Abstract
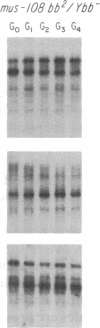
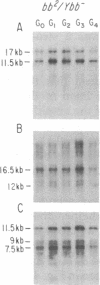
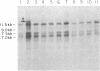
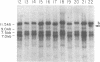
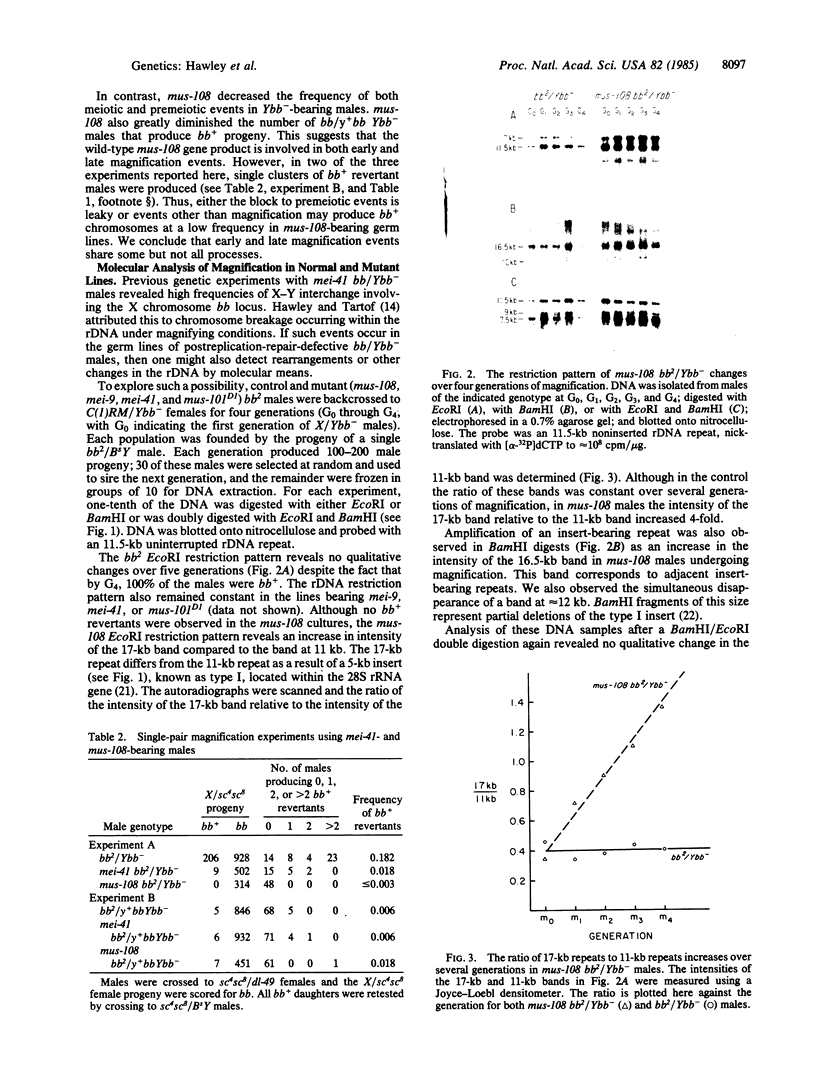
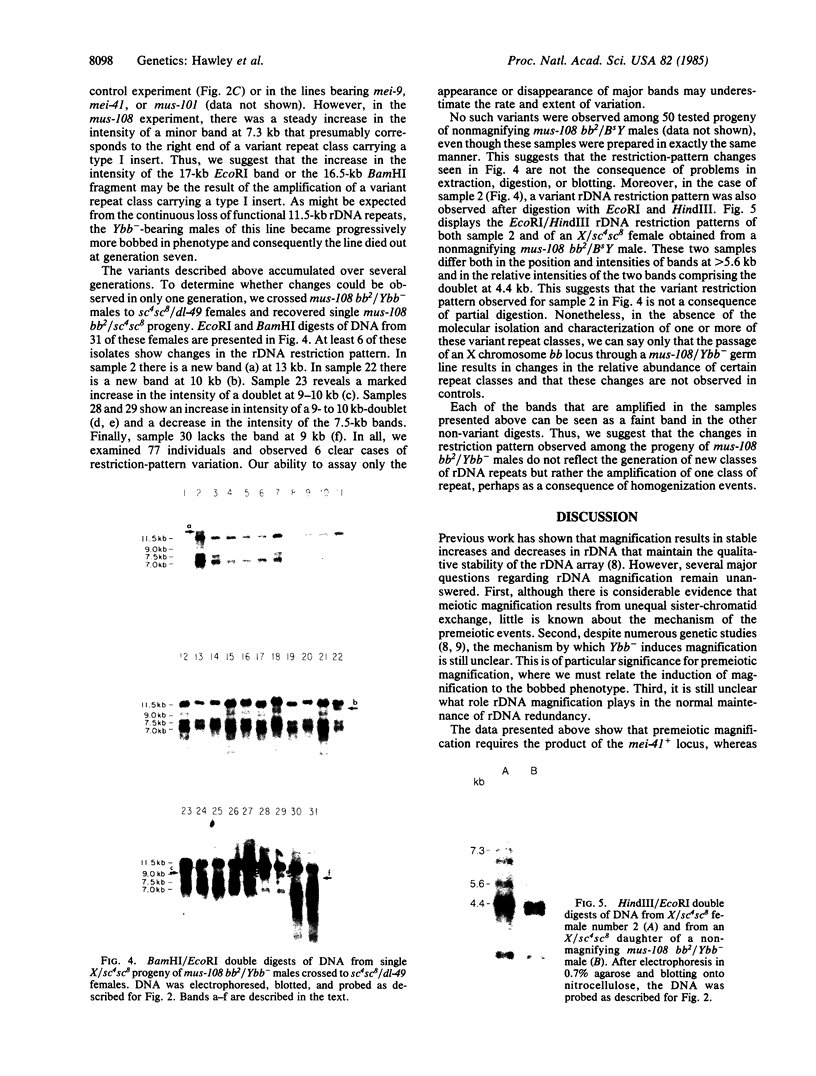
We have examined rDNA magnification in Drosophila melanogaster males carrying one of 11 recombination- or repair-defective mutations representing seven loci. We show that mutations defined by a defect in postreplication repair (mus-101, mei-41, and mus-108) are also defective in rDNA magnification, whereas mutations that do not affect postreplication repair have little or no effect on magnification. mei-41 inhibits only premeiotic magnification events, while mus-108 blocks both premeiotic and meiotic events. This suggests that meiotic and premeiotic events share some but not all functions. A molecular analysis of rDNA magnification reveals that in mus-108 males, changes in the rDNA restriction pattern can occur within one or a few generations under magnifying conditions. We interpret these data in terms of the role of DNA repair systems in rDNA magnification and in terms of stable maintenance of tandemly repeated genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd J. B., Golino M. D., Nguyen T. D., Green M. M. Isolation and characterization of X-linked mutants of Drosophila melanogaster which are sensitive to mutagens. Genetics. 1976 Nov;84(3):485–506. doi: 10.1093/genetics/84.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd J. B., Setlow R. B. Characterization of postreplication repair in mutagen-sensitive strains of Drosophila melanogaster. Genetics. 1976 Nov;84(3):507–526. doi: 10.1093/genetics/84.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd J. B., Shaw K. E. Postreplication repair defects in mutants of Drosophila melanogaster. Mol Gen Genet. 1982;186(2):289–294. doi: 10.1007/BF00331864. [DOI] [PubMed] [Google Scholar]
- Brown T. C., Boyd J. B. Abnormal recovery of DNA replication in ultraviolet-irradiated cell cultures of Drosophila melanogaster which are defective in DNA repair. Mol Gen Genet. 1981;183(2):363–368. doi: 10.1007/BF00270641. [DOI] [PubMed] [Google Scholar]
