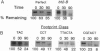Abstract
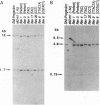
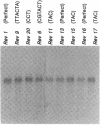
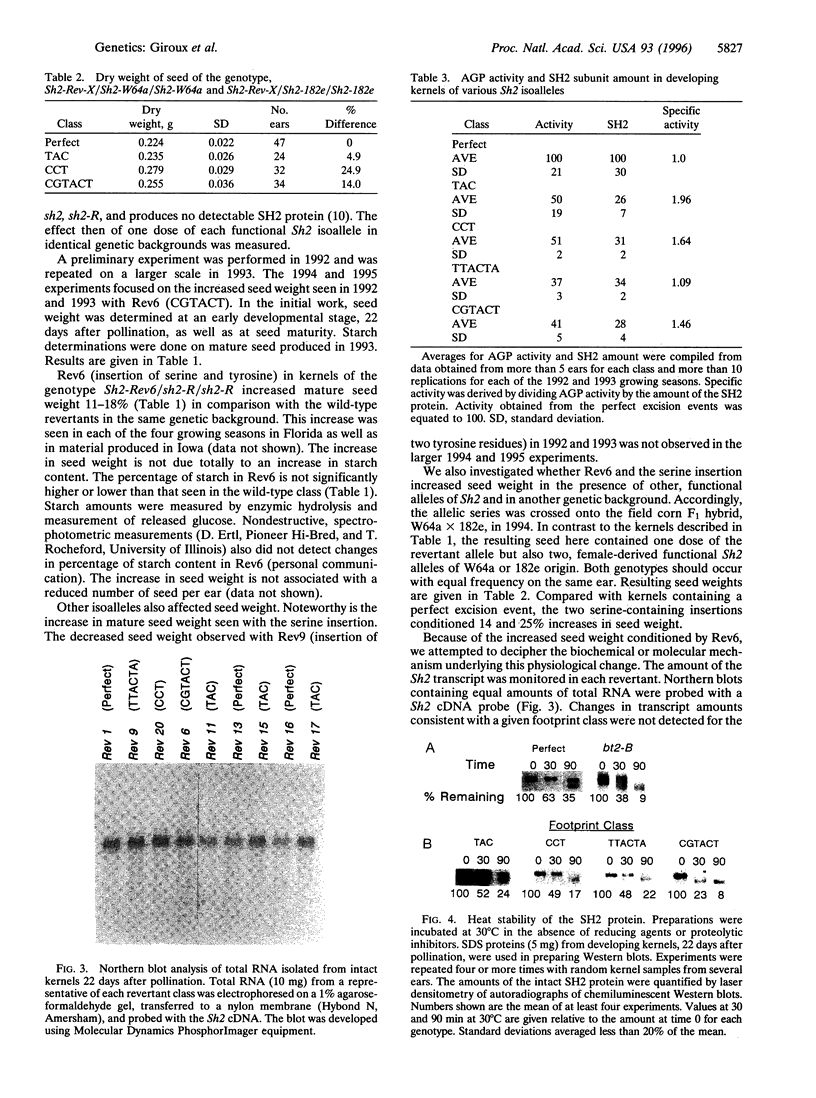
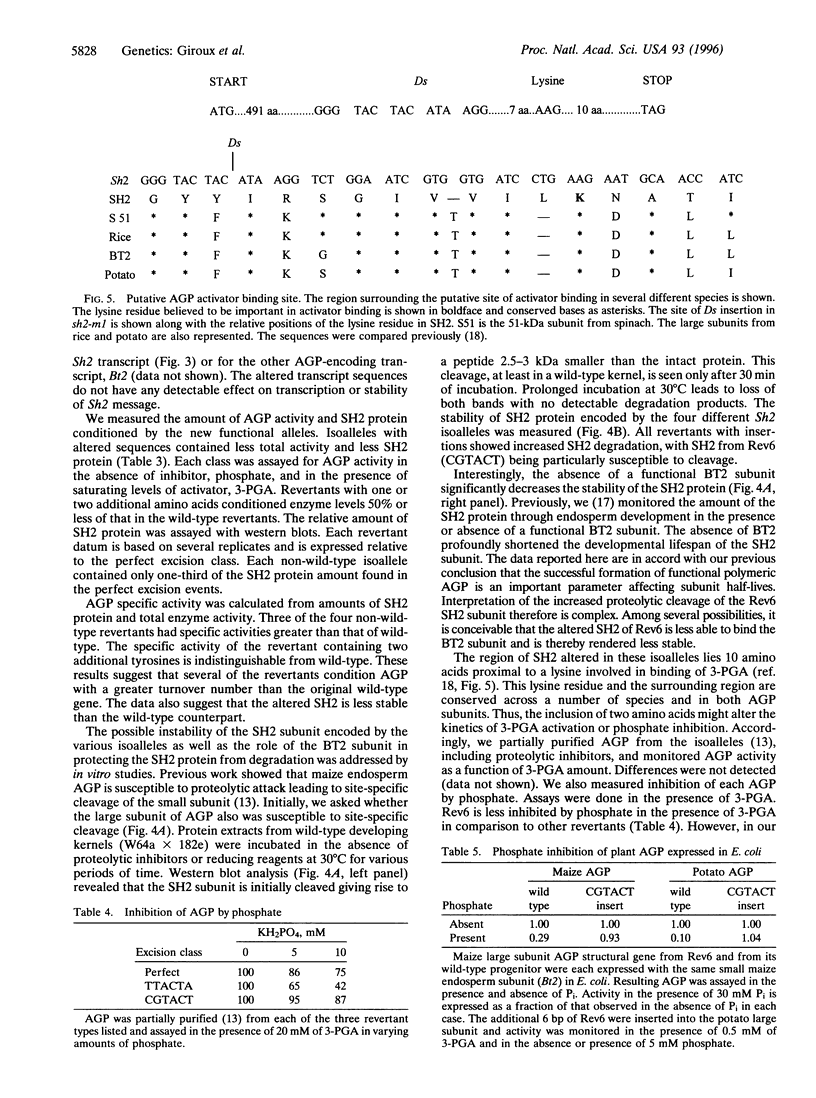
The maize endosperm-specific gene shrunken2 (Sh2) encodes the large subunit of the heterotetrameric starch synthetic enzyme adenosine diphosphoglucose pyrophosphorylase (AGP; EC 2.7.7.27). Here we exploit an in vivo, site-specific mutagenesis system to create short insertion mutations in a region of the gene known to be involved in the allosteric regulation of AGP. The site-specific mutagen is the transposable element dissociation (Ds). Approximately one-third (8 of 23) of the germinal revertants sequenced restored the wild-type sequence, whereas the remaining revertants contained insertions of 3 or 6 bp. All revertants retained the original reading frame 3' to the insertion site and involved the addition of tyrosine and/or serine. Each insertion revertant reduced total AGP activity and the amount of the SH2 protein. The revertant containing additional tyrosine and serine residues increased seed weight 11-18% without increasing or decreasing the percentage of starch. Other insertion revertants lacking an additional serine reduced seed weight. Reduced sensitivity to phosphate, a long-known inhibitor of AGP, was found in the high seed-weight revertant. This alteration is likely universally important since insertion of tyrosine and serine in the potato large subunit of AGP at the comparable position and expression in Escherichia coli also led to a phosphate-insensitive enzyme. These results show that single gene mutations giving rise to increased seed weight, and therefore perhaps yield, are clearly possible in a plant with a long history of intensive and successful breeding efforts.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball K., Preiss J. Allosteric sites of the large subunit of the spinach leaf ADPglucose pyrophosphorylase. J Biol Chem. 1994 Oct 7;269(40):24706–24711. [PubMed] [Google Scholar]
