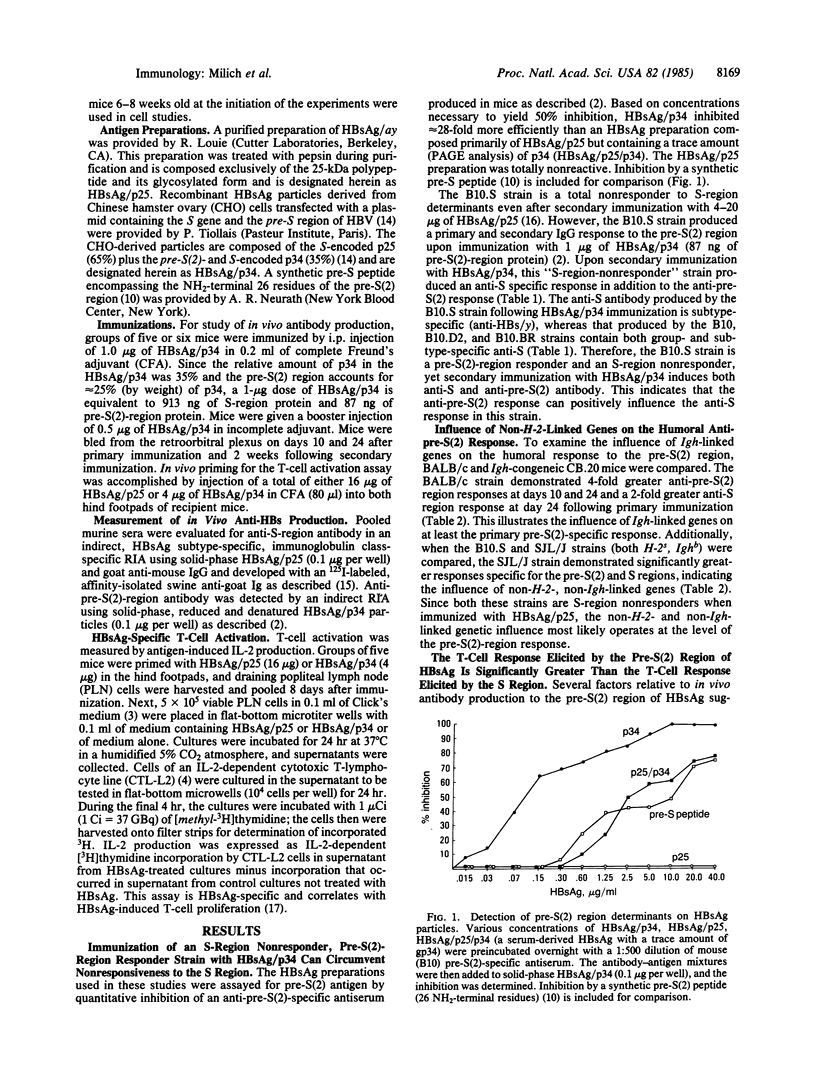
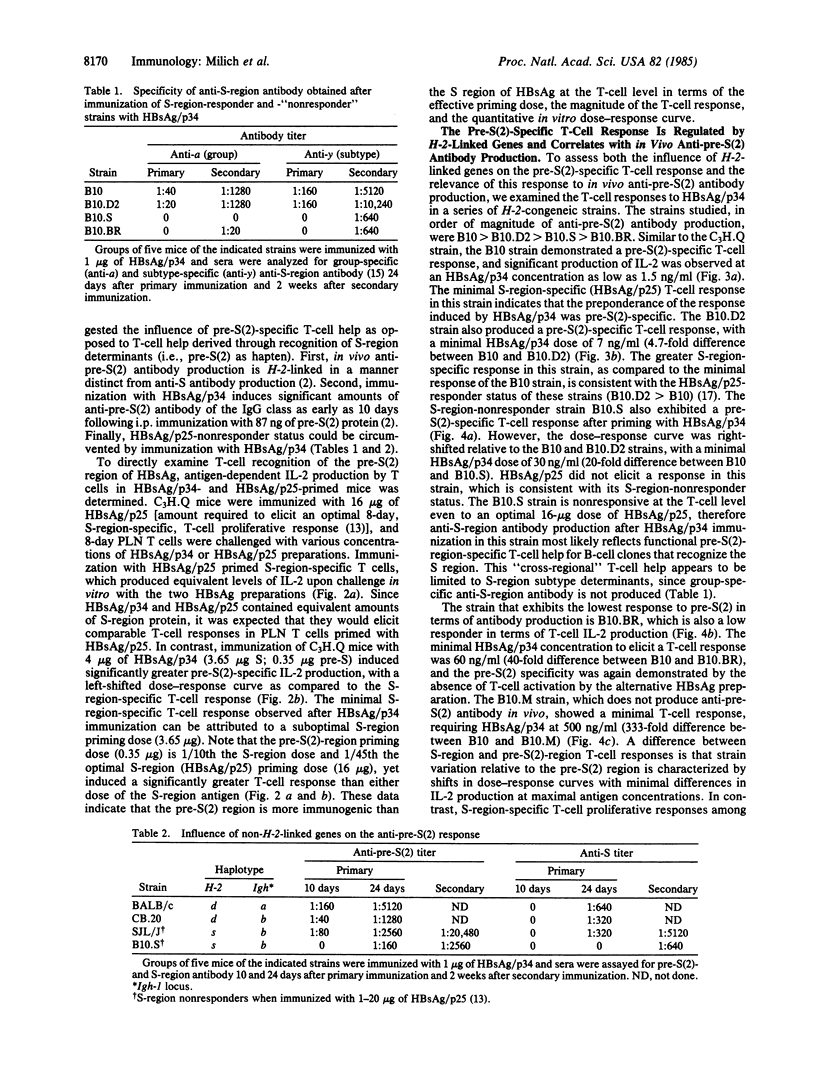
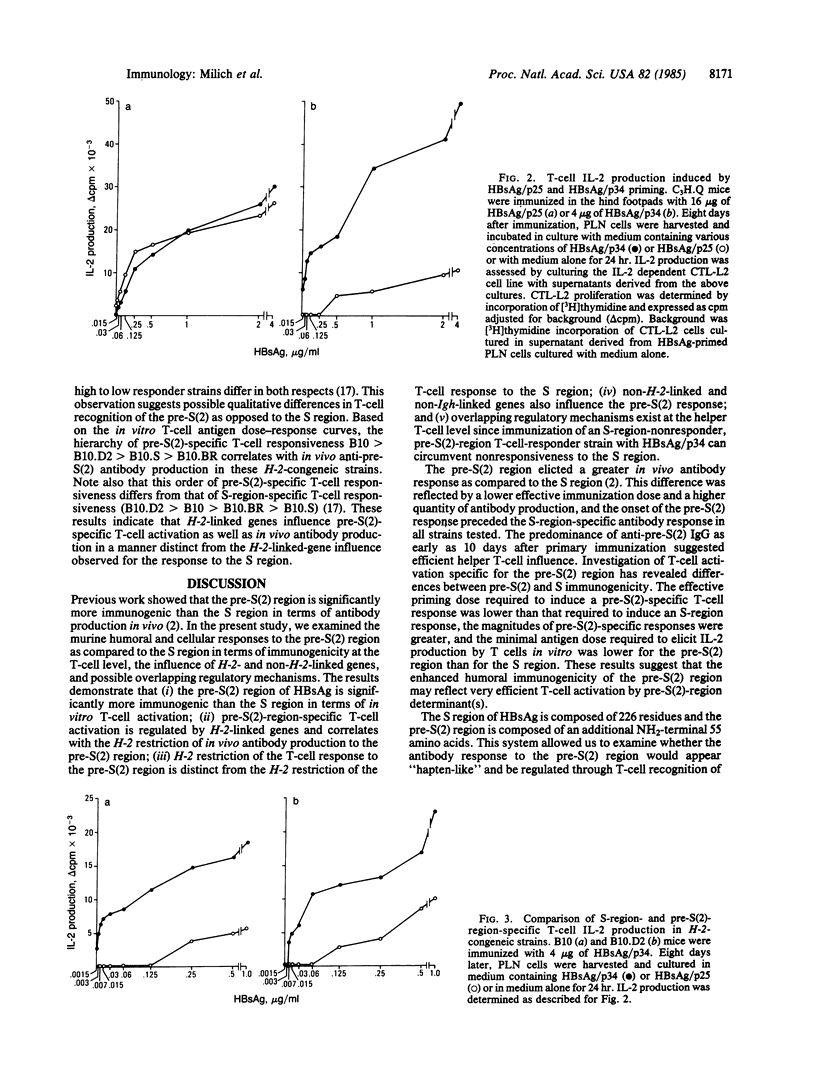
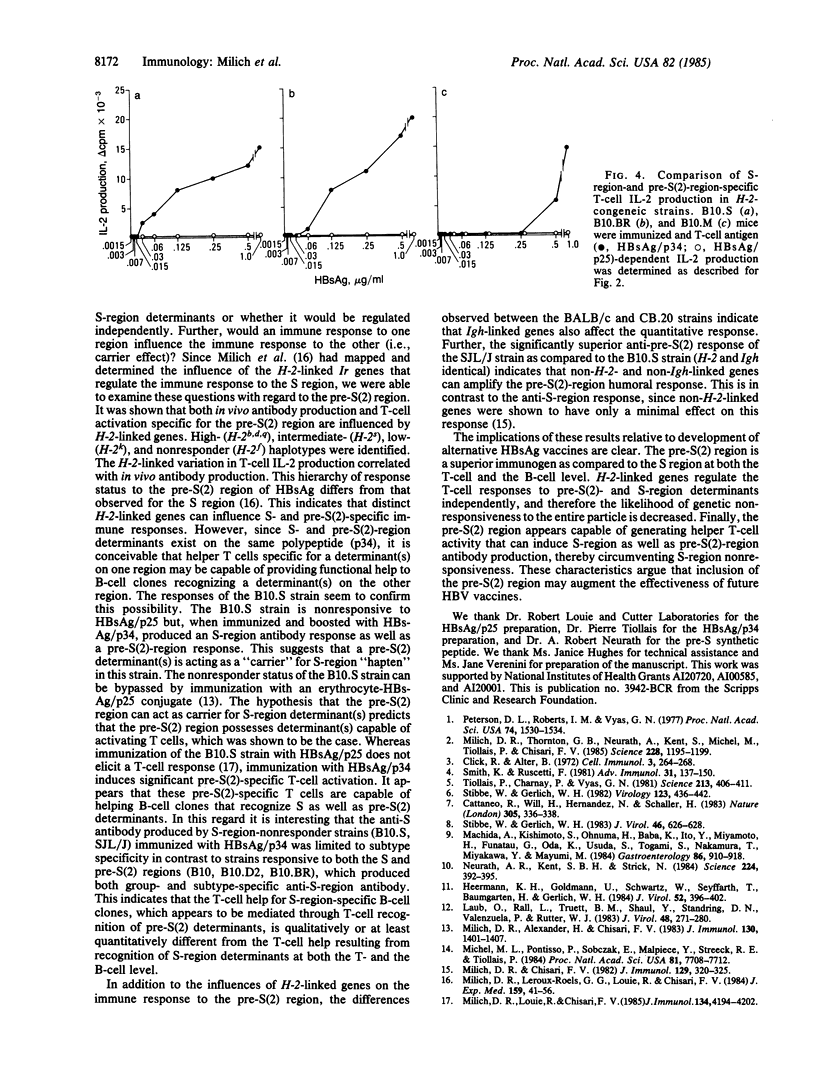
Abstract
Recently, additional polypeptide components of the surface envelope of hepatitis B virus (HBV) have been identified. The pre-S(1) and pre-S(2) regions of the HBV genome encode NH2-terminal amino acid residues that together with the S-gene product (25 kDa) comprise polypeptides of 33 kDa and 39 kDa. The possible immunopathologic significance of these larger polypeptides and their relevance to vaccine development prompted us to examine the murine immune response to pre-S(2)-encoded determinants as compared to S-encoded determinants on the same polypeptide. Previous work showed that the pre-S(2) region elicits greater antibody production in vivo than does the S region of hepatitis B surface antigen. In this study, we examined immunogenicity of the pre-S(2) region at the T-cell level, H-2- and non-H-2-linked genetic influences on the pre-S(2) response, and the effect of the immune response to one region on the immune response to the other region. The results indicate that (i) the pre-S(2) region is significantly more immunogenic than the S region at the T-cell level; (ii) pre-S(2)-region-specific T-cell activation is regulated by H-2-linked genes and correlates with the H-2 restriction of in vivo antibody production to the pre-S(2) region; (iii) the H-2 restriction of the T-cell response to the pre-S(2) region is distinct from the H-2 restriction of the T-cell response to S-region determinants; (iv) non-H-2-linked and non-Igh-linked genes also influence the humoral immune response to the pre-S(2) region; and (v) immunization of an S-region-nonresponder, pre-S(2)-region T-cell-responder strain with HBV envelope particles containing both the pre-S(2) and S regions can circumvent nonresponsiveness to the S region through pre-S(2)-specific T-cell helper function.