Abstract
The identification and validation of gene–gene interactions is a major challenge in human studies. Here, we explore an approach for studying epistasis in humans using a Drosophila melanogaster model of neonatal diabetes mellitus. Expression of the mutant preproinsulin (hINSC96Y) in the eye imaginal disc mimics the human disease: it activates conserved stress-response pathways and leads to cell death (reduction in eye area). Dominant-acting variants in wild-derived inbred lines from the Drosophila Genetics Reference Panel produce a continuous, highly heritable distribution of eye-degeneration phenotypes in a hINSC96Y background. A genome-wide association study (GWAS) in 154 sequenced lines identified a sharp peak on chromosome 3L, which mapped to a 400-bp linkage block within an intron of the gene sulfateless (sfl). RNAi knockdown of sfl enhanced the eye-degeneration phenotype in a mutant-hINS-dependent manner. RNAi against two additional genes in the heparan sulfate (HS) biosynthetic pathway (ttv and botv), in which sfl acts, also modified the eye phenotype in a hINSC96Y-dependent manner, strongly suggesting a novel link between HS-modified proteins and cellular responses to misfolded proteins. Finally, we evaluated allele-specific expression difference between the two major sfl-intronic haplotypes in heterozygtes. The results showed significant heterogeneity in marker-associated gene expression, thereby leaving the causal mutation(s) and its mechanism unidentified. In conclusion, the ability to create a model of human genetic disease, map a QTL by GWAS to a specific gene, and validate its contribution to disease with available genetic resources and the potential to experimentally link the variant to a molecular mechanism demonstrate the many advantages Drosophila holds in determining the genetic underpinnings of human disease.
Keywords: mutant insulin, Drosophila, genome-wide association study, heparan sulfate proteoglycan, sulfateless
LIMITATIONS imposed by human subject research can be overcome by investigating models of human disease in experimental organisms. Drosophila can provide genetic insights relevant to human biology and disease, owing to the conservation of fundamental cellular and developmental processes. We constructed a fly model of protein-misfolding disease, by creating a transgene of a diabetes-causing, human mutant preproinsulin (hINSC96Y) that could be expressed in the eye imaginal discs and other tissues (Park et al. 2013). This misfolded proinsulin protein causes the loss of insulin-secreting pancreatic beta cells and diabetes in humans and mice (Støy et al. 2007). When misexpressed in the Drosophila eye imaginal disc, it disrupts eye development, resulting in a reduced eye area in adult flies (Park et al. 2013).
In the accompanying article (Park et al. 2013), we crossed the transgenic line bearing the mutant preproinsulin and an eye-specific Gal4 driver (GMR >> hINSC96Y) with a subset of the lines from the Drosophila Genetics Reference Panel (DGRP). The F1 lines displayed a wide, nearly continuous, range of heritable eye-degeneration phenotypes, suggesting a polygenic basis for this genetic background variation (Park et al. 2013). To investigate the genetic basis of this background variation, here we performed a genome-wide association study in a larger set of 154 DGRP lines.
Drosophila’s many favorable attributes for mapping quantitative trait loci (QTL)—a high density of common variants, relatively little population subdivision, a decay of linkage disequilibrium (LD) over a scale of only hundreds of base pairs, controlled crosses allowing repeat measurements, and excellent resources for confirmatory genetics—allowed us to identify a variant in the heparan sulfate (HS) biosynthesis pathway gene, sulfateless (sfl), contributing to the eye-degeneration phenotype and then confirm a genetic interaction between mutant hINS and sfl by RNAi knockdown analysis. Two other genes in the HS biosynthetic pathway, tout-velo (ttv) and brother of tout-velo (botv), displayed a similar interaction upon genetic analysis, implicating HS-modified proteins, or proteoglycans (HSPG), in the response to misfolded proteins.
We then tested the hypothesis that the intronic sfl variants act by decreasing gene expression by measuring the relative expression level of each allele in 15 heterozygotes containing both alleles. The results are mixed, with seven crosses showing a difference that is consistent with the hypothesis; however, overall there is only modest correlation between the genotype and the expression level, which leaves the causal mutation(s) and its mechanism yet to be identified.
Although our model of neonatal diabetes in the fly—transgenic expression of a mutant disease-causing human insulin allele—is Mendelian, the severity of the disease trait is exquisitely sensitive to genetic background and behaves as a complex trait. We discuss the prospects for modeling complex human disease in the fly with this general approach.
Materials and Methods
Drosophila stocks and crosses
The {GMR–Gal4, UAS–hINSC96Y} line was generated by crossing the GMR–Gal4 line (stock 1104, Bloomington Stock Center) with the UAS–hINSC96Y line (Park et al. 2013) and obtaining the recombinant second chromosome, which was balanced over CyO. DGRP lines were obtained from the Bloomington Stock Center. RNAi lines against sfl (GD5070), ttv (GD4871), and botv (GD37186) were from the Vienna Drosophila RNAi Center. Mutant lines for ttv (ttv681) and botv (botv510) were described previously (Ren et al. 2009).
Eye area measurement
All crosses were reared at 25°. Total eye area was measured as described in Park et al. (2013). At least 10 images (independent flies) passing the quality check were collected for each cross. Raw data are available in Supporting Information, Table S1.
Principal Component Analysis
The whole-genome SNP data set for the 154 DGRP lines used for genome-wide association study (GWAS) (see Table S2 for the list of line numbers) was downloaded from the DGRP website (http://dgrp.gnets.ncsu.edu/, freeze 1). To characterize population structure, 900K SNPs (after LD pruning using PLINK v. 1.07, with parameter–indep-pairwise 50 5 0.5) were used to identify the top 15 principal components (PCs) (SmartPCA software in Eigensoft v. 3.0, no outlier exclusion). We then estimated the correlation between the hINSC96Y phenotype (line mean) and projection length in the direction of the top five principle components in each DGRP line to test whether population structure is a confounding source of association in GWAS.
Genome-wide association using linear regression
The mean eye area of 154 DGRP lines crossed to the hINSC96Y line was regressed on each SNP with a minor allele frequency (MAF) >5% (PLINK 1.07, quantitative trait mode). On the X chromosome, 1,616,121 autosomal and 256,948 SNPs were tested. The F1 males inherited their X chromosome from the common transgene-containing strain. The identity by descent of this X chromosome allowed us to test whether the X-linked SNPs in the DGRP sample conformed to a null distribution assuming no association (although linkage is likely to cause deviation from this expectation). This was tested in quantile–quantile (Q–Q) plot analysis.
Association by mixed linear model to control for genetic relatedness
A Python implementation of EMMAX (Kang et al. 2010; Segura et al. 2012) was used to estimate the genetic related matrix (GRM) using inverse variance-weighted SNPs. The GRM is plotted using the pheatmap package in R to visualize any cryptic relatedness (Kolde 2011). When performing mixed linear model regression, we used the GRM estimated from just the X-chromosome SNPs, for which the mixed model yields a narrow sense heritability of 0.83 (SNPs with MAF >0.05). By doing so, we increase our power to detect associations at loci on the other chromosomes, because those are not included in the GRM (Listgarten et al. 2012). The ∼250K SNPs on the X chromosome are sufficient for inferring the population structure in the sample and thereby controlling population stratification. This is evident by the uniform P-value distribution in the Q–Q plots (Figure S4). To assess the genome-wide significance threshold while accounting for both the relatedness structure in the data as well as the nonindependence between SNPs due to LD, we performed a permutation procedure (details in File S1).