- Coen E. S., Dover G. A. Unequal exchanges and the coevolution of X and Y rDNA arrays in Drosophila melanogaster. Cell. 1983 Jul;33(3):849–855. doi: 10.1016/0092-8674(83)90027-2. [DOI] [PubMed] [Google Scholar]
- Dawid I. B., Wellauer P. K., Long E. O. Ribosomal DNA in Drosophila melanogaster. I. Isolation and characterization of cloned fragments. J Mol Biol. 1978 Dec 25;126(4):749–768. doi: 10.1016/0022-2836(78)90018-9. [DOI] [PubMed] [Google Scholar]
- Dawid I. B., Wellauer P. K. Ribosomal DNA and related sequences in Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1185–1194. doi: 10.1101/sqb.1978.042.01.119. [DOI] [PubMed] [Google Scholar]
- Dover G. Molecular drive: a cohesive mode of species evolution. Nature. 1982 Sep 9;299(5879):111–117. doi: 10.1038/299111a0. [DOI] [PubMed] [Google Scholar]
- Endow S. A., Komma D. J., Atwood K. C. Ring chromosomes and rDNA magnification in Drosophila. Genetics. 1984 Dec;108(4):969–983. doi: 10.1093/genetics/108.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley R. S., Tartof K. D. A two-stage model for the control of rDNA magnification. Genetics. 1985 Apr;109(4):691–700. doi: 10.1093/genetics/109.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley R. S., Tartof K. D. The effect of mei-41 on rDNA redundancy in Drosophila melanogaster. Genetics. 1983 May;104(1):63–80. doi: 10.1093/genetics/104.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indik Z. K., Tartof K. D. Long spacers among ribosomal genes of Drosophila melanogaster. Nature. 1980 Apr 3;284(5755):477–479. doi: 10.1038/284477a0. [DOI] [PubMed] [Google Scholar]
- Locker D., Marrakechi M. Evidence for an excess of rDNA in the testis of Drosophila melanogaster during rDNA magnification. Mol Gen Genet. 1977 Sep 9;154(3):249–254. doi: 10.1007/BF00571279. [DOI] [PubMed] [Google Scholar]
- Locker D., Prud'homme N. Etude de plusieurs facteurs faisant varier la fréquence de reversion au locus bobbed chez Drosophila melanogaster. Mol Gen Genet. 1973 Jul 31;124(1):11–19. doi: 10.1007/BF00267160. [DOI] [PubMed] [Google Scholar]
