Abstract
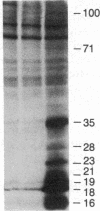
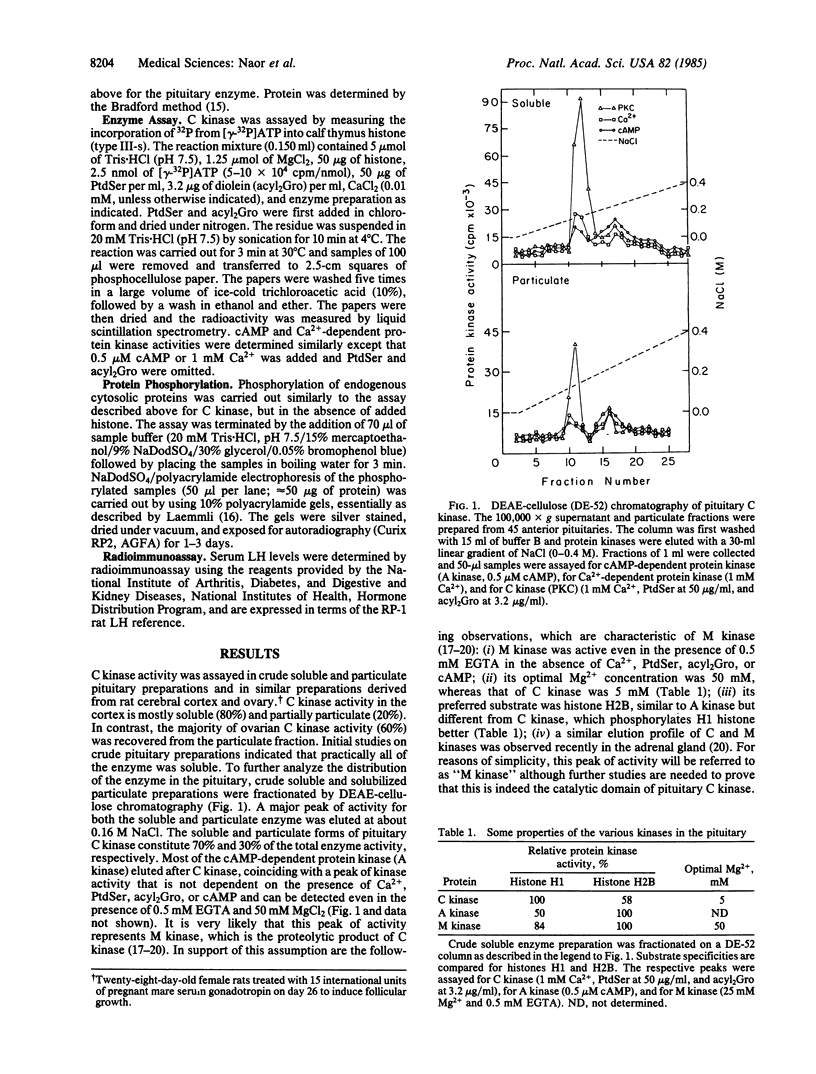
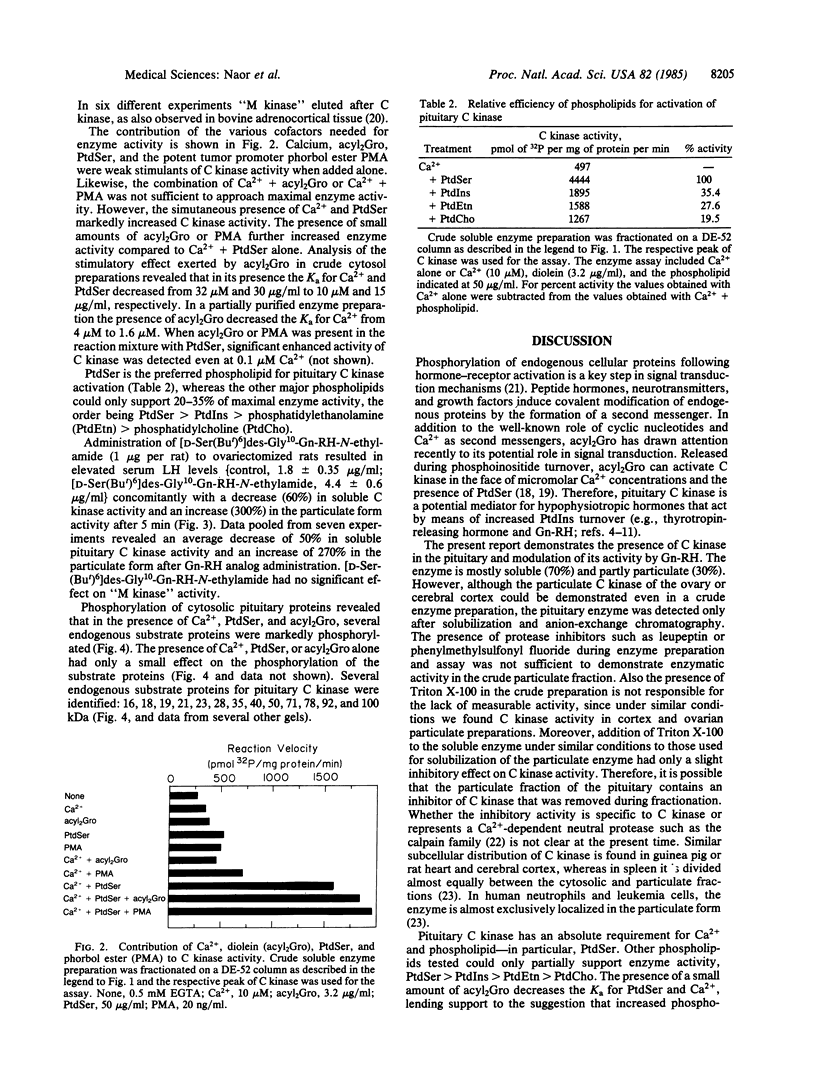
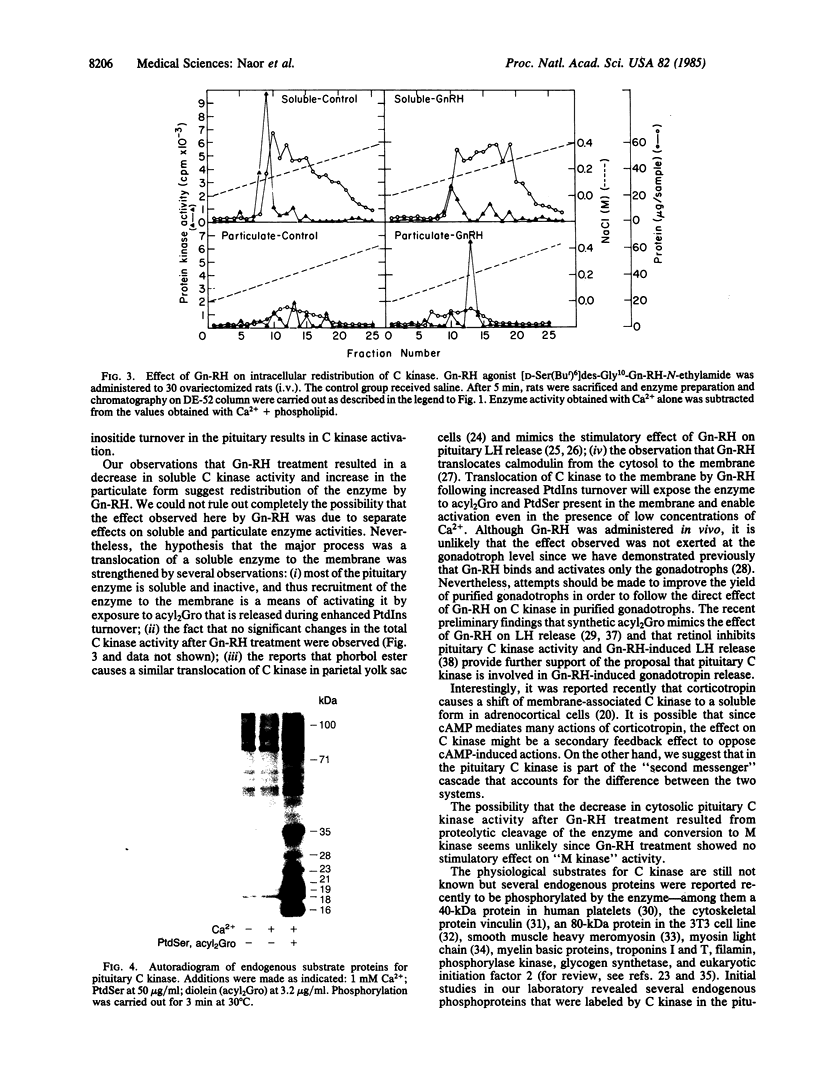
We report the presence in the rat pituitary of a calcium-activated, phospholipid-dependent protein kinase (C kinase), originally described by Takai et al. [Takai, Y., Kishimoto, A., Iwasa, Y., Kawahara, Y., Mori, T. & Nishizuka, Y. (1979) J. Biol. Chem. 254, 3692-3695]. Enzyme activity is absolutely dependent on the simultaneous presence of Ca2+ and phospholipid--in particular, phosphatidylserine. The presence of small amounts of unsaturated diacylglycerol greatly increases the apparent affinity of the enzyme for Ca2+ and phosphatidylserine. Pituitary C kinase is mostly soluble (70%) and partly particulate (30%). Although the soluble form of the enzyme can be detected in a crude cytosol preparation, the particulate form is detectable only after solubilization and anion-exchange chromatography. Administration of a gonadotropin-releasing hormone (Gn-RH) agonist analog, [D-Ser(But)6]des-Gly10-Gn-RH-N-ethylamide, to ovariectomized rats resulted in elevated serum luteinizing hormone levels (245%) accompanied by a decrease in the cytosolic form of the enzyme (60%) and an increase in the particulate form (300%) after 5 min. This apparent activation of the particulate form seems to result from translocation of a soluble C kinase to the membrane. Several endogenous substrate proteins for C kinase ranging from 16 to 100 kDa were identified in pituitary cytosol. Pituitary C kinase might be involved in signal-transduction mechanisms in Gn-RH action, in particular, and in other hypophysiotropic hormones, in general, which operate by means of stimulation of phosphoinositide turnover during which diacylglycerol is liberated.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brazeau P., Ling N., Esch F., Böhlen P., Mougin C., Guillemin R. Somatocrinin (growth hormone releasing factor) in vitro bioactivity; Ca++ involvement, cAMP mediated action and additivity of effect with PGE2. Biochem Biophys Res Commun. 1982 Nov 30;109(2):588–594. doi: 10.1016/0006-291x(82)91762-4. [DOI] [PubMed] [Google Scholar]