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Bishop J. M., Smith D. H., Chen E. Y., Colby W. W., Levinson A. D. Nucleotide sequence to the v-myc oncogene of avian retrovirus MC29. Proc Natl Acad Sci U S A. 1983 Jan;80(1):100–104. doi: 10.1073/pnas.80.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Becker P., Renkawitz R., Schütz G. Tissue-specific DNaseI hypersensitive sites in the 5'-flanking sequences of the tryptophan oxygenase and the tyrosine aminotransferase genes. EMBO J. 1984 Sep;3(9):2015–2020. doi: 10.1002/j.1460-2075.1984.tb02084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch J. B., Weintraub H. Temporal order of chromatin structural changes associated with activation of the major chicken vitellogenin gene. Cell. 1983 May;33(1):65–76. doi: 10.1016/0092-8674(83)90335-5. [DOI] [PubMed] [Google Scholar]
- Cattaneo R., Will H., Hernandez N., Schaller H. Signals regulating hepatitis B surface antigen transcription. Nature. 1983 Sep 22;305(5932):336–338. doi: 10.1038/305336a0. [DOI] [PubMed] [Google Scholar]
- Cheley S., Anderson R. A reproducible microanalytical method for the detection of specific RNA sequences by dot-blot hybridization. Anal Biochem. 1984 Feb;137(1):15–19. doi: 10.1016/0003-2697(84)90339-7. [DOI] [PubMed] [Google Scholar]
- Click R. E., Benck L., Alter B. J. Immune responses in vitro. I. Culture conditions for antibody synthesis. Cell Immunol. 1972 Feb;3(2):264–276. doi: 10.1016/0008-8749(72)90165-7. [DOI] [PubMed] [Google Scholar]
- Fritton H. P., Igo-Kemenes T., Nowock J., Strech-Jurk U., Theisen M., Sippel A. E. Alternative sets of DNase I-hypersensitive sites characterize the various functional states of the chicken lysozyme gene. Nature. 1984 Sep 13;311(5982):163–165. doi: 10.1038/311163a0. [DOI] [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Ginder G. D., Whitters M. J., Pohlman J. K. Activation of a chicken embryonic globin gene in adult erythroid cells by 5-azacytidine and sodium butyrate. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3954–3958. doi: 10.1073/pnas.81.13.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. W., Goeddel D. V. Structure of the human immune interferon gene. Nature. 1982 Aug 26;298(5877):859–863. doi: 10.1038/298859a0. [DOI] [PubMed] [Google Scholar]
