Abstract
Recombinant interferon gamma (rIFN-gamma) activates macrophage antimicrobial and antitumor functions and related metabolic processes, including secretion of reactive oxygen intermediates in mice and in cultured mouse and human macrophages. To look for similar actions in man, we monitored the H2O2 secretory capacity of monocytes from cancer patients receiving intravenous rIFN-gamma at 0.1, 0.5, or 1.0 mg/m2 of body area over 6 hr daily or over 1 hr on alternate days. Monocytes taken just before the first infusion served as controls and were comparable to normal donor monocytes in secretion of H2O2. Monocytes from 11 of the 13 subjects (85%) studied through 20 treatment cycles responded to rIFN-gamma with elevation in H2O2 secretion in greater than or equal to 67% of the tests conducted greater than 1 hr after the start of treatment. Five of the five subjects tested had monocytes with diminished H2O2 secretory capacity when tested immediately after a 1-hr infusion of rIRN-gamma, at which time the amount of adherent mononuclear cell protein recovered from the blood averaged only 24% of the control. At all other times tested (from 6 hr to 5 days after infusion) combined results for all subjects showed enhancement of H2O2 releasing capacity. Statistically significant mean increases ranged from 1.4- to 2.8-fold above the control and included the sets in which monocytes collected 24 hr following a single infusion were assayed the same day or the next. By the criterion of enhanced H2O2 secretory capacity, the ability of rIFN-gamma to activate mononuclear phagocytes is manifest upon its administration to patients with advanced malignancy.
Full text
PDF
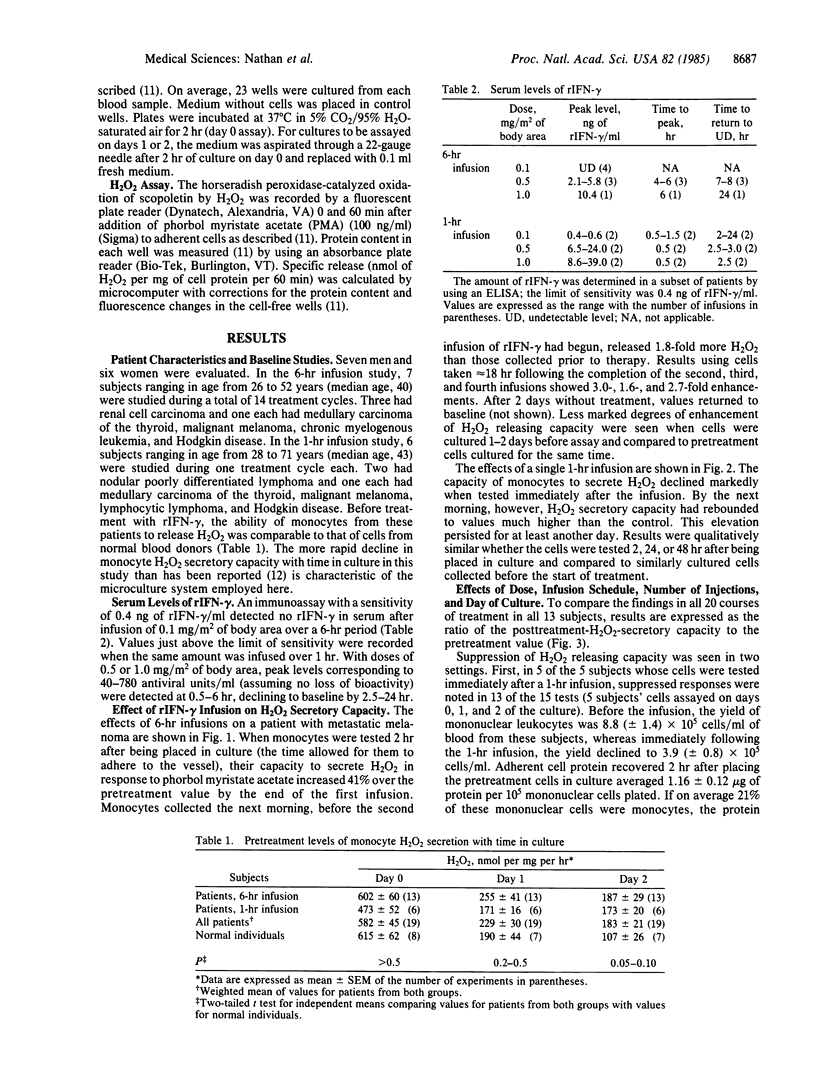
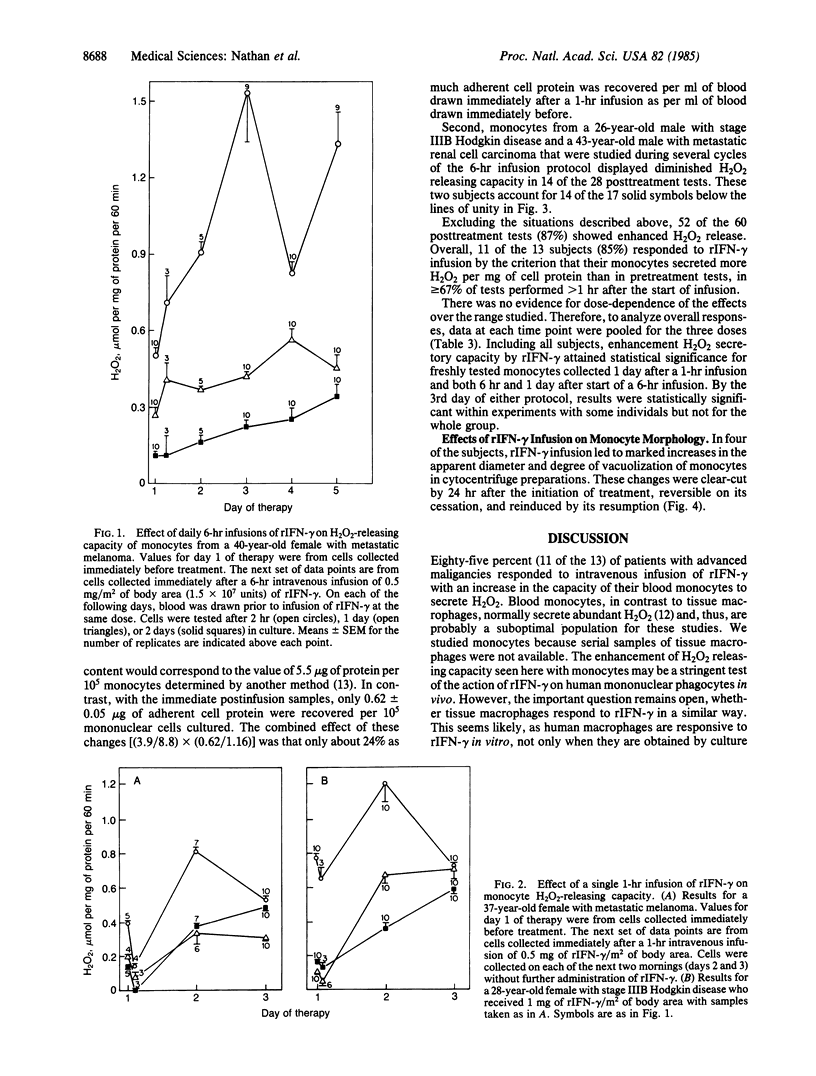
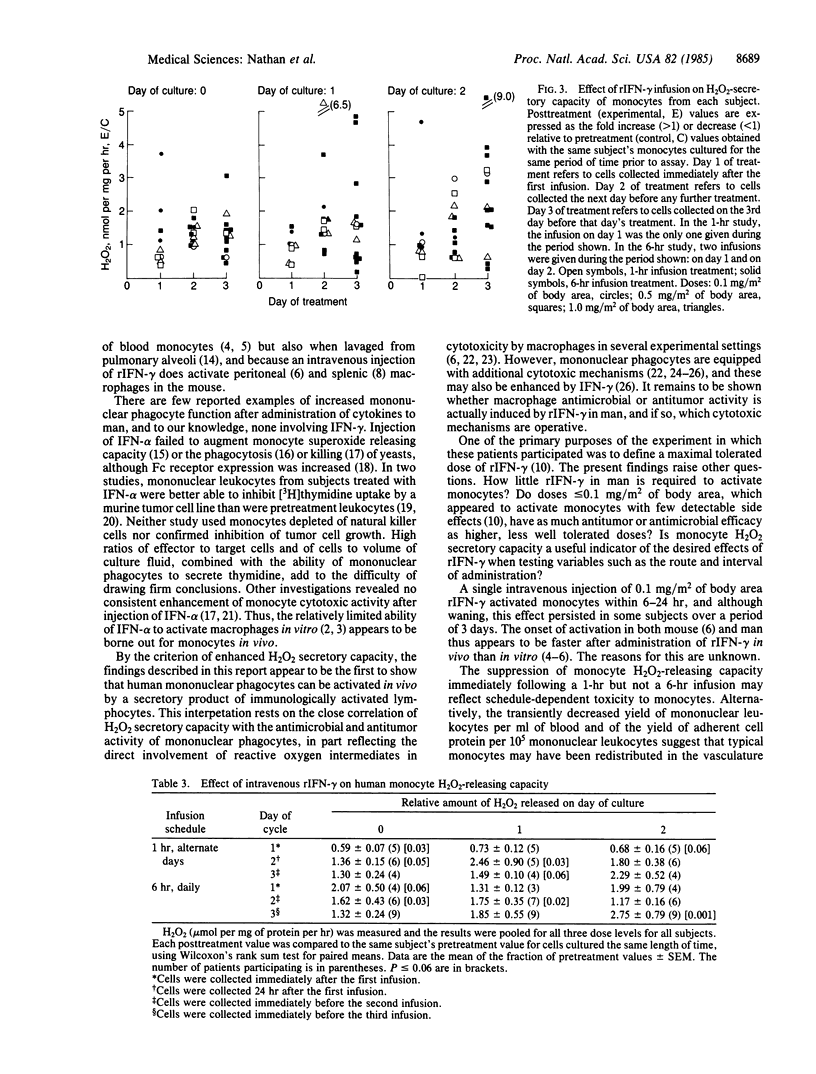