Conditional analysis using sfl intronic SNPs as covariates
To identify possible secondary associations in sfl or elsewhere in the genome independent of the intronic QTL variants in sfl, we fit a linear model with the most significant variant, an 18-bp/4-bp insertion/deletion polymorphism, as a covariate. This analysis was performed either within the sfl locus or genome wide. The P-values were corrected for multiple testing using Bonferroni’s method.
Estimate proportion of variance explained by common SNPs
We first used GCTA (v. 1.0) to estimate the genetic relatedness matrix with all SNPs with minor allele frequency >5% (–MAF 0.05). We then used the restricted maximum-likelihood (REML) method implemented in GCTA to estimate the quantity VG/VP (–REML), i.e., the narrow sense heritability.
Expression of sfl and CG32396
Expression profiles in adult tissues were assessed using data from FlyAtlas (Chintapalli et al. 2007) and modENCODE (Roy et al. 2010). To assay expression in the eye imaginal discs, we isolated total RNA from 10 pairs of discs from third-instar larvae. The individual larva was sexed and dissected in 1× phosphate buffer saline (PBS); the eye portions of the eye-antennal disc were collected and the isolated discs immediately dissolved in 300 µl Trizol (Invitrogen). Total RNA was extracted according to the manufacturer’s instructions. cDNA libraries were constructed using (dT)20 primers after DNase I treatment (Invitrogen). Real-time quantitative PCR was performed with primer pairs targeting either sfl or CG32396, with expression of the gene rp49 as an endogenous reference (SYBR-Green assay). Primers used for qRT–PCR are listed in Table S3.
RNAi and validation studies
All RNAi lines were originally from the Vienna Drosophila RNAi Center as P-element insertion lines on a co-isogenic w1118 background. Each RNAi line was first tested to determine whether it alone had an effect on eye development by crossing it to GMR–Gal4 and comparing the eye area of the F1 males (or females) to the control cross between w1118 and GMR–Gal4. In all crosses, GMR–Gal4 was used as the maternal parent. To test its effect on the hINSC96Y-induced eye-degeneration phenotype, the RNAi line was crossed to the GMR >> hINSC96Y line (used as maternal parent), so that both hINSC96Y and the RNAi constructs are driven by GMR–Gal4. The resulting phenotype was compared to the cross between hINSC96Y females and w1118 males. At least 10 individual flies were measured per cross and a t-test was used to determine significance at 0.05 level with multiple testing correction. For mutant lines, GMR–Gal4 was replaced with w1118 in the first test and used as a control. The same scheme was used for the second test. It is worth noting that because the mutants were tested in heterozygous states, only dominant interaction with hINSC96Y are revealed.
sfl expression studies
Six lines carrying the 18-bp indel allele and eight carrying the 4-bp allele were chosen and paired to form 15 crosses (Figure S1A). Three sets of 10 late third-instar (wandering stage) larvae were collected from each cross and dissected in 1× PBS to isolate eye imaginal discs. RNA isolation and cDNA library preparation are the same as described above. Genomic DNA was extracted from adult flies from the same cross. Because the 18-bp/4-bp polymorphism is in the intron of sfl, a SNP in the cDNA that could be used to distinguish the two alleles in each cross was identified (Figure S1B). Four such SNPs were chosen and pyrosequencing assays were designed (primers listed in Table S3). Pyrosequencing was performed as previously described (Wittkopp 2011). Briefly, each of the three cDNA and one gDNA sample per cross were analyzed by pyrosequencing in four replicate PCR amplifications to determine relative expression. The ratio in genomic DNA analysis was used to account for amplification bias. The resulting 12 ratios were first log2 transformed and analyzed using ANOVA according to the model yij = α + Li + εij, where α is the estimate of the relative expression ratio, which is expected to be significantly different from zero when the two alleles are differentially expressed; Li is a random effect term for the biological replicates (i = 1, 2, 3). For 13 of the 15 crosses the P-value >0.1; for these crosses the data were fit to a reduced ANOVA model yi = α + εi, from which the estimate and the 95% confidence interval for the ratio of expression (α) were calculated. In the two cases where the random effect term was nominally significant (P < 0.1), a linear mixed-effect model was fit using the lme package in R to obtain an estimate and 95% confidence interval for the same ratio.
Results
Effect of natural variation on hINSC96Y-induced eye phenotype
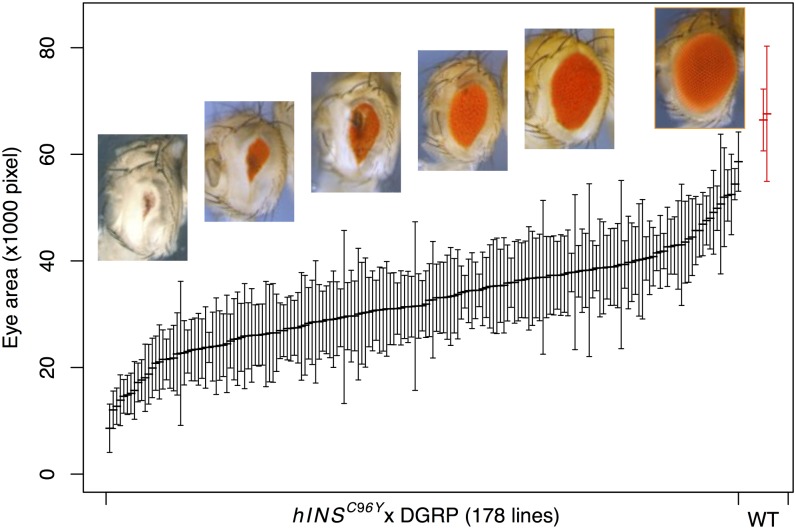
We crossed the transgenic fly line (w; P{GMR–Gal4}, P{UAS–hINSC96Y }/CyO) as the maternal parent to 178 inbred lines from DGRP (only 154 were used in the subsequent GWAS analyses due to genome sequence availability). These lines represent a spectrum of natural variation, except for recessive lethal variants, which were eliminated in the formation of the DGRP. Among several eye phenotypes observed—rough eye, reduced total area, distortion of the oval shape, and black lesion spots—we chose total eye area as the phenotype to carry out a GWAS. We quantified eye area in 10 male progeny from each hINSC96Y × DGRP cross. We observed a continuously varying distribution of this phenotype, ranging from 13 to 86% of wild-type fly eye area (Figure 1). ANOVA indicated that 58.6% of the variance is between genotypes [approximately equal to the broad sense heritability (Falconer 1981, p. 115)], indicating a large genetic component. Males were chosen for measurement and analysis because they showed a more severe phenotype than females (Park et al. 2013). However, we also measured F1 females for a subset of 38 lines and found a strong correlation between the two sexes from the same cross (r = 0.8, Figure S2).
Figure 1.
Distribution of eye area in hINSC96Y × DGRP crosses. Mean ±1 SD, sorted by the mean, is shown for crosses between the transgenic {GMR >> hINSC96Y} line to 178 DGRP lines, and two randomly chosen DGRP inbred lines (red). Representative photographs of eyes from across the range of the distribution are shown. The rightmost image is of a nontransgenic wild-type fly eye.