- Groudine M., Kohwi-Shigematsu T., Gelinas R., Stamatoyannopoulos G., Papayannopoulou T. Human fetal to adult hemoglobin switching: changes in chromatin structure of the beta-globin gene locus. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7551–7555. doi: 10.1073/pnas.80.24.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heermann K. H., Goldmann U., Schwartz W., Seyffarth T., Baumgarten H., Gerlich W. H. Large surface proteins of hepatitis B virus containing the pre-s sequence. J Virol. 1984 Nov;52(2):396–402. doi: 10.1128/jvi.52.2.396-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook N. J., Lieber M., Crabtree G. R. DNA sequence of the 5' flanking region of the human interleukin 2 gene: homologies with adult T-cell leukemia virus. Nucleic Acids Res. 1984 Jun 25;12(12):5005–5013. doi: 10.1093/nar/12.12.5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Laub O., Rall L. B., Truett M., Shaul Y., Standring D. N., Valenzuela P., Rutter W. J. Synthesis of hepatitis B surface antigen in mammalian cells: expression of the entire gene and the coding region. J Virol. 1983 Oct;48(1):271–280. doi: 10.1128/jvi.48.1.271-280.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida A., Kishimoto S., Ohnuma H., Baba K., Ito Y., Miyamoto H., Funatsu G., Oda K., Usuda S., Togami S. A polypeptide containing 55 amino acid residues coded by the pre-S region of hepatitis B virus deoxyribonucleic acid bears the receptor for polymerized human as well as chimpanzee albumins. Gastroenterology. 1984 May;86(5 Pt 1):910–918. [PubMed] [Google Scholar]
- Mercola M., Wang X. F., Olsen J., Calame K. Transcriptional enhancer elements in the mouse immunoglobulin heavy chain locus. Science. 1983 Aug 12;221(4611):663–665. doi: 10.1126/science.6306772. [DOI] [PubMed] [Google Scholar]