- Conn P. M., Chafouleas J. G., Rogers D., Means A. R. Gonadotropin releasing hormone stimulates calmodulin redistribution in rat pituitary. Nature. 1981 Jul 16;292(5820):264–265. doi: 10.1038/292264a0. [DOI] [PubMed] [Google Scholar]
- Conn P. M., Ganong B. R., Ebeling J., Staley D., Neidel J. E., Bell R. M. Diacylglycerols release LH: structure-activity relations reveal a role for protein kinase C. Biochem Biophys Res Commun. 1985 Jan 16;126(1):532–539. doi: 10.1016/0006-291x(85)90638-2. [DOI] [PubMed] [Google Scholar]
- Gershengorn M. C. Thyrotropin releasing hormone. A review of the mechanisms of acute stimulation of pituitary hormone release. Mol Cell Biochem. 1982 Jun 25;45(3):163–179. doi: 10.1007/BF00230085. [DOI] [PubMed] [Google Scholar]
- Giguère V., Labrie F., Côté J., Coy D. H., Sueiras-Diaz J., Schally A. V. Stimulation of cyclic AMP accumulation and corticotropin release by synthetic ovine corticotropin-releasing factor in rat anterior pituitary cells: site of glucocorticoid action. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3466–3469. doi: 10.1073/pnas.79.11.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P. Phosphorylated proteins as physiological effectors. Science. 1978 Jan 13;199(4325):146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- Hirota K., Hirota T., Aguilera G., Catt K. J. Hormone-induced redistribution of calcium-activated phospholipid-dependent protein kinase in pituitary gonadotrophs. J Biol Chem. 1985 Mar 25;260(6):3243–3246. [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Sawamura M., Hoshijima M., Fujikura T., Nishizuka Y. Synergistic functions of protein phosphorylation and calcium mobilization in platelet activation. J Biol Chem. 1983 Jun 10;258(11):6701–6704. [PubMed] [Google Scholar]