The observed variation in eye degeneration is consistent with the hypothesis that it reflects differences in cellular response to the expression of hINSC96Y. The severity of the eye-degeneration phenotype is not correlated with body size of the same individual or the mean eye size of the same line, nor is it correlated with GAL4 protein levels in eye imaginal discs (Park et al. 2013). The GWAS described below showed no evidence for association between eye area and SNPs in or surrounding the glass (gl) locus, the trans-activator of GMR–Gal4, a result consistent with Gal4 protein measurements and the fact that the eye-degeneration phenotype is insensitive to GMR–Gal4 gene dose when hINSC96Y is present in single copy (Park et al. 2013, Figure 3). Finally, when we expressed hINSC96Y in the notum (rather than the eye) and measured the loss of macrochaetae in F1 crosses to 38 DGRP lines for which we also collected eye-degeneration data, we observed no correlation between the two traits, indicating that the degeneration phenotypes are not caused by line-specific differences in mutant insulin expression (Park et al. 2013).
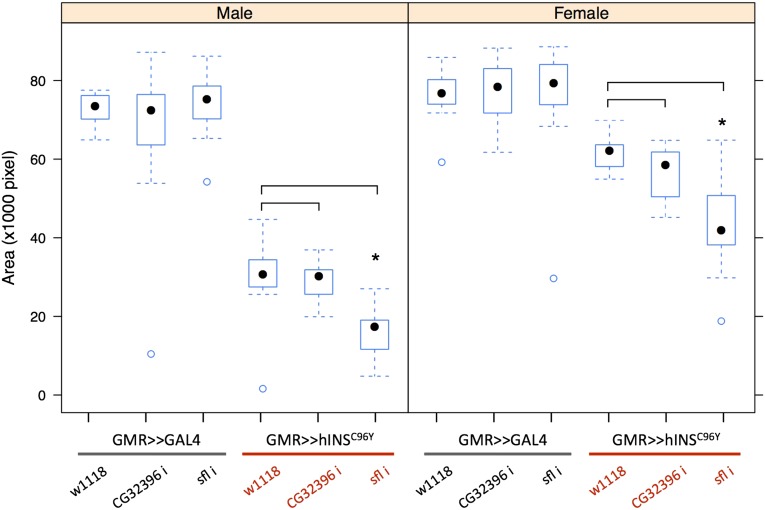
Figure 3.
RNAi knockdown confirms sfl and excludes CG32396 as the causal gene. The effect of knocking down either CG32396 or sfl was tested in the absence ({UAS–RNAi} × {GMR–Gal4}) or presence ({UAS–RNAi} × {GMR–Gal4, UAS–hINSC96Y}) of hINSC96Y. Compared to the control crosses (first and third columns in both sexes), significant difference in mean eye area was observed only with RNAi against sfl and only in the presence of hINSC96Y (n = 15, asterisks above a box plot indicate significant differences at 0.05 level determined by a student’s t-test, with Bonferroni correction for multiple testing). In box plots, the median (black dot), interquartile (box), and 1.5 times the interquartile range (whiskers) are indicated; data points outside the range are represented by circles.
Genome-wide association analysis
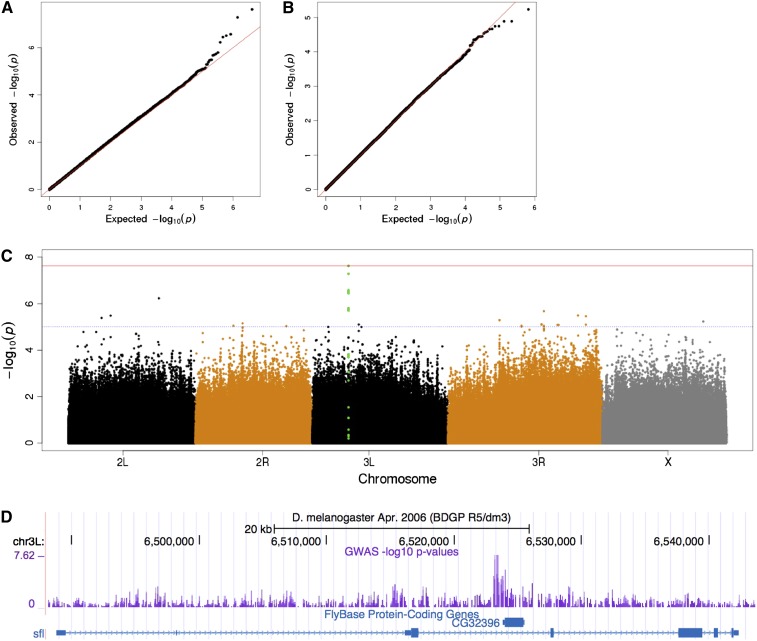
To identify candidate genetic loci and variants underlying the phenotypic variation, we carried out GWAS on the F1 males from the crosses of hINSC96Y and 154 DGRP lines. We used mean eye area as a quantitative trait to perform single-marker regression for 1.6 million autosomal SNPs, restricted to biallelic sites for which the minor allele frequency is at least 5%. The result revealed a strong peak on chromosome 3L and minor ones on other major chromosome arms (Figure 2C). The most significant SNP underlying the chromosome 3L peak has a raw P-value of 2.4 × 10−8 (t-test); the Bonferroni-corrected P = 0.04.
Figure 2.
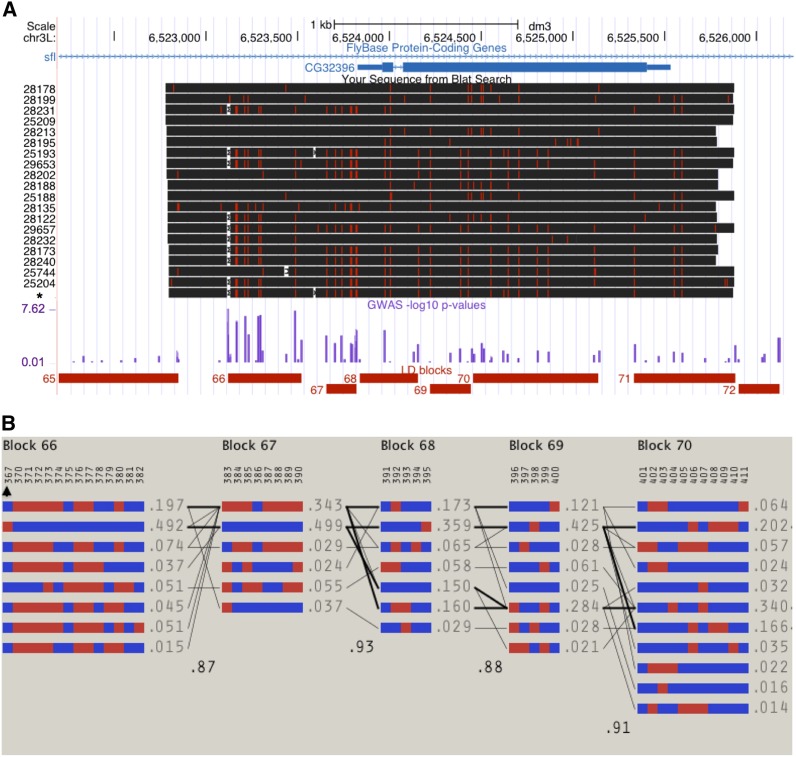
Genome-wide scan identifies candidate locus associated with the hINSC96Y-induced phenotype. Quantile–quantile (Q–Q) plot reveals an excess of small P-values on autosomes (A) but not on the X chromosome (B), which is not variable in the mapping population due to cross design. (C) Manhattan plot shows a strong peak (green) on chromosome 3L. The blue and red horizontal lines indicate raw P < 10−5 and Bonferroni corrected P < 0.05, respectively. (D) UCSC browser view of the sfl locus containing the association peak. The intron containing the peak also contains a nested gene CG32396.
