid-β1–42 had occurred for a month. These data suggest there is either no equilibrium, or at least a significantly delayed equilibrium, between the central and peripheral pools of amyloid-β and that depletion in the periphery does not influence central amyloid-β levels. This is the first time that the levels of amyloid-β have been measured simultaneously in plasma and CSF and that peripheral depletion has been shown to have no effect on central levels of amyloid-β in non-human primates. While there remains the possibility that amyloid-β exported from the brain is somehow protected from degradation by HSA-NEPv, for example by complexation to a binding protein, such a population of amyloid-β has not previously been reported.
At first sight these data contrast with studies showing that systemically administered antibodies capable of binding and sequestering amyloid-β in the periphery are able to reduce central amyloid-β (DeMattos et al., 2001). However, it is possible that the small proportion (∼0.1%) of antibody, or other amyloid-β sequestering agent, which crosses the blood–brain barrier may be solely responsible for the reduction in central amyloid-β because of their high affinity for amyloid-β (Bard et al., 2000). In addition, the observation that IgGs are rapidly transported from brain to blood across the blood–brain barrier (Zhang and Pardridge, 2001) may explain how amyloid-β is transported out of the brain. The mechanism of antibody transport across the blood–brain barrier is not fully characterized but the neonatal receptor FcRn, which is expressed at the blood–brain barrier (Schlachetzki et al., 2002), seems to be essential for efflux of IgG from brain to plasma (Deane et al., 2005). In separate studies we have confirmed that peripheral administration of antibodies that sequester amyloid-β is capable of reducing free amyloid-β levels in brains of APP transgenic mice, rats and cynomolgus monkeys (manuscript in preparation). This confirms published data and the use of these species in detecting decreases in brain amyloid-β, and indicates a fundamental difference in how antibodies and enzymes affect brain amyloid-β levels. The neprilysin fusions used in this study are able to efficiently lower the peripheral amyloid-β levels yet do not lower the brain levels of amyloid-β. They are able to penetrate the brain to a similar extent to antibodies (<0.5% in these studies) but their enzymatic properties (Km for amyloid-β of ∼14 μM and kcat 2.1 per sec) are such that this extent of brain penetration is unlikely to have any effect on central levels of amyloid-β. Therefore, it seems most likely that the effect of antibodies on the levels of amyloid-β in the brain is mediated by their high affinity and rapid efflux across the blood–brain barrier and not by a peripheral sink mechanism.
Many approaches to treat Alzheimer’s disease have focused on the role of amyloid and the potential of these approaches has been demonstrated in animal models of the disease (Bard et al., 2000; DeMattos et al., 2001; Comery et al., 2005; Fukumoto et al., 2010). However, interest in the treatment of Alzheimer’s disease with amyloid-depleting drugs is starting to wane with the disappointing outcome of phase III clinical trials with the amyloid-β targeting antibodies bapineuzumab and solanezumab. The demonstration in this paper of apparent flaws in the peripheral sink hypothesis suggests that peripheral administration of non-antibody biological drugs is unlikely to reduce the central pool of amyloid-β and as such has important implications in the search for potential treatments for Alzheimer’s disease. It may mean that many of the biological approaches need to take a more targeted approach to depletion of amyloid, or other proteins implicated in the pathogenesis of Alzheimer’s disease such as tau, in the brain itself. Direct delivery of neprilysin to the brain (Barua et al., 2012) or by coupling it to a blood–brain barrier delivery domain (Spencer et al., 2011) may offer the only realistic way of depleting central amyloid by enzymatic means.
Supplementary Material
Acknowledgements
The authors would like to thank the MedImmune CMC team for the provision of materials for use in these studies, also Barbara Vercoutère, Virginie Roger, Flordeliza de Villa, Kristina Eliason, Susanne Gruber, Carina Raynoschek, Marie Porsemyr, Anna-Lena Berg, Valentina Screpanti, Malin Eklund, Sufyan Maqbool, Judy Paterson and Lemy Tsikna for technical assistance with the work described in this paper, and Fraser Welsh and Michael Perkinton for critical review of the manuscript.
Glossary
Abbreviations
- APP
amyloid precursor protein
- HSA-hNepv
human serum albumin-human neprilysin variant fusion protein
- MSA-mNEPv
mouse serum albumin-mouse neprilysin variant fusion protein
Funding
All work was funded by MedImmune Ltd, and AstraZeneca R&D.
Supplementary material
Supplementary material is available at Brain online.