- Kiesel L., Catt K. J. Phosphatidic acid and the calcium-dependent actions of gonadotropin-releasing hormone in pituitary gonadotrophs. Arch Biochem Biophys. 1984 May 15;231(1):202–210. doi: 10.1016/0003-9861(84)90379-5. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Kajikawa N., Shiota M., Nishizuka Y. Proteolytic activation of calcium-activated, phospholipid-dependent protein kinase by calcium-dependent neutral protease. J Biol Chem. 1983 Jan 25;258(2):1156–1164. [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983 Feb 17;301(5901):621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- Kuo J. F., Schatzman R. C., Turner R. S., Mazzei G. J. Phospholipid-sensitive Ca2+-dependent protein kinase: a major protein phosphorylation system. Mol Cell Endocrinol. 1984 May;35(2-3):65–73. doi: 10.1016/0303-7207(84)90001-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Murachi T., Tanaka K., Hatanaka M., Murakami T. Intracellular Ca2+-dependent protease (calpain) and its high-molecular-weight endogenous inhibitor (calpastatin). Adv Enzyme Regul. 1980;19:407–424. doi: 10.1016/0065-2571(81)90026-1. [DOI] [PubMed] [Google Scholar]
- Naka M., Nishikawa M., Adelstein R. S., Hidaka H. Phorbol ester-induced activation of human platelets is associated with protein kinase C phosphorylation of myosin light chains. Nature. 1983 Dec 1;306(5942):490–492. doi: 10.1038/306490a0. [DOI] [PubMed] [Google Scholar]
- Naor Z., Catt K. J. Mechanism of action of gonadotropin-releasing hormone. Involvement of phospholipid turnover in luteinizing hormone release. J Biol Chem. 1981 Mar 10;256(5):2226–2229. [PubMed] [Google Scholar]
- Naor Z., Childs G. V., Leifer A. M., Clayton R. N., Amsterdam A., Catt K. J. Gonadotropin-releasing hormone binding and activation of enriched population of pituitary gonadotrophs. Mol Cell Endocrinol. 1982 Jan;25(1):85–97. doi: 10.1016/0303-7207(82)90171-x. [DOI] [PubMed] [Google Scholar]