Population stratification is a potential confounder for GWAS—it can inflate the test statistic for nonassociated variants if the population structure correlates with the phenotype. We assessed its impact in our study in three ways. First, we evaluated the Q–Q plots for autosomal and X-linked variants. Neither showed a systematic shift toward low P-values compared to the null expectation, which would be expected if population structure induces false association signals (Figure 2, A and B). Second, we used principle component analysis (PCA) to calculate the top eigenvectors explaining the most genetic variation in the sample. Plotting the phenotype of each cross against the coordinate of each of the top five eigenvectors revealed no correlation between the two (Materials and Methods and Figure S3). Third, because all F1 males inherited their X-chromosome from the GMR >> hINSC96Y tester line, we expect no association between the phenotype and X-linked SNPs. Indeed, we found only an excess of low P-values in autosomal variants, but not in X-linked ones (Figure 2, A and B). The above analyses suggest that population stratification does not correlate with the trait and does not influence the results of the association study.
Cryptic relatedness, i.e., unknown genetic relationships between individuals in a sample, can also confound the association analysis due to nonindependence and larger than expected phenotypic variance (Voight and Pritchard 2005; Cheng et al. 2010). We estimated the GRM from whole-genome SNP data using mixmogam (a Python implementation of EMMAX) (Kang et al. 2010; Segura et al. 2012). We found that while the majority of the 154 lines are genetically unrelated (Figure S4A), several pairs of lines showed higher levels of relatedness, e.g., RAL-350/RAL-358 and RAL-352/RAL-712 (Figure S4B). Next, we performed mixed linear model (MLM) regression to explicitly account for the cryptic relatedness as well as population stratification (Yu et al. 2006; Atwell et al. 2010). A permutation procedure specifically designed to preserve the phenotype covariance structure is used to establish a genome-wide 5% significance threshold (File S1). The resulting P-value distribution is qualitatively similar to the linear regression analysis, and it identified sfl as significantly associated with the trait under a permutation-based 5% genome-wide threshold (Figure S4E). The most significant SNP (3L:6523119, dm3) has a raw P-value of 1.4 × 10−8. Below we focus on identifying the gene(s) underlying the peak and genetically testing its association with the phenotype.
sfl modifies eye area phenotype
The peak on chromosome 3L is confined to the third intron of the gene, sfl (Figure 2D). This intron also contains a nested gene (CG32396) lying close to the association peak. CG32396 is predicted to encode a protein with a probable tubulin β-chain. To determine which of the two genes, or possibly both, is responsible for the association, we examined the expression pattern of each gene and also used RNAi to knock down gene expression. sfl is expressed in the eye-antennal imaginal disc and eye and brain in adults (Figure S5 and Figure S6). CG32396 has a testis-specific expression pattern in adults, with very low expression in the adult eye (Figure S5) and no detectable expression in eye imaginal discs by RT–PCR (Figure S6 and Figure S7).
RNAi knockdown of either sfl or CG32396 in the eye imaginal disc had no measurable effect on eye area. In contrast, RNAi against sfl, but not CG32396, significantly decreased mean eye area in the presence of hINSC96Y but not hINSWT (Figure 3). These results rule out CG32396 as the causal gene and strongly implicate sfl as the genetic modifier of hINSC96Y-induced eye degeneration.
To test if sfl also modifies the hINSC96Y-induced phenotype in other tissues, we carried out RNAi knockdown of sfl in the developing wing (using a dpp–Gal4 driver) and notum (using an ap–Gal4 driver). In both experiments we observed more severe phenotypes than that caused by hINSC96Y alone (Figure S8 and Figure S9). However, the interpretation is complicated by the fact that sfl knockdown alone causes mutant phenotypes in these tissues, consistent with previous knowledge (Lin 2004). At present we cannot distinguish the alternative hypotheses of additive vs. epistatic interactions between sfl and hINSC96Y.
Heparan sulfate biosynthetic pathway modifies the hINSC96Y-induced eye degeneration
Sulfateless encodes a bifunctional enzyme in the heparin sulfate biosynthesis pathway. An important component of the cell surface and extracellular matrix (Kirkpatrick and Selleck 2007), HSPGs regulate signaling during development by influencing the levels and activity of growth factors and morphogens at cell surfaces and in the extracellular matrix (Nakato et al. 1995; Häcker et al. 1997; Giráldez et al. 2002; Fujise et al. 2003; Kirkpatrick et al. 2004). The involvement of HSPGs in the cellular responses to misfolded proteins (proteostasis) has not been previously described.
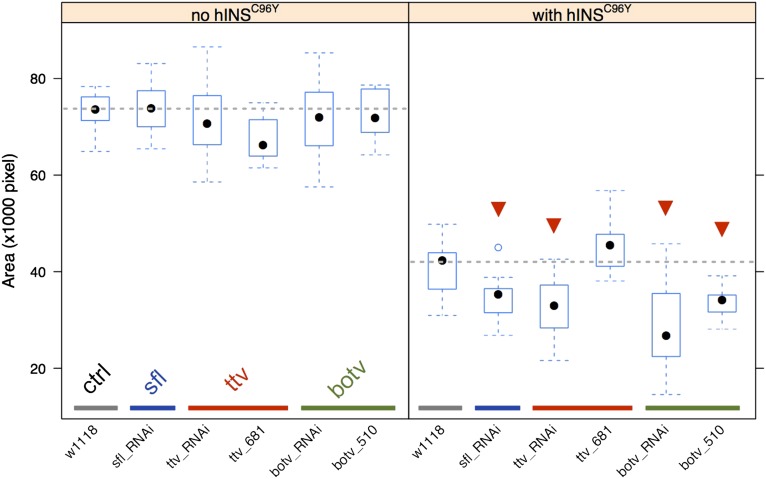
To further examine the hINSC96Y-dependent interaction of sfl, we examined RNAi knockdowns and mutants for two additional genes in the HS biosynthetic pathway, ttv and botv, producing the glycosaminoglycan polymer that is modified by sfl (Lin 2004). SNPs in neither of the genes showed evidence of association in our GWAS (lowest adjusted P > 0.5 in both loci, adjusted for multiple-testing using Bonferroni’s method). RNAi knockdown of both genes shows a hINSC96Y-dependent effect on eye area in the same direction as sfl RNAi (Figure 4). In addition, a mutant allele of botv also showed a significant dominant enhancement of the eye-degeneration phenotype. These results implicate HSPGs in modifying the cellular response to misfolded proteins. Neither of the genes, however, was identified in the GWAS.
Figure 4.
RNAi and mutant analysis for heparin sulfate biosynthesis pathway genes. The experimental design is the same as in Figure 3. Left: the effect of RNAi or mutant alleles in the absence of hINSC96Y expression. Right: the effect when hINSC96Y is expressed in the eye imaginal disc. Mutants were tested in heterozygous states for a dominant interaction with hINSC96Y. Fifteen male flies are measured for each group. The statistical significance of differences from the control cross (gray, w1118) was determined by a two-sided student’s t-test. Those that are significant at 0.05 level after Bonferroni correction are marked with a red arrowhead.
