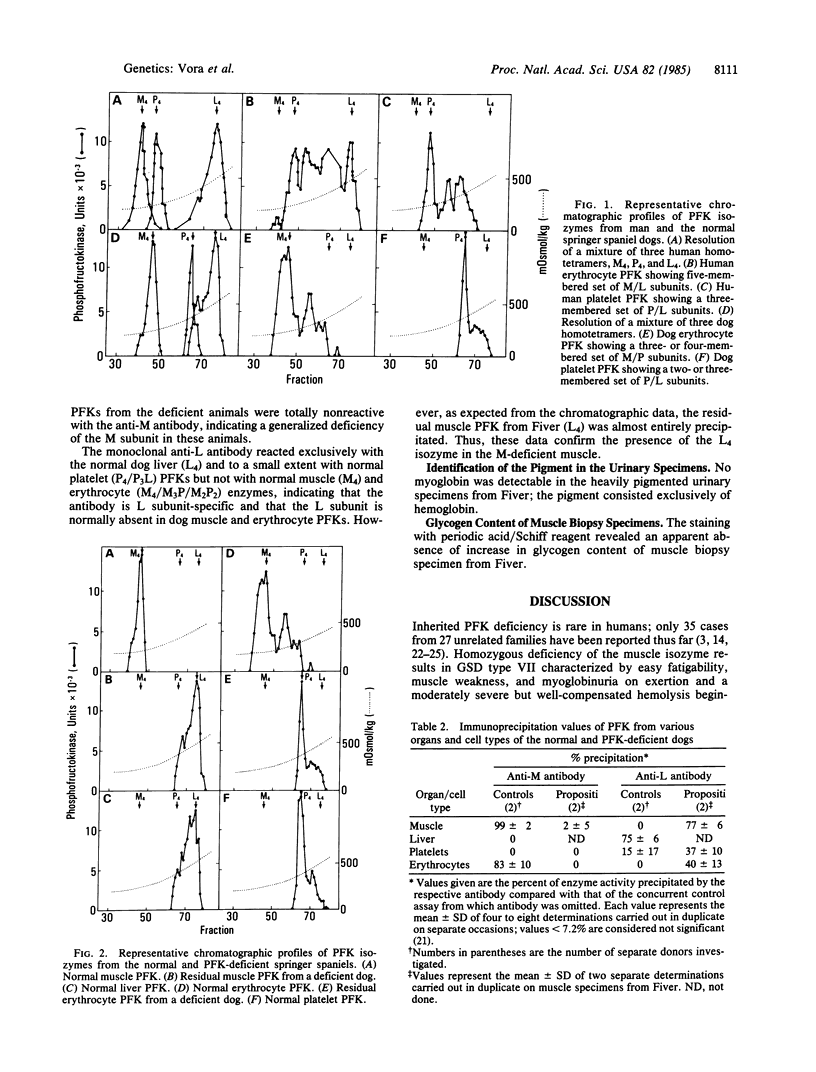
Abstract
Mammalian phosphofructokinase (PFK; ATP:D-fructose-6-phosphate 1-phosphotransferase, EC 2.7.1.11) exists in multimolecular forms, which result from random tetramerization of three distinct subunits, M (muscle-type), L (liver-type), and P (platelet-type), each under a separate genetic control. Human muscle and liver contain homotetramers M4 and L4, respectively, whereas erythrocytes contain a mixture of M4, M3L, M2L2, ML3, and L4 isozymes. Homozygous deficiency of the M subunit in man results in glycogen storage disease (GSD) type VII, which is characterized by exertional muscle weakness and compensated hemolysis; the residual erythrocyte PFK consists of isolated L4 isozyme. Recently, PFK deficiency associated with isolated hemolytic anemia has been identified among English springer spaniel dogs. We investigated the genetic control of the dog PFK system and the nature of the enzymatic defect in two PFK-deficient animals, using chromatographic and immunological techniques. Our studies indicate the existence of a trilocus isozyme system for the dog, as is the case with other mammals. Muscle PFK consists of M4 isozyme, whereas the predominant species of liver and platelet consists, respectively, of the L4 and P4 isozyme; erythrocyte PFK consists of a three- or four-membered set composed of M and P subunits. PFK deficiency in the dogs was found to result from a total and universal lack of the M subunit, as is the case in man. However, the probands consistently exhibited L4 isozyme in their muscle; P4, L4, and hybrids thereof in their erythrocytes; and an increase in the L-containing isozymes in their platelets, indicating a generalized anomalous presence of the L subunit. The apparent absence of muscle disease in these animals is most likely accounted for by both the well-known high oxidative potential of the canine muscle in general and the presence of liver PFK in the M-deficient muscle in particular. In contrast, presence of hemolysis despite residual P4 and hybrids of P and L in the erythrocytes may be inferred to result in severe glycolytic handicap under existing intraerythrocytic conditions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CERRETELLI P., PIIPER J., MANGILI F., RICCI B. AEROBIC AND ANAEROBIC METABOLISM IN EXERCISING DOGS. J Appl Physiol. 1964 Jan;19:25–28. doi: 10.1152/jappl.1964.19.1.25. [DOI] [PubMed] [Google Scholar]
- Chance B., Eleff S., Bank W., Leigh J. S., Jr, Warnell R. 31P NMR studies of control of mitochondrial function in phosphofructokinase-deficient human skeletal muscle. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7714–7718. doi: 10.1073/pnas.79.24.7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M., Collins M., Byrne J., Vora S. Alterations in phosphofructokinase isoenzymes during early human development. Establishment of adult organ-specific patterns. Biochem J. 1983 Sep 15;214(3):703–710. doi: 10.1042/bj2140703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desnick R. J., McGovern M. M., Schuchman E. H., Haskins M. E. Animal analogues of human inherited metabolic diseases: molecular pathology and therapeutic studies. Prog Clin Biol Res. 1982;94:27–65. [PubMed] [Google Scholar]