References
- Bard F, Cannon C, Barbour R, Burke R, Games D, Grajeda H, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer’s disease. Nat Med. 2000;6:916–9. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- Barua NU, Miners JS, Bienemann AS, Wyatt MJ, Welser K, Tabor AB, et al. Convection-enhanced delivery of neprilysin: a novel amyloid-beta- degrading therapeutic strategy. J Alzheimers Dis. 2012;32:43–56. doi: 10.3233/JAD-2012-120658. [DOI] [PubMed] [Google Scholar]
- Callahan MJ, Lipinski WJ, Bian F, Durham RA, Pack A, Walker LC. Augmented senile plaque load in aged female β-amyloid precursor protein-transgenic mice. Am J Pathol. 2001;158:1173–7. doi: 10.1016/s0002-9440(10)64064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comery TA, Martone RL, Aschmies S, Atchison KP, Diamantidis G, Gong X, et al. Acute-secretase inhibition improves contextual fear conditioning in the Tg2576 mouse model of Alzheimer's disease. J Neurosci. 2005;25:8898–902. doi: 10.1523/JNEUROSCI.2693-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–13. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- Deane R, Sagare A, Hamm K, Parisi M, LaRue B, Guo H, et al. IgG-assisted age-dependent clearance of Alzheimer's amyloid beta peptide by the blood-brain barrier neonatal Fc receptor. J Neurosci. 2005;25:11495–503. doi: 10.1523/JNEUROSCI.3697-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, Wu Z, Zlokovic BV. RAGE (yin) versus LRP (yang) balance regulates Alzheimer amyloid beta-peptide clearance through transport across the blood-brain barrier. Stroke. 2004;35:2628–31. doi: 10.1161/01.STR.0000143452.85382.d1. [DOI] [PubMed] [Google Scholar]
- DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-Abeta antibody alters CNS and plasma Abeta clearance and decreases brain Abeta burden in a mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 2001;98:8850–5. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Amouri SS, Zhu H, Yu J, Marr R, Verma IM, Kindy MS. Neprilysin: an enzyme candidate to slow the progression of Alzheimer's disease. Am J Pathol. 2008;172:1342–54. doi: 10.2353/ajpath.2008.070620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto H, Takahashi H, Tarui N, Matsui J, Tomita T, Hirode M, et al. A noncompetitive BACE1 inhibitor TAK-070 ameliorates abeta pathology and behavioral deficits in a mouse model of Alzheimer's disease. J Neurosci. 2010;30:11157–66. doi: 10.1523/JNEUROSCI.2884-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–5. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Hemming ML, Patterson M, Reske-Nielsen C, Lin L, Isacson O, Selkoe DJ. Reducing amyloid plaque burden via ex vivo gene delivery of an abeta-degrading protease: a novel therapeutic approach to Alzheimer disease. PLoS Med. 2007;4:e262. doi: 10.1371/journal.pmed.0040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh LB. Peptidases, proteases and amyloid beta-peptide catabolism. Curr Pharm Des. 2003;9:449–54. doi: 10.2174/1381612033391676. [DOI] [PubMed] [Google Scholar]
- Hong CS, Goins WF, Goss JR, Burton EA, Glorioso JC. Herpes simplex virus RNAi and neprilysin gene transfer vectors reduce accumulation of Alzheimer's disease-related amyloid-beta peptide in vivo. Gene Ther. 2006;13:1068–79. doi: 10.1038/sj.gt.3302719. [DOI] [PubMed] [Google Scholar]
- Howell S. Neutral endopeptidase can hydrolyze beta-amyloid(1-40) but shows no effect on beta-amyloid precursor protein metabolism. Peptides. 1995;16:647–52. doi: 10.1016/0196-9781(95)00021-b. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, et al. Correlative memory deficits, abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Iwata N, Mizukami H, Shirotani K, Takaki Y, Muramatsu S, Lu B, et al. Presynaptic localization of neprilysin contributes to efficient clearance of amyloid-beta peptide in mouse brain. J Neurosci. 2004;24:991–8. doi: 10.1523/JNEUROSCI.4792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP, et al. Metabolic regulation of brain abeta by neprilysin. Science. 2001;292:1550–2. doi: 10.1126/science.1059946. [DOI] [PubMed] [Google Scholar]
- Iwata N, Tsubuki S, Takaki Y, Watanabe K, Sekiguchi M, Hosoki E, et al. Identification of the major Abeta1-42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat Med. 2000;6:143–50. doi: 10.1038/72237. [DOI] [PubMed] [Google Scholar]
- Jeppsson F, Eketjäll S, Janson J, Karlström S, Gustavsson S, Olsson L, et al. Discovery of AZD3839, a potent and selective BACE1 inhibitor clinical candidate for the treatment of Alzheimer disease. J Biol Chem. 2012;287:41245–57. doi: 10.1074/jbc.M112.409110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlnoski RA, Rosenthal A, Kobayashi D, Pons J, Alamed J, Mercer M, et al. Suppression of amyloid deposition leads to long-term reductions in Alzheimer's pathologies in Tg2576 mice. J Neurosci. 2009;29:4964–71. doi: 10.1523/JNEUROSCI.4560-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karran E, Mercken M, De Strooper B. The amyloid cascade hypothesis for Alzheimer's disease: an appraisal for the development of therapeutics. Nat Rev Drug Disc. 2011;10:698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- Leissring MA, Farris W, Chang AY, Walsh DM, Wu X, Sun X, et al. Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron. 2003a;40:1087–93. doi: 10.1016/s0896-6273(03)00787-6. [DOI] [PubMed] [Google Scholar]