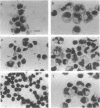
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Kao K. J., Farb R., Pizzo S. V. Effector mechanisms of cytolytically activated macrophages. II. Secretion of a cytolytic factor by activated macrophages and its relationship to secreted neutral proteases. J Immunol. 1980 Jan;124(1):293–300. [PubMed] [Google Scholar]
- De la Harpe J., Nathan C. F. A semi-automated micro-assay for H2O2 release by human blood monocytes and mouse peritoneal macrophages. J Immunol Methods. 1985 Apr 22;78(2):323–336. doi: 10.1016/0022-1759(85)90089-4. [DOI] [PubMed] [Google Scholar]
- Einhorn S., Jarstrand C. Decrease in the phagocytic activity of peripheral monocytes in patients treated with human interferon-alpha. Cancer Immunol Immunother. 1982;13(3):149–152. doi: 10.1007/BF00205379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengst J. C., Kempf R. A., Kan-Mitchell J., Pham A. T., Grunberg S. M., Kortes V. L., Mitchell M. S. Immunological effects of recombinant interferon-alpha 2 in cancer patients. J Biol Response Mod. 1983;2(6):516–527. [PubMed] [Google Scholar]
- Kiderlen A. F., Kaufmann S. H., Lohmann-Matthes M. L. Protection of mice against the intracellular bacterium Listeria monocytogenes by recombinant immune interferon. Eur J Immunol. 1984 Oct;14(10):964–967. doi: 10.1002/eji.1830141019. [DOI] [PubMed] [Google Scholar]
- Laszlo J., Huang A. T., Brenckman W. D., Jeffs C., Koren H., Cianciolo G., Metzgar R., Cashdollar W., Cox E., Buckley C. E., 3rd Phase I study of pharmacological and immunological effects of human lymphoblastoid interferon given to patients with cancer. Cancer Res. 1983 Sep;43(9):4458–4466. [PubMed] [Google Scholar]
- Maluish A. E., Leavitt R., Sherwin S. A., Oldham R. K., Herberman R. B. Effects of recombinant interferon-alpha on immune function in cancer patients. J Biol Response Mod. 1983;2(5):470–481. [PubMed] [Google Scholar]