- Michel M. L., Pontisso P., Sobczak E., Malpièce Y., Streeck R. E., Tiollais P. Synthesis in animal cells of hepatitis B surface antigen particles carrying a receptor for polymerized human serum albumin. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7708–7712. doi: 10.1073/pnas.81.24.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich D. R., Alexander H., Chisari F. V. Genetic regulation of the immune response to hepatitis B surface antigen (HBsAg). III. Circumvention of nonresponsiveness in mice bearing HBsAg nonresponder haplotypes. J Immunol. 1983 Mar;130(3):1401–1407. [PubMed] [Google Scholar]
- Milich D. R., Chisari F. V. Genetic regulation of the immune response to hepatitis B surface antigen (HBsAg). I. H-2 restriction of the murine humoral immune response to the a and d determinants of HBsAg. J Immunol. 1982 Jul;129(1):320–325. [PubMed] [Google Scholar]
- Milich D. R., Leroux-Roels G. G., Louie R. E., Chisari F. V. Genetic regulation of the immune response to hepatitis B surface antigen (HBsAg). IV. Distinct H-2-linked Ir genes control antibody responses to different HBsAg determinants on the same molecule and map to the I-A and I-C subregions. J Exp Med. 1984 Jan 1;159(1):41–56. doi: 10.1084/jem.159.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich D. R., Louie R. E., Chisari F. V. Genetic regulation of the immune response to hepatitis B surface antigen (HBsAg). V. T cell proliferative response and cellular interactions. J Immunol. 1985 Jun;134(6):4194–4202. [PubMed] [Google Scholar]
- Milich D. R., Thornton G. B., Neurath A. R., Kent S. B., Michel M. L., Tiollais P., Chisari F. V. Enhanced immunogenicity of the pre-S region of hepatitis B surface antigen. Science. 1985 Jun 7;228(4704):1195–1199. doi: 10.1126/science.2408336. [DOI] [PubMed] [Google Scholar]
- Mills F. C., Fisher L. M., Kuroda R., Ford A. M., Gould H. J. DNase I hypersensitive sites in the chromatin of human mu immunoglobulin heavy-chain genes. Nature. 1983 Dec 22;306(5945):809–812. doi: 10.1038/306809a0. [DOI] [PubMed] [Google Scholar]