- Leissring MA, Lu A, Condron MM, Teplow DB, Stein RL, Farris W, et al. Kinetics of amyloid beta-protein degradation determined by novel fluorescence- and fluorescence polarization-based assays. J Biol Chem. 2003b;278:37314–20. doi: 10.1074/jbc.M305627200. [DOI] [PubMed] [Google Scholar]
- Levites Y, Das P, Price RW, Rochette MJ, Kostura LA, McGowan EM, et al. Anti-Abeta42- and anti-Abeta40-specific mAbs attenuate amyloid deposition in an Alzheimer disease mouse model. J Clin Invest. 2006;116:193–201. doi: 10.1172/JCI25410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr RA, Rockenstein E, Mukherjee A, Kindy MS, Hersh LB, Gage FH, et al. Neprilysin gene transfer reduces human amyloid pathology in transgenic mice. J Neurosci. 2003;23:1992–6. doi: 10.1523/JNEUROSCI.23-06-01992.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, et al. Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalivaeva NN, Beckett C, Belyaev ND, Turner AJ. Are amyloid-degrading enzymes viable therapeutic targets in Alzheimer's disease? J Neurochem. 2012;120:167–85. doi: 10.1111/j.1471-4159.2011.07510.x. [DOI] [PubMed] [Google Scholar]
- Roques BP, Noble F, Dauge V, Fournie-Zaluski MC, Beaumont A. Neutral endopeptidase 24.11: Structure, inhibition, and experimental and clinical pharmacology. Pharmacol Rev. 1993;45:87–146. [PubMed] [Google Scholar]
- Sagare A, Deane R, Bell RD, Johnson B, Hamm K, Pendu R, et al. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat Med. 2007;13:1029–31. doi: 10.1038/nm1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk D, Basi GS, Pangalos MN. Treatment strategies targeting amyloid beta-protein. Cold Spring Harb Perspect Med. 2012;2:a006387. doi: 10.1101/cshperspect.a006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlachetzki F, Zhu C, Pardridge WM. Expression of the neonatal Fc receptor (FcRn) at the blood-brain barrier. J Neurochem. 2002;81:203–6. doi: 10.1046/j.1471-4159.2002.00840.x. [DOI] [PubMed] [Google Scholar]
- Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, et al. Clearance of Alzheimer's amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106:1489–99. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirotani K, Tsubuki S, Iwata N, Takaki Y, Harigaya W, Maruyama K, et al. Neprilysin degrades both amyloid beta peptides 1-40 and 1-42 most rapidly and efficiently among thiorphan- and phosphoramidon-sensitive endopeptidases. J Biol Chem. 2001;276:21895–901. doi: 10.1074/jbc.M008511200. [DOI] [PubMed] [Google Scholar]
- Spencer B, Marr RA, Gindi R, Potkar R, Michael S, Adame A, et al. Peripheral delivery of a CNS targeted, metalo-protease reduces abeta toxicity in a mouse model of Alzheimer's disease. PLoS One. 2011;6:e16575. doi: 10.1371/journal.pone.0016575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki Y, Iwata N, Tsubuki S, Taniguchi S, Toyoshima S, Lu B, et al. Biochemical identification of the neutral endopeptidase family member responsible for the catabolism of amyloid beta peptide in the brain. J Biochem. 2000;128:897–902. doi: 10.1093/oxfordjournals.jbchem.a022839. [DOI] [PubMed] [Google Scholar]
- Tamayev R, Matsuda S, Giliberto L, Arancio O, D’Adamio L. APP heterozygosity averts memory deficit in knockin mice expressing the Danish dementia BRI2 mutant. EMBO J. 2011;30:2501–9. doi: 10.1038/emboj.2011.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner AJ, Isaac RE, Coates D. The neprilysin (NEP) family of zinc metalloendopeptidases: genomics and function. Bioessays. 2001;23:261–9. doi: 10.1002/1521-1878(200103)23:3<261::AID-BIES1036>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Walker JR, Pacoma R, Watson J, Ou W, Alves J, Mason DE, et al. Enhanced proteolytic clearance of plasma a by peripherally administered neprilysin does not result in reduced levels of brain Aβ in mice. J Neurosci. 2013;33:2457–64. doi: 10.1523/JNEUROSCI.3407-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock DM, Rojiani A, Rosenthal A, Levkowitz G, Subbarao S, Alamed J, et al. Passive amyloid immunotherapy clears amyloid and transiently activates microglia in a transgenic mouse model of amyloid deposition. J Neurosci. 2004;24:6144–51. doi: 10.1523/JNEUROSCI.1090-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lee DH. Sink hypothesis and therapeutic strategies for attenuating abeta levels. Neuroscientist. 2011;17:163–73. doi: 10.1177/1073858410381532. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Pardridge WM. Mediated efflux of IgG molecules from brain to blood across the blood-brain barrier. J Neuroimmunol. 2001;114:168–72. doi: 10.1016/s0165-5728(01)00242-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.