- Dill D. B., Edwards H. T., Talbott J. H. Studies in muscular activity: VII. Factors limiting the capacity for work. J Physiol. 1932 Dec 19;77(1):49–62. doi: 10.1113/jphysiol.1932.sp002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaway G. A. A review of animal phosphofructokinase isozymes with an emphasis on their physiological role. Mol Cell Biochem. 1983;52(1):75–91. doi: 10.1007/BF00230589. [DOI] [PubMed] [Google Scholar]
- Edwards J. R., Richards R. B. Bovine generalized glycogenosis type II: a clinico-pathological study. Br Vet J. 1979 Jul-Aug;135(4):338–348. doi: 10.1016/s0007-1935(17)32836-1. [DOI] [PubMed] [Google Scholar]
- GONDKO R., SCHMIDT M., LEYKO W. SEPARATION OF MYOGLOBIN AND HEMOGLOBIN ON DEAE-SEPHADEX. Biochim Biophys Acta. 1964 Apr 4;86:190–191. doi: 10.1016/0304-4165(64)90178-3. [DOI] [PubMed] [Google Scholar]
- Gerrity L. W., Friedman J. M. Choosing animal models of human inborn errors of metabolism. Prog Clin Biol Res. 1982;94:459–461. [PubMed] [Google Scholar]
- Giger U., Harvey J. W., Yamaguchi R. A., McNulty P. K., Chiapella A., Beutler E. Inherited phosphofructokinase deficiency in dogs with hyperventilation-induced hemolysis: increased in vitro and in vivo alkaline fragility of erythrocytes. Blood. 1985 Feb;65(2):345–351. [PubMed] [Google Scholar]
- Kahn A., Meienhofer M. C., Cottreau D., Lagrange J. L., Dreyfus J. C. Phosphofructokinase (PFK) isozymes in man. I. Studies of adult human tissues. Hum Genet. 1979 Apr 17;48(1):93–108. doi: 10.1007/BF00273280. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meienhofer M. C., Cottreau D., Dreyfus J. C., Kahn A. Kinetic properties of human F4 phosphofructokinase: a poor regulatory enzyme. FEBS Lett. 1980 Feb 11;110(2):219–222. doi: 10.1016/0014-5793(80)80077-9. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Kuwajima M., Kono N., Mineo I., Sumi S., Yonezawa T., Nonaka K., Tarui S. Kinetic properties of mutant enzymes in erythrocyte phosphofructokinase deficiency and erythrocyte pyruvate kinase deficiency. Med J Osaka Univ. 1983 Mar;33(3-4):49–58. [PubMed] [Google Scholar]
- Snow D. H., Billeter R., Mascarello F., Carpenè E., Rowlerson A., Jenny E. No classical type IIB fibres in dog skeletal muscle. Histochemistry. 1982;75(1):53–65. doi: 10.1007/BF00492533. [DOI] [PubMed] [Google Scholar]
- Tani K., Fujii H., Takegawa S., Miwa S., Koyama W., Kanayama M., Imanaka A., Imanaka F., Kuramoto A. Two cases of phosphofructokinase deficiency associated with congenital hemolytic anemia found in Japan. Am J Hematol. 1983 Apr;14(2):165–174. doi: 10.1002/ajh.2830140208. [DOI] [PubMed] [Google Scholar]