- Miyatake S., Yokota T., Lee F., Arai K. Structure of the chromosomal gene for murine interleukin 3. Proc Natl Acad Sci U S A. 1985 Jan;82(2):316–320. doi: 10.1073/pnas.82.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath A. R., Kent S. B., Strick N. Location and chemical synthesis of a pre-S gene coded immunodominant epitope of hepatitis B virus. Science. 1984 Apr 27;224(4647):392–395. doi: 10.1126/science.6200931. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Pardue M. L., Lafer E. M., Möller A., Stollar B. D., Rich A. Antibodies to left-handed Z-DNA bind to interband regions of Drosophila polytene chromosomes. Nature. 1981 Dec 3;294(5840):417–422. doi: 10.1038/294417a0. [DOI] [PubMed] [Google Scholar]
- Parslow T. G., Granner D. K. Chromatin changes accompany immunoglobulin kappa gene activation: a potential control region within the gene. Nature. 1982 Sep 30;299(5882):449–451. doi: 10.1038/299449a0. [DOI] [PubMed] [Google Scholar]
- Peterson D. L., Roberts I. M., Vyas G. N. Partial amino acid sequence of two major component polypeptides of hepatitis B surface antigen. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1530–1534. doi: 10.1073/pnas.74.4.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard D., Schaffner W. A lymphocyte-specific enhancer in the mouse immunoglobulin kappa gene. Nature. 1984 Jan 5;307(5946):80–82. doi: 10.1038/307080a0. [DOI] [PubMed] [Google Scholar]
- Reddy E. P., Reynolds R. K., Watson D. K., Schultz R. A., Lautenberger J., Papas T. S. Nucleotide sequence analysis of the proviral genome of avian myelocytomatosis virus (MC29). Proc Natl Acad Sci U S A. 1983 May;80(9):2500–2504. doi: 10.1073/pnas.80.9.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Hennighausen L., Battey J., Leder P. Chromatin structure and protein binding in the putative regulatory region of the c-myc gene in Burkitt lymphoma. Cell. 1984 Jun;37(2):381–391. doi: 10.1016/0092-8674(84)90368-4. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Ruscetti F. W. T-cell growth factor and the culture of cloned functional T cells. Adv Immunol. 1981;31:137–175. doi: 10.1016/s0065-2776(08)60920-7. [DOI] [PubMed] [Google Scholar]
- Starcich B., Ratner L., Josephs S. F., Okamoto T., Gallo R. C., Wong-Staal F. Characterization of long terminal repeat sequences of HTLV-III. Science. 1985 Feb 1;227(4686):538–540. doi: 10.1126/science.2981438. [DOI] [PubMed] [Google Scholar]