- Naor Z., Eli Y. Synergistic stimulation of luteinizing hormone (LH) release by protein kinase C activators and Ca2+-ionophore. Biochem Biophys Res Commun. 1985 Jul 31;130(2):848–853. doi: 10.1016/0006-291x(85)90494-2. [DOI] [PubMed] [Google Scholar]
- Naor Z., Molcho J., Zakut H., Yavin E. Calcium-independent phosphatidylinositol response in gonadotropin-releasing-hormone-stimulated pituitary cells. Biochem J. 1985 Oct 1;231(1):19–23. doi: 10.1042/bj2310019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa M., Hidaka H., Adelstein R. S. Phosphorylation of smooth muscle heavy meromyosin by calcium-activated, phospholipid-dependent protein kinase. The effect on actin-activated MgATPase activity. J Biol Chem. 1983 Dec 10;258(23):14069–14072. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Raymond V., Leung P. C., Veilleux R., Lefèvre G., Labrie F. LHRH rapidly stimulates phosphatidylinositol metabolism in enriched gonadotrophs. Mol Cell Endocrinol. 1984 Jul;36(3):157–164. doi: 10.1016/0303-7207(84)90031-5. [DOI] [PubMed] [Google Scholar]
- Rebecchi M. J., Kolesnick R. N., Gershengorn M. C. Thyrotropin-releasing hormone stimulates rapid loss of phosphatidylinositol and its conversion to 1,2-diacylglycerol and phosphatidic acid in rat mammotropic pituitary cells. Association with calcium mobilization and prolactin secretion. J Biol Chem. 1983 Jan 10;258(1):227–234. [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena M., Smith K. A. Phorbol esters, phospholipase C, and growth factors rapidly stimulate the phosphorylation of a Mr 80,000 protein in intact quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7244–7248. doi: 10.1073/pnas.80.23.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. A., Vale W. W. Desensitization to gonadotropin-releasing hormone observed in superfused pituitary cells on Cytodex beads. Endocrinology. 1981 Mar;108(3):752–759. doi: 10.1210/endo-108-3-752. [DOI] [PubMed] [Google Scholar]