- Mason J. M., Green M. M., Shaw K. E., Boyd J. B. Genetic analysis of X-linked mutagen-sensitive mutants of Drosophila melanogaster. Mutat Res. 1981 May;81(3):329–343. doi: 10.1016/0027-5107(81)90120-2. [DOI] [PubMed] [Google Scholar]
- Ritossa F. M., Atwood K. C., Lindsley D. L., Spiegelman S. On the chromosomal distribution of DNA complementary to ribosomal and soluble RNA. Natl Cancer Inst Monogr. 1966 Dec;23:449–472. [PubMed] [Google Scholar]
- Ritossa F. M. Unstable redundancy of genes for ribosomal RNA. Proc Natl Acad Sci U S A. 1968 Jun;60(2):509–516. doi: 10.1073/pnas.60.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. M., Buck L. B., Axel R. A structure for amplified DNA. Cell. 1983 May;33(1):53–63. doi: 10.1016/0092-8674(83)90334-3. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Wilde C. E., 3rd, Reno D. L., Howard-Flanders P. Exchanges between DNA strands in ultraviolet-irradiated Escherichia coli. J Mol Biol. 1971 Oct 14;61(1):25–44. doi: 10.1016/0022-2836(71)90204-x. [DOI] [PubMed] [Google Scholar]
- Tartof K. D., Dawid I. G. Similarities and differences in the structure of X and Y chromosome rRNA genes of Drosophila. Nature. 1976 Sep 2;263(5572):27–30. doi: 10.1038/263027a0. [DOI] [PubMed] [Google Scholar]
- Tartof K. D. Increasing the multiplicity of ribosomal RNA genes in Drosophila melanogaster. Science. 1971 Jan 22;171(3968):294–297. doi: 10.1126/science.171.3968.294. [DOI] [PubMed] [Google Scholar]
- Tartof K. D. Redundant genes. Annu Rev Genet. 1975;9:355–385. doi: 10.1146/annurev.ge.09.120175.002035. [DOI] [PubMed] [Google Scholar]
- Tartof K. D. Regulation of ribosomal RNA gene multiplicity in Drosophila melanogaster. Genetics. 1973 Jan;73(1):57–71. doi: 10.1093/genetics/73.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartof K. D. Unequal mitotic sister chromatin exchange as the mechanism of ribosomal RNA gene magnification. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1272–1276. doi: 10.1073/pnas.71.4.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. L., Hogness D. S. R loop mapping of the 18S and 28S sequences in the long and short repeating units of Drosophila melanogaster rDNA. Cell. 1977 Feb;10(2):177–192. doi: 10.1016/0092-8674(77)90213-6. [DOI] [PubMed] [Google Scholar]