Intronic variation and sfl expression
We resequenced a 3-kb region containing the GWAS peak in sfl (and the nested gene CG32396) in 19 of the 154 DGRP lines and the transgenic hINSC96Y stock to identify all the variants in this region. We found that the SNP achieving the lowest P-value genome-wide was an 18-bp/4-bp-length polymorphism (relative to the Drosophila simulans orthologous sequence) (Figure 5A). We also found three other insertion/deletion (INDEL) polymorphisms in this region, with sizes ranging from 4 to 30 bp and the minor alleles (deletion in all three cases) being present only once or twice in the sample. In contrast, the 18-/4-bp polymorphism is present at 50% frequency in the DGRP sample. Below we use the term single-feature polymorphism (SFP) to refer to both INDEL and single-nucleotide polymorphism in the sfl locus.
Figure 5.
Sequencing of a 3-kb region in sfl and the LD patterns in the region. (A) Alignment of 19 DGRP sequences ordered by their eye-degeneration phenotype (mean, most severe on the bottom). The hINSC96Y transgenic line (asterisk) was also sequenced. Red ticks and white spaces indicate SNPs and deletions relative to the reference sequence. No insertions relative to the reference were found. The purple track shows the −log10 of GWAS P-values. The bottom track shows the linkage blocks as determined by Haploview (4.02) using the solid spine method with default settings (D’ > 0.8). (B) Detailed haplotype block structures. Each numbered column represents a polymorphic site, with the alleles colored as blue or red; each row represents a haplotype with frequency >0.01. An arrowhead marks the 18-/4-bp indel polymorphism (see text; 18 bp, blue; 4 bp, red). Finally, the number between any two blocks represents the multiallelic D’, which quantifies the associations between adjacent blocks.
A plot of haplotype structure surrounding the association peak (Haploview v. 4.2) pinpoints an LD block of 400 bp (block 66 in Figure 5A, chr3L:6523119–6523518). There are two major haplotypes in this block, each represented by two equal-sized groups among the 154 DGRP lines (Figure 5B). For convenience, we refer to these two haplotypes as the 18-bp or 4-bp allele, although it is worth noting that we do not have the ability to distinguish between the SFPs within this block, unless further recombinant individuals are sampled or generated.
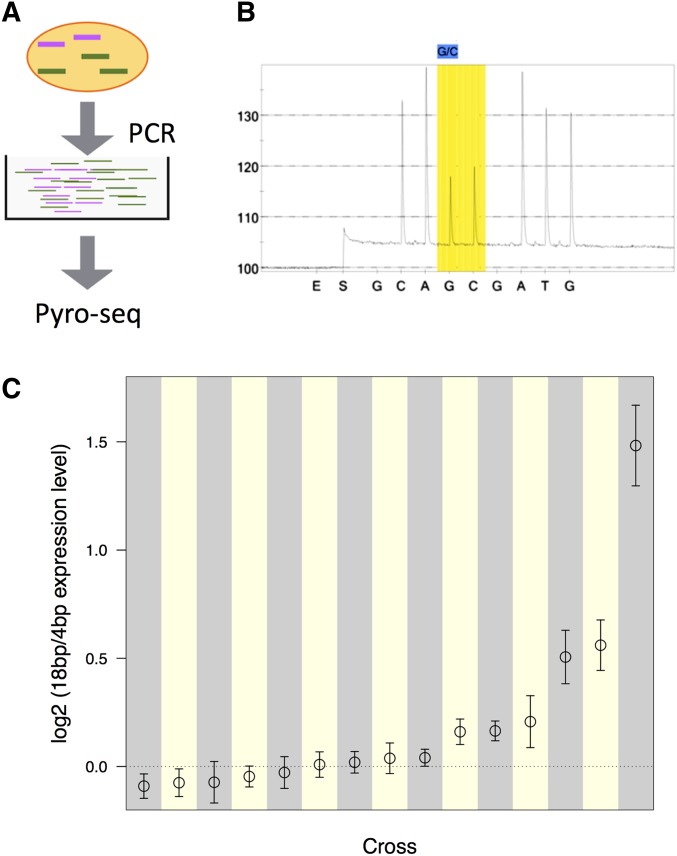
Because all coding variants in sfl lie outside of this 400-bp LD block, we hypothesized that one or more of these intronic SFPs are the causal variant(s) and modify the hINSC96Y-induced eye phenotype by altering sfl expression. We tested this hypothesis by examining the correlation between the allelic states and the allele-specific expression level. We selected pairs of 4- and 18-bp lines from the respective phenotypic spectrum, crossed them to obtain F1 individuals heterozygous for the two alleles, and used pyrosequencing to estimate the relative expression of the two alleles in eye imaginal discs. This method allowed us to measure the ratio of expression of sfl associated with each allele in the same animal, thereby controlling for both the trans-environment as well as experimental noise, resulting in highly reproducible results (Figure S10). Based on RNAi knock-down of sfl, which enhanced the hINSC96Y phenotype, we expected the 4-bp allele (associated with more severe phenotypes in the GWAS) to produce less transcript than the 18-bp allele.
Allele-specific expression of sfl differed in both magnitude and direction among the 15 crosses (Figure 6). Seven crosses supported the hypothesis by exhibiting significantly greater expression from the 18-bp allele, with an 18-/4-bp ratio ranging from 1.03 to 2.8 (median 1.15). Two crosses, however, showed slightly greater expression from the 4-bp allele (18-bp/4-bp ratios of 0.94 and 0.96). The remaining six crosses showed no significant differences in expression of the two alleles in our test. While more strains showed higher expression of the transcript linked to the 18-bp allele and the difference in this direction is stronger, the small sample size and the modest correlation between the allelic states and the transcription level prevented us from drawing a conclusion. Proving the causal mutation(s) and identifying the mechanisms require further experiments making precise changes at the candidate loci and assaying the effects in the same genetic background.
Figure 6.
Pyro-sequencing measure of sfl allele-specific transcript ratio in 18-/4-bp heterozygotes. (A) Schematic diagram of the pyrosequencing approach. Colored lines represent transcripts (mRNA) associated with either the 18 or the 4bp allele, expressed at different levels. Common primers were used to amplify both transcripts of the gene of interest from the cDNA library made from eye imaginal disc tissues. Pyrosequencing was carried out on the amplified products. (B) A pyrogram of a heterozygote with the polymorphic site (G/C) that is diagnostic for the 18-/4-bp indel highlighted. The ratio of the two peaks (light intensity, y-axis) are used to calculate the relative ratio of the two alleles. (E, enzyme; S, substrate; A/C/G/T, nucleotides). (C) Log2-transformed ratio of 18-/4-bp allele expression in 15 crosses between randomly paired 18- and 4-bp lines. Estimates of the ratio and 95% confidence intervals are plotted. The dotted line corresponds to equal expression from the two alternative alleles.
Search for additional association by conditional analysis
In light of the above finding, we carried out a conditional analysis to identify variants that act independently of the 18-/4-bp SFP. To do so, we tested variants other than the 18-/4-bp SFP, either within the sfl locus or genome wide, by treating the 18-/4-bp SFP as a covariate in a linear regression model. After accounting for multiple testing, we observed no significant signals in either case (Figure S11). The lack of significance genome wide may be attributable to the lack of power after correcting for multiple testing. The analysis restricted to the 40-kb sfl locus reduces the burden of multiple testing by several orders of magnitude, but also fails to identify a significant association. Considering the large range of allele-specific expression differences between the 18- and 4-bp alleles observed in the 15 crosses, the additional cis-acting expression variants must either be low frequency alleles or have epistatic properties, two situations this analysis would be underpowered to detect.