- Bhave M. R., Lawrence S., Barton C., Hannah L. C. Identification and molecular characterization of shrunken-2 cDNA clones of maize. Plant Cell. 1990 Jun;2(6):581–588. doi: 10.1105/tpc.2.6.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb B. G., Hannah L. C. Sugar utilization by developing wild type and shrunken-2 maize kernels. Plant Physiol. 1986 Mar;80(3):609–611. doi: 10.1104/pp.80.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroux M. J., Boyer C., Feix G., Hannah L. C. Coordinated Transcriptional Regulation of Storage Product Genes in the Maize Endosperm. Plant Physiol. 1994 Oct;106(2):713–722. doi: 10.1104/pp.106.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroux M. J., Clancy M., Baier J., Ingham L., McCarty D., Hannah L. C. De novo synthesis of an intron by the maize transposable element Dissociation. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):12150–12154. doi: 10.1073/pnas.91.25.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroux M. J., Hannah L. C. ADP-glucose pyrophosphorylase in shrunken-2 and brittle-2 mutants of maize. Mol Gen Genet. 1994 May 25;243(4):400–408. doi: 10.1007/BF00280470. [DOI] [PubMed] [Google Scholar]
- Hannah L. C., Nelson O. E., Jr Characterization of ADP-glucose pyrophosphorylase from shrunken-2 and brittle-2 mutants of maize. Biochem Genet. 1976 Aug;14(7-8):547–560. doi: 10.1007/BF00485834. [DOI] [PubMed] [Google Scholar]