- Tsai M. Y., Kemp R. G. Isozymes of rabbit phosphofructokinase. Electrophoretic and immunochemical studies. J Biol Chem. 1973 Feb 10;248(3):785–792. [PubMed] [Google Scholar]
- Tsai M. Y., Kemp R. G. Rabbit brain phosphofructokinase. Comparison of regulatory properties with those of other phosphofructokinase isozymes. J Biol Chem. 1974 Oct 25;249(20):6590–6596. [PubMed] [Google Scholar]
- Uyeda K. Phosphofructokinase. Adv Enzymol Relat Areas Mol Biol. 1979;48:193–244. doi: 10.1002/9780470122938.ch4. [DOI] [PubMed] [Google Scholar]
- Vora S., Corash L., Engel W. K., Durham S., Seaman C., Piomelli S. The molecular mechanism of the inherited phosphofructokinase deficiency associated with hemolysis and myopathy. Blood. 1980 Apr;55(4):629–635. [PubMed] [Google Scholar]
- Vora S., Davidson M., Seaman C., Miranda A. F., Noble N. A., Tanaka K. R., Frenkel E. P., Dimauro S. Heterogeneity of the molecular lesions in inherited phosphofructokinase deficiency. J Clin Invest. 1983 Dec;72(6):1995–2006. doi: 10.1172/JCI111164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vora S., Durham S., de Martinville B., George D. L., Francke U. Assignment of the human gene for muscle-type phosphofructokinase (PFKM) to chromosome 1 (region cen leads to q32) using somatic cell hybrids and monoclonal anti-M antibody. Somatic Cell Genet. 1982 Jan;8(1):95–104. doi: 10.1007/BF01538653. [DOI] [PubMed] [Google Scholar]
- Vora S. Isozymes of human phosphofructokinase in blood cells and cultured cell lines: molecular and genetic evidence for a trigenic system. Blood. 1981 Apr;57(4):724–732. [PubMed] [Google Scholar]
- Vora S. Isozymes of phosphofructokinase. Isozymes Curr Top Biol Med Res. 1982;6:119–167. [PubMed] [Google Scholar]
- Vora S., Oskam R., Staal G. E. Isoenzymes of phosphofructokinase in the rat. Demonstration of the three non-identical subunits by biochemical, immunochemical and kinetic studies. Biochem J. 1985 Jul 15;229(2):333–341. doi: 10.1042/bj2290333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vora S., Seaman C., Durham S., Piomelli S. Isozymes of human phosphofructokinase: identification and subunit structural characterization of a new system. Proc Natl Acad Sci U S A. 1980 Jan;77(1):62–66. doi: 10.1073/pnas.77.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vora S., Wims L. A., Durham S., Morrison S. L. Production and characterization of monoclonal antibodies to the subunits of human phosphofructokinase: new tools for the immunochemical and genetic analyses of isozymes. Blood. 1981 Oct;58(4):823–829. [PubMed] [Google Scholar]
- Walvoort H. C. Glycogen storage diseases in animals and their potential value as models of human disease. J Inherit Metab Dis. 1983;6(1):3–16. doi: 10.1007/BF02391186. [DOI] [PubMed] [Google Scholar]
- de Faria J. B., Crivellaro O., Bacila M. Multiple forms of phosphofructokinase in animals from the phylum Chordata. Comp Biochem Physiol B. 1976;55(2):323–329. doi: 10.1016/0305-0491(76)90276-5. [DOI] [PubMed] [Google Scholar]