Discussion
sfl and hINSC96Y-induced eye degeneration
Statistical (GWAS) and genetic (RNAi) evidence support a role for sfl as a natural genetic modifier for hINSC96Y-induced eye degeneration. Although we conducted a GWAS for dominant-acting modifiers in a relatively small sample of lines (154) considering the large number of segregating common SNPs (1.6 million), we found statistical support for a QTL in sfl in a mixed model analysis, which addresses effects of both population structure and genetic relatedness in the sample. One possible reason that the sfl QTL achieves statistical significance is because the two alternative alleles occur at a ∼50% frequency in the sample, where GWAS is maximally powerful.
RNAi knockdown experiments showed that perturbation of sfl expression, and also two other genes in the HS biosynthesis pathway, has a measurable effect on eye degeneration, but only in the presence of hINSC96Y expression, indicating a specific interaction between protein misfolding and HS biosynthesis (also see Park et al. 2013). RNAi against CG32396, the gene nested inside the intron of sfl, had no effect on eye area in both the absence and presence of hINSC96Y, suggesting that the hINSC96Y-induced eye-degeneration phenotype is not simply a consequence of RNAi expression. We caution, however, that genetic proof of sfl as modifying the phenotype in this population will require additional studies.
A direct test for sfl and the intronic variation being causal would be to genetically engineer two lines in the same genetic background, differing only at the sfl locus. A potential caveat of this approach lies in the assumption that the differential activity of the two alleles is independent of the genetic background (i.e., no epistasis), which, if violated, will lead to a false-negative result (Chandler et al. 2013). We used instead an indirect approach by examining the correlation between the allelic states and the expression level. To take into account the genetic background differences, we measured allele-specific gene expression of 18- and 4-bp sfl alleles in 15 different “controlled” genetic backgrounds, but keeping the background the same for the two alleles by comparing their expression ratios in heterozygotes. The results are mixed: the ratio of expression from the 18-/4-bp alleles differed in the 15 crosses, ranging from 2.8 to 0.94 (Figure 6); nearly half (7/15) showed greater expression from the allele associated with the 18-bp variant, consistent with the expectation based on the RNAi result; two showed a small difference in the contrary direction (18-/4-bp ratio = 0.94 and 0.96); and the remaining six were insignificant in our test. This marked heterogeneity in expression means we can neither accept nor reject the hypothesis of a causal role for the intronic variants and the expression level of sfl. Hence we are also not able to conclude that expression difference is the mechanism underlying the genotype–phenotype association, although it remains a possibility. Future experiments employing genome-editing technologies will allow better resolution of the mechanism(s) underlying the association (Jinek et al. 2012; Gratz et al. 2013; Ran et al. 2013).
Finally, we investigated whether additional eQTLs exist in sfl or in other genes acting epistatically with sfl. Likely due to lack of power, a conditional analysis failed to identify additional variants in the sfl locus or elsewhere in the genome. However, it is now well established that gene expression is a highly polygenic trait in Drosophila melanogaster, with many eQTLs contributing to expression variability both in cis and in trans (Brem et al. 2002, 2005; West et al. 2007), and intralocus genetic complexity influencing a quantitative trait has long been known, as in the Adh example (King et al. 2012). In the 40-kb region spanning the sfl locus alone, 1358 SNPs are present among the 14 lines used in this experiment, which individually or in combination could influence expression of the gene. Thus, predictions based on one or two strongly associated variant(s) is not adequate. A polygenic risk predictor may be needed to summarize contributions even from a single locus.
HSPG function and misfolded protein response
Our study identified the HS biosynthesis pathway (sfl, ttv, and botv) as a modifier of eye degeneration induced by expression of a misfolded human proinsulin protein. Although we do not yet know whether this response is to a specific misfolded protein (hINSC96Y) or whether it applies to a broader class of misfolded proteins, our discovery now implicates the HSPGs in the regulation of cellular proteostasis.
We propose that genetic variation in HS biosynthesis influences the response to misfolded protein through its biological activity in vesicular trafficking of misfolded protein. HS-modified proteins (HSPGs) are abundant components of cell surfaces and extracellular matrices and are best understood for their roles in cell signaling and in functioning as coreceptors, processes integral to normal development (Häcker et al. 2005; Kirkpatrick and Selleck 2007). HSPGs are also involved in endocytocis (Ren et al. 2009; Stanford et al. 2009) and vesicular trafficking (Nybakken and Perrimon 2002; Sarrazin et al. 2011), roles that may link them to cellular response to misfolded proteins (Higashio and Kohno 2002; Kim et al. 2009; Kimmig et al. 2012).
HSPGs may also influence membrane trafficking indirectly, perhaps by regulating signaling events that impinge on trafficking processes. The generation of phosphatidylinositol (3,4,5) triphosphate [PtdIns(3,4,5)P3] by type I phosphoinositide (PI) 3-kinases is affected by a number of growth factors and cytokines, many of which are influenced by HSPGs as accessory molecules. PtdIns(3,4,5)P3 affects a number of trafficking events, including endocytosis and autophagy (Downes et al. 2005).
In a yeast study of the mutant protein folding assistant, protein disulfide isomerase (Pdi1a’), the authors found that more than 50% of the 130 genes identified as synthetic-lethal were related to vesicle trafficking, while only 10 belonged to the canonical unfolded protein response (UPR) pathway (Kim et al. 2009). In another study, Kimmig et al. (2012) found an enrichment of vesicle-trafficking-related genes among those that changed expression significantly after induction of ER stress. Both studies indicate that a global regulation of vesicle trafficking is important to a cell’s response to unfolded or misfolded protein. Activation of UPR has also been shown to affect ER-to-Golgi transport via stimulation of COPII vesicle formation from the ER (Higashio and Kohno 2002). We propose that either natural variation or genetic perturbation of HS biosynthesis influences the global regulation of vesicle trafficking, which in turn affects the cell’s ability to process an excess of unfolded or misfolded protein. Prolonged ER stress may then lead to apoptosis.
Genetic architecture of the hINSC96Y-induced eye-degeneration phenotypes
Phenotypic heterogeneity that is dependent on the genetic background is a common phenomenon and, in humans, imposes a significant challenge in both diagnosis and treatment. Our fly model provides a tractable system for studying the genetic and molecular basis for such phenotypic heterogeneity, but with limitations imposed by the sample size of the study. To assess the power for identifying QTL using this population, we did a simple calculation for a t-test-based statistic at P = 0.05 level, with Bonferroni’s correction for multiple testing, which indicates that we have 66% power to identify a variant at 50% population frequency, with an effect size of 1.0 (measured as the shift in phenotypic mean in units of standard deviation of the trait; see Table S4). This example was chosen to match the estimates for the 18-/4-bp indel polymorphism in the sfl intron in the sample of 154 crosses. Any variant with a smaller effect size and/or lower frequency than the 18-/4-bp polymorphism would likely have been missed in this study.