- Hannah L. C., Tuschall D. M., Mans R. J. Multiple forms of maize endosperm adp-glucose pyrophosphorylase and their control by shrunken-2 and brittle-2. Genetics. 1980 Aug;95(4):961–970. doi: 10.1093/genetics/95.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias A. A., Barry G. F., Meyer C., Bloksberg L., Nakata P. A., Greene T., Laughlin M. J., Okita T. W., Kishore G. M., Preiss J. Expression of the potato tuber ADP-glucose pyrophosphorylase in Escherichia coli. J Biol Chem. 1993 Jan 15;268(2):1081–1086. [PubMed] [Google Scholar]
- Morell M., Bloom M., Preiss J. Affinity labeling of the allosteric activator site(s) of spinach leaf ADP-glucose pyrophosphorylase. J Biol Chem. 1988 Jan 15;263(2):633–637. [PubMed] [Google Scholar]
- Plaxton W. C., Preiss J. Purification and Properties of Nonproteolytic Degraded ADPglucose Pyrophosphorylase from Maize Endosperm. Plant Physiol. 1987 Jan;83(1):105–112. doi: 10.1104/pp.83.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. R., Hannah L. C. Genomic Nucleotide Sequence of a Wild-Type Shrunken-2 Allele of Zea mays. Plant Physiol. 1992 Mar;98(3):1214–1216. doi: 10.1104/pp.98.3.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-White B. J., Preiss J. Comparison of proteins of ADP-glucose pyrophosphorylase from diverse sources. J Mol Evol. 1992 May;34(5):449–464. doi: 10.1007/BF00162999. [DOI] [PubMed] [Google Scholar]
- Stark D. M., Timmerman K. P., Barry G. F., Preiss J., Kishore G. M. Regulation of the Amount of Starch in Plant Tissues by ADP Glucose Pyrophosphorylase. Science. 1992 Oct 9;258(5080):287–292. doi: 10.1126/science.258.5080.287. [DOI] [PubMed] [Google Scholar]
- Tuschall D. M., Hannah L. C. Altered Maize Endosperm Adp-Glucose Pyrophosphorylases from Revertants of a SHRUNKEN-2-DISSOCIATION ALLELE. Genetics. 1982 Jan;100(1):105–111. doi: 10.1093/genetics/100.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]