- Stibbe W., Gerlich W. H. Structural relationships between minor and major proteins of hepatitis B surface antigen. J Virol. 1983 May;46(2):626–628. doi: 10.1128/jvi.46.2.626-628.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stibbe W., Gerlich W. H. Variable protein composition of hepatitis B surface antigen from different donors. Virology. 1982 Dec;123(2):436–442. doi: 10.1016/0042-6822(82)90275-6. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Charnay P., Vyas G. N. Biology of hepatitis B virus. Science. 1981 Jul 24;213(4506):406–411. doi: 10.1126/science.6264599. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Watson D. K., Reddy E. P., Duesberg P. H., Papas T. S. Nucleotide sequence analysis of the chicken c-myc gene reveals homologous and unique coding regions by comparison with the transforming gene of avian myelocytomatosis virus MC29, delta gag-myc. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2146–2150. doi: 10.1073/pnas.80.8.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A., Imboden J., Shoback D., Stobo J. Role of T3 surface molecules in human T-cell activation: T3-dependent activation results in an increase in cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4169–4173. doi: 10.1073/pnas.81.13.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A., Imboden J., Wiskocil R., Stobo J. The role of T3 in the activation of human T cells. J Clin Immunol. 1984 May;4(3):165–173. doi: 10.1007/BF00914963. [DOI] [PubMed] [Google Scholar]
- Weiss A., Wiskocil R. L., Stobo J. D. The role of T3 surface molecules in the activation of human T cells: a two-stimulus requirement for IL 2 production reflects events occurring at a pre-translational level. J Immunol. 1984 Jul;133(1):123–128. [PubMed] [Google Scholar]
- Wu C. The 5' ends of Drosophila heat shock genes in chromatin are hypersensitive to DNase I. Nature. 1980 Aug 28;286(5776):854–860. doi: 10.1038/286854a0. [DOI] [PubMed] [Google Scholar]