- McCabe R. E., Luft B. J., Remington J. S. Effect of murine interferon gamma on murine toxoplasmosis. J Infect Dis. 1984 Dec;150(6):961–962. doi: 10.1093/infdis/150.6.961. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Carriero S. M., Harris A. M., Jaffee E. A. Human mononuclear phagocyte antiprotozoal mechanisms: oxygen-dependent vs oxygen-independent activity against intracellular Toxoplasma gondii. J Immunol. 1985 Mar;134(3):1982–1988. [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Rothermel C. D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-gamma is the activating lymphokine. J Clin Invest. 1983 Oct;72(4):1506–1510. doi: 10.1172/JCI111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Spitalny G. L., Nathan C. F. Activation of mouse peritoneal macrophages in vitro and in vivo by interferon-gamma. J Immunol. 1985 Mar;134(3):1619–1622. [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F. A simple method for counting adherent cells: application to cultured human monocytes, macrophages and multinucleated giant cells. J Immunol Methods. 1983 Jan 28;56(2):261–268. doi: 10.1016/0022-1759(83)90418-0. [DOI] [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F., Cohn Z. A. Hydrogen peroxide metabolism in human monocytes during differentiation in vitro. J Clin Invest. 1981 Nov;68(5):1243–1252. doi: 10.1172/JCI110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F. Mechanisms of macrophage antimicrobial activity. Trans R Soc Trop Med Hyg. 1983;77(5):620–630. doi: 10.1016/0035-9203(83)90190-6. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Prendergast T. J., Wiebe M. E., Stanley E. R., Platzer E., Remold H. G., Welte K., Rubin B. Y., Murray H. W. Activation of human macrophages. Comparison of other cytokines with interferon-gamma. J Exp Med. 1984 Aug 1;160(2):600–605. doi: 10.1084/jem.160.