Sulfateless was the only QTL identified as genome-wide significant in this study (Figure 2 and Figure S4); its association with the trait is robust with respect to population structure and cryptic relatedness (Figure S3 and Figure S4). This does not mean, however, that the genetic architecture for the hINSC96Y-induced eye phenotype involves a single locus. Rather, we have several reasons to believe that the genetic architecture must involve many loci. First, the distribution of the phenotype, i.e., eye areas expressed as line means, suggests a non-Mendelian genetic basis (Figure 1). Second, while ANOVA estimates that nearly 60% of the total phenotypic variance is between crosses, <20% within the 60% (i.e., <12% of the total variance) can be attributed to the sfl locus. Even this 20% estimate, because it is derived from the same population used to identify the locus, is liable to be an overestimate due to the Winner’s curse effect (Garner 2007).
To estimate what percentage of the between-cross variance can be explained by the additive effects of common variants combined, we applied the GCTA tool, which uses a mixed linear model method, to the line means of the 154 crosses (Yang et al. 2011). The result showed that 83% (standard error 37%) of the variance between crosses could be attributed to common, autosomal variants with minor allele frequencies >5%. Analysis using GEMMA (v. 0.94beta), which used a Bayesian method, achieved nearly identical results (posterior mode 0.83, SE 0.41). We then did the same analysis with GCTA, but including the 18-/4-bp indel polymorphism as a covariate to remove the effect of sfl, to estimate the remaining additive heritability. As a result, we got 62% (SE 47%). The large standard error as a result of the limited sample size leaves the proportion of variance explained by all common SNPs undetermined. However, the estimates are encouraging and suggest that a potentially large proportion of phenotype variance may be explained by additional loci, which require larger sample size to identify.
Relationship to common, complex diseases
While our fly model is of a monogenic form of diabetes, it exhibits a complex genetic architecture when placed on a diverse set of genetic backgrounds. We posit that fly models of monogenetic disease are suitable subjects for the genetic dissection of common disorders in humans.
One role of the Mendelian mutation is to sensitize the fly to allow phenotypic effects of background genetic modifiers to become visible. Although common disorders are normally considered as lacking a major mutation, a careful consideration suggests that this view is inaccurate. What common disorders lack are large-effect mutations shared by a substantial proportion of the affected individuals. For many iseases, perturbation may be required to boost the expressivity of additive genetic variation that would otherwise be cryptic, i.e., below a disease-causing threshold. Such a perturbation could be genetic, such as driver mutations in cancer, but could also be environmental, such as diet and lifestyle changes in the case of cardiovascular disease and type 2 diabetes. Consistent with this view, it has been proposed that recent genome evolution and rapid environmental as well as cultural changes in human history have decanalizing effects on physiology, which release cryptic genetic variation and underlie the rising incidence of common human disorders (Gibson 2009).
A genetic screen for naturally occurring modifiers in a sensitized background, such as the one we employed here, should apply equally well in the study of Mendelian or complex disease. Were this not the case, two different classes of genetic modifiers would have to be posited. An intriguing question, which we found little empirical evidence for or against, could be addressed in the fly by constructing a series of sensitized backgrounds utilizing different disease-causing mutant hINS alleles of varying effect on disease [e.g., neonatal diabetes vs. maturity-onset diabetes of the young (Støy et al. 2007)] and comparing the composition of naturally occurring modifiers.
Advantages of a fly model of complex disease
A primary mutation can manifest itself in different ways and with tissue-specific effects (Mefford et al. 2008), possibly a consequence of its interdependence with the individual’s genetic background. The binary Gal4–UAS system enables the creation of a series of models using the same disease mechanism, but directed to different tissues with high tissue specificity. The ability to construct and study multiple related models in parallel can provide insight into the basis of disease heterogeneity. In the accompanying article we show, for example, that the developing eye and notum have different sets of genetic background modifiers of hINSC96Y-dependent disease (Park et al. 2013). Sex-specific differences in disease risk and severity are also readily modeled in the fly. In both the fly and mouse model of hINSC96Y-induced disease, males consistently show more severe disease phenotypes (Wang et al. 1999; Park et al. 2013).
Drosophila models of human disease provide a useful alternative to the study of complex disease in patient populations. First, many models of human disease have been established in the fly, most notably neurodegeneration and cancer (Bilen and Bonini 2005; Gonzalez 2013). We predict that natural variation will influence the severity of disease phenotypes in all of them. Second, many models of disease can be created by expression of a mutant allele, which makes them suitable for F1 screens between a tester stock and inbred population collections, such as we employed here. Our study shows that dominant genetic variation for disease severity is abundant. This outcrossing design also avoids unwanted effects of inbreeding on traits and better mimics the natural heterozygosity of low-frequency variants. Third, this experimental design facilitates repeated measurement of a disease phenotype, thereby increasing the power to detect a causal association (Mackay et al. 2009). Fourth, LD is low in D. melanogaster and SNP are 20–40× more abundant than in humans. Finally, both forward and reverse genetics can be applied to investigate the biology and pathway genetics of candidate variants. For all these reasons we believe fly models will prove useful in understanding the genetic architecture of complex human disease.
Supplementary Material
Acknowledgments
We thank Dan Nicolae for technical help and advice on GWAS and Xiang Zhou and Matthew Stephens for advice on the mixed linear model approach using Gemma. We thank Jian Yang and Peter Visscher for help with the interpretation of the GCTA results. Joseph Coolon in the Wittkopp lab helped design the pyro-sequencing assays, and Ellen Pederson at the DNA sequencing center at the University of Michigan provided technical assistance. We also thank the anonymous reviewer and Dr. Sabatti for helpful comments. This work was funded by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK013914 and P30 DK020595), the National Institute of General Medical Sciences (GM081892), the Chicago Biomedical Consortium with support from the Searle Funds at The Chicago Community Trust, and a gift from the Kovler Family Foundation. S.B.S. is supported by GM054832 and P.J.W. is supported by National Science Foundation MCB-1021398.
Note added in proof: See Park et al. 2014 (pp. 539–555) in this issue for a related work.