- Snyder G. D., Bleasdale J. E. Effect of LHRH on incorporation of [32P]-orthophosphate into phosphatidylinositol by dispersed anterior pituitary cells. Mol Cell Endocrinol. 1982 Sep;28(1):55–63. doi: 10.1016/0303-7207(82)90040-5. [DOI] [PubMed] [Google Scholar]
- Sutton C. A., Martin T. F. Thyrotropin-releasing hormone (TRH) selectively and rapidly stimulates phosphatidylinositol turnover in GH pituitary cells: a possible second step of TRH action. Endocrinology. 1982 Apr;110(4):1273–1280. doi: 10.1210/endo-110-4-1273. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Inoue M., Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. I. Purification and characterization of an active enzyme from bovine cerebellum. J Biol Chem. 1977 Nov 10;252(21):7603–7609. [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Iwasa Y., Kawahara Y., Mori T., Nishizuka Y. Calcium-dependent activation of a multifunctional protein kinase by membrane phospholipids. J Biol Chem. 1979 May 25;254(10):3692–3695. [PubMed] [Google Scholar]
- Turgeon J. L., Ashcroft S. J., Waring D. W., Milewski M. A., Walsh D. A. Characteristics of the adenohypophyseal Ca2+-phospholipid-dependent protein kinase. Mol Cell Endocrinol. 1984 Feb;34(2):107–112. doi: 10.1016/0303-7207(84)90061-3. [DOI] [PubMed] [Google Scholar]
- Vilgrain I., Cochet C., Chambaz E. M. Hormonal regulation of a calcium-activated, phospholipid-dependent protein kinase in bovine adrenal cortex. J Biol Chem. 1984 Mar 25;259(6):3403–3406. [PubMed] [Google Scholar]
- Werth D. K., Niedel J. E., Pastan I. Vinculin, a cytoskeletal substrate of protein kinase C. J Biol Chem. 1983 Oct 10;258(19):11423–11426. [PubMed] [Google Scholar]
- Wrenn R. W., Katoh N., Wise B. C., Kuo J. F. Stimulation by phosphatidylserine and calmodulin of calcium-dependent phosphorylation of endogenous proteins from cerebral cortex. J Biol Chem. 1980 Dec 25;255(24):12042–12046. [PubMed] [Google Scholar]