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F. Secretion of oxygen intermediates: role in effector functions of activated macrophages. Fed Proc. 1982 Apr;41(6):2206–2211. [PubMed] [Google Scholar]
- Neefe J. R., Sullivan J. E., Silgals R. Preliminary observations of immunomodulatory activity of lymphoblastoid interferon-alpha administered every other day or weekly. J Biol Response Mod. 1983;2(5):441–449. [PubMed] [Google Scholar]
- Rhodes J., Jones D. H., Bleehen N. M. Increased expression of human monocyte HLA-DR antigens and Fc gamma receptors in response to human interferon in vivo. Clin Exp Immunol. 1983 Sep;53(3):739–743. [PMC free article] [PubMed] [Google Scholar]
- Schreiber R. D., Hicks L. J., Celada A., Buchmeier N. A., Gray P. W. Monoclonal antibodies to murine gamma-interferon which differentially modulate macrophage activation and antiviral activity. J Immunol. 1985 Mar;134(3):1609–1618. [PubMed] [Google Scholar]
- Territo M., Sarna G., Figlin R. Effect of in vivo administration of interferon on human monocyte function. J Biol Response Mod. 1983;2(5):450–457. [PubMed] [Google Scholar]
- Varesio L., Blasi E., Thurman G. B., Talmadge J. E., Wiltrout R. H., Herberman R. B. Potent activation of mouse macrophages by recombinant interferon-gamma. Cancer Res. 1984 Oct;44(10):4465–4469. [PubMed] [Google Scholar]
- Zacharchuk C. M., Drysdale B. E., Mayer M. M., Shin H. S. Macrophage-mediated cytotoxicity: role of a soluble macrophage cytotoxic factor similar to lymphotoxin and tumor necrosis factor. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6341–6345. doi: 10.1073/pnas.80.20.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]