Footnotes
Communicating editor: C. Sabatti
Literature Cited
- Atwell S., Huang Y. S., Vilhjalmsson B. J., Willems G., Horton M., et al. , 2010. Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature 465: 627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilen J., Bonini N. M., 2005. Drosophila as a model for human neurodegenerative disease. Annu. Rev. Genet. 39: 153–171. [DOI] [PubMed] [Google Scholar]
- Brem R. B., Yvert G., Clinton R., Kruglyak L., 2002. Genetic dissection of transcriptional regulation in budding yeast. Science 296: 752–755. [DOI] [PubMed] [Google Scholar]
- Brem R. B., Storey J. D., Whittle J., Kruglyak L., 2005. Genetic interactions between polymorphisms that affect gene expression in yeast. Nature 436: 701–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler C. H., Chari S., Dworkin I., 2013. Does your gene need a background check?: how genetic background impacts the analysis of mutations, genes, and evolution. Trends Genet. 29: 358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng R., Lim J. E., Samocha K. E., Sokoloff G., Abney M., et al. , 2010. Genome-wide association studies and the problem of relatedness among advanced intercross lines and other highly recombinant populations. Genetics 185: 1033–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapalli V. R., Wang J., Dow J. A. T., 2007. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 39: 715–720. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Gray A., Lucocq J. M., 2005. Probing phosphoinositide functions in signaling and membrane trafficking. Trends Cell Biol. 15: 259–268. [DOI] [PubMed] [Google Scholar]
- Fujise M., Takeo S., Kamimura K., Matsuo T., Aigaki T., Izumi S., et al. , 2003. Dally regulates Dpp morphogen gradient formation in the Drosophila wing. Development 130: 1515–1522. [DOI] [PubMed] [Google Scholar]
- Garner C., 2007. Upward bias in odds ratio estimates from genome-wide association studies. Genet. Epidemiol. 31: 288–295. [DOI] [PubMed] [Google Scholar]
- Gibson G., 2009. Decanalization and the origin of complex disease. Nat. Rev. Genet. 10: 134–140. [DOI] [PubMed] [Google Scholar]
- Giráldez A. J., Copley R. R., Cohen S. M., 2002. HSPG modification by the secreted enzyme Notum shapes the Wingless morphogen gradient. Dev. Cell 2: 667–676. [DOI] [PubMed] [Google Scholar]
- Gonzalez C., 2013. Drosophila melanogaster: a model and a tool to investigate malignancy and identify new therapeutics. Nat. Rev. Cancer 13: 172–183. [DOI] [PubMed] [Google Scholar]
- Gratz S. J., Cummings A. M., Nguyen J. N., Hamm D. C., Donohue L. K., et al. , 2013. Genome Engineering of Drosophila with the CRISPR RNA-Guided Cas9 Nuclease. Genetics 194: 1029–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häcker U., Lin X., Perrimon N., 1997. The Drosophila sugarless gene modulates Wingless signaling and encodes an enzyme involved in polysaccharide biosynthesis. Development 124: 3565–3573. [DOI] [PubMed] [Google Scholar]
- Häcker U., Nybakken K., Perrimon N., 2005. Heparan sulphate proteoglycans: the sweet side of development. Nat. Rev. Mol. Cell Biol. 6: 530–541. [DOI] [PubMed] [Google Scholar]
- Higashio H., Kohno K., 2002. A genetic link between the unfolded protein response and vesicle formation from the endoplasmic reticulum. Biochem. Biophys. Res. Commun. 296: 568–574. [DOI] [PubMed] [Google Scholar]
- Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J. A., Charpentier E., 2012. A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 337: 816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H. M. M., Sul J. H. H., Service S. K., Zaitlen N. A., Kong S.-Y. Y., et al. , 2010. Variance component model to account for sample structure in genome-wide association studies. Nat. Genet. 42: 348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.-H. H., Zhao Y., Pan X., He X., Gilbert H. F., 2009. The unfolded protein response is necessary but not sufficient to compensate for defects in disulfide isomerization. J. Biol. Chem. 284: 10400–10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmig P., Diaz M., Zheng J., Williams C. C., Lang A., et al. , 2012. The unfolded protein response in fission yeast modulates stability of select mRNAs to maintain protein homeostasis. eLife 1: e00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King E. G., Merkes C. M., McNeil C. L., Hoofer S. R., Sen S., Broman K. W., et al. , 2012. Genetic dissection of a model complex trait using the Drosophila Synthetic Population Resource. Genome Res. 22: 1558–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick C. A., Selleck S. B., 2007. Heparan sulfate proteoglycans at a glance. J. Cell Sci. 120: 1829–1832. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick C. A., Dimitroff B. D., Rawson J. M., Selleck S. B., 2004. Spatial regulation of Wingless morphogen distribution and signaling by Dally-like protein. Dev. Cell 7: 513–523. [DOI] [PubMed] [Google Scholar]
- Kolde, R., 2011 pheatmap: Pretty Heatmaps. http://cran.r-project.org/package=pheatmap.
- Lin X., 2004. Functions of heparan sulfate proteoglycans in cell signaling during development. Development 131: 6009–6021. [DOI] [PubMed] [Google Scholar]
- Listgarten J., Lippert C., Kadie C. M., Davidson R. I., Eskin E., Heckerman D., 2012. Improved linear mixed models for genome-wide association studies. Nat. Methods 9: 525–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay T. F. C., Stone E. A., Ayroles J. F., 2009. The genetics of quantitative traits: challenges and prospects. Nat. Rev. Genet. 10: 565–577. [DOI] [PubMed] [Google Scholar]
- Mefford H. C., Sharp A. J., Baker C., Itsara A., Jiang Z., et al. , 2008. Recurrent rearrangements of chromosome 1q21.1 and variable pediatric phenotypes. N. Engl. J. Med. 359: 1685–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakato H., Futch T. A., Selleck S. B., 1995. The division abnormally delayed (dally) gene: a putative integral membrane proteoglycan required for cell division patterning during postembryonic development of the nervous system in Drosophila. Development 121: 3687–3702. [DOI] [PubMed] [Google Scholar]
- Nybakken K., Perrimon N., 2002. Heparan sulfate proteoglycan modulation of developmental signaling in Drosophila. Biochim. Biophys. Acta 1573: 280–291. [DOI] [PubMed] [Google Scholar]
- Park S.-Y., Ludwig M. Z., Tamarina N. A., He B. Z., Carl S., et al. , 2013. Genetic complexity in a Drosophila model of diabetes-associated misfolded human proinsulin. Genetics 539–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran F. A., Hsu P. D., Lin C.-Y., Gootenberg J. S., Konermann S., et al. , 2013. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154: 1380–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y., Kirkpatrick C. A., Rawson J. M., Sun M., Selleck S. B., 2009. Cell type-specific requirements for heparan sulfate biosynthesis at the Drosophila neuromuscular junction: effects on synapse function, membrane trafficking, and mitochondrial localization. J. Neurosci. 29: 8539–8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Ernst J., Kharchenko P. V., Kheradpour P., Negre N., Eaton M. L., et al. , 2010. Identification of functional elements and regulatory circuits by Drosophila modENCODE. Science 330: 1787–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrazin S., Lamanna W. C., Esko J. D., 2011. Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. DOI: 10.1101/cshperspect.a004952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura V., Vilhjalmsson B. J., Platt A., Korte A., Seren U., et al. , 2012. An efficient multi-locus mixed-model approach for genome-wide association studies in structured populations. Nat. Genet. 44: 825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford K. I., Bishop J. R., Foley E. M., Gonzales J. C., Niesman I. R., et al. , 2009. Syndecan-1 is the primary heparan sulfate proteoglycan mediating hepatic clearance of triglyceride-rich lipoproteins in mice. J. Clin. Invest. 119: 3236–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Støy J., Edghill E. L., Flanagan S. E., Ye H., Paz V. P., et al. , 2007. Insulin gene mutations as a cause of permanent neonatal diabetes. Proc. Natl. Acad. Sci. USA 104: 15040–15044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voight B. F., Pritchard J. K., 2005. Confounding from cryptic relatedness in case-control association studies. PLoS Genet. 1: e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Takeuchi T., Tanaka S., Kubo S. K., Kayo T., et al. , 1999. A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J. Clin. Invest. 103: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West M. A., Kim K., Kliebenstein D. J., van Leeuwen H., Michelmore R. W., et al. , 2007. Global eQTL mapping reveals the complex genetic architecture of transcript-level variation in Arabidopsis. Genetics 175: 1441–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittkopp P. J., 2011. Using pyrosequencing to measure allele-specific mRNA abundance and infer the effects of cis- and trans-regulatory differences. Methods Mol. Biol. 772: 297–317. [DOI] [PubMed] [Google Scholar]
- Yang J., Lee S. H., Goddard M. E., Visscher P. M., 2011. GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 88: 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Pressoir G., Briggs W. H., Vroh Bi I., Yamasaki M., et al. , 2006. A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat. Genet. 38: 203–208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.