Abstract
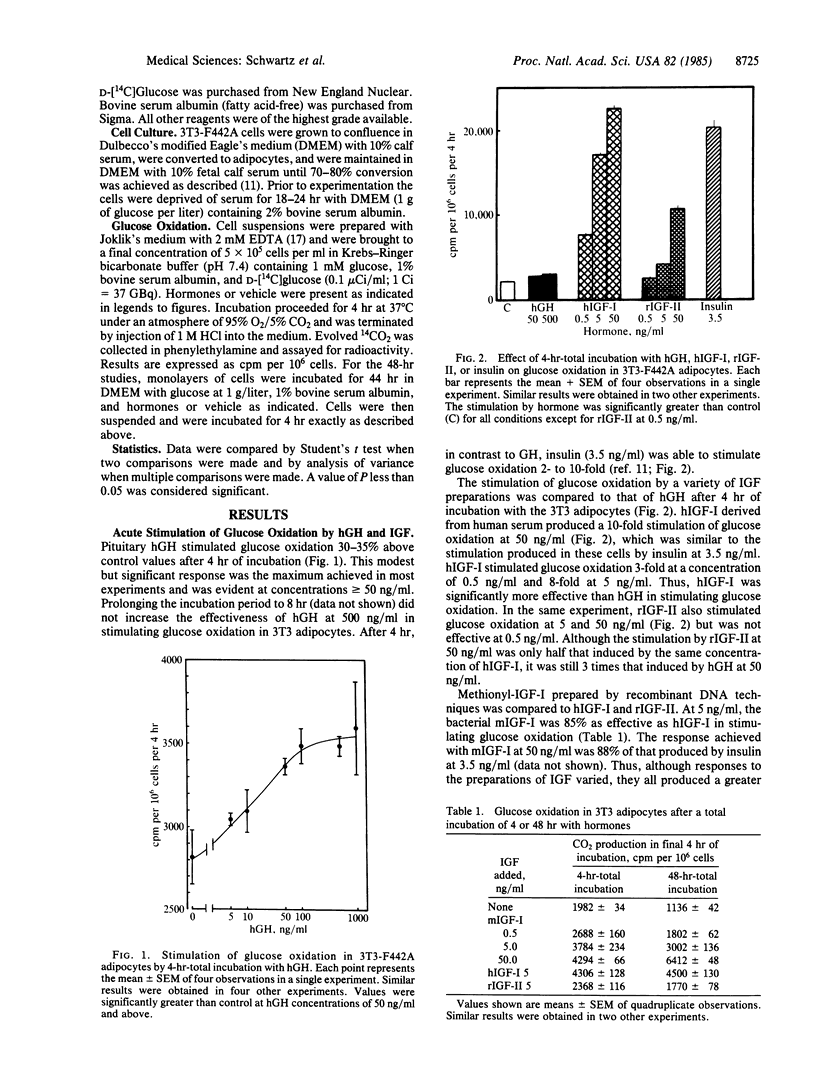
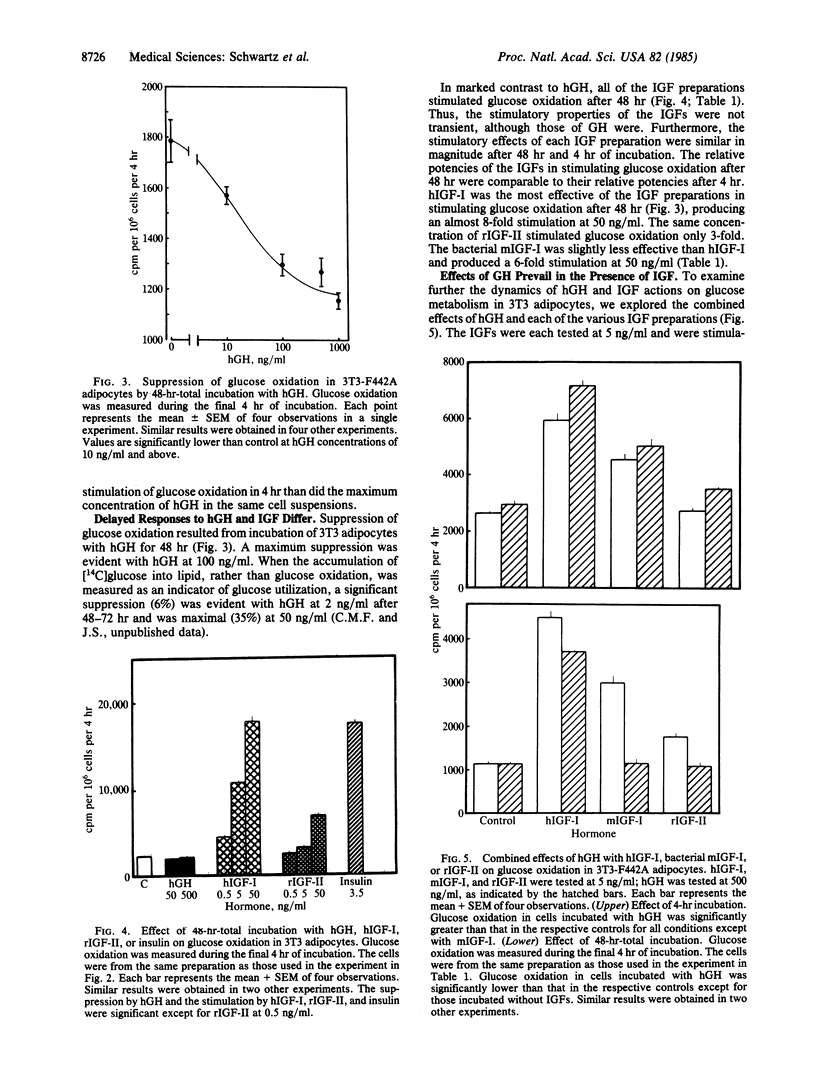
In 3T3-F442A adipocytes, human growth hormone (hGH) stimulates glucose oxidation in 4 hr. A maximal increase is evident at hGH concentrations of 50-100 ng/ml and rarely exceeds 50% above control. The stimulation is transient; after 48 hr of incubation with GH, glucose oxidation is significantly suppressed to 35% below control values. In view of the concept that insulin-like growth factors (IGF) may mediate the effects of GH, we compared the effects of hGH (500 ng/ml) and several preparations of IGF on glucose metabolism in 3T3 adipocytes. After 4 hr of incubation, IGF-I from human plasma stimulated glucose oxidation in a dose-related manner, producing a 10-fold increase at 50 ng/ml. Methionyl-IGF-I produced by recombinant DNA techniques was 85-88% as effective as IGF-I. IGF-II stimulated glucose oxidation 3-fold at 50 ng/ml after 4 hr of incubation. In contrast to the suppression observed with hGH after 48 hr, all three of the IGF preparations stimulated glucose oxidation after 48 hr of incubation and were as effective as they were after 4 hr. When each of the IGF preparations was tested (at 5 ng/ml) in combination with hGH, both the stimulatory and suppressive effects of GH were superimposed on the stimulation by the IGFs. Thus, the stimulatory properties of IGF differed from those of GH in that the maximum extent to which IGF increased glucose oxidation, compared with hGH, was as much as 20-fold greater. Furthermore, all of the IGF preparations stimulated glucose oxidation after 48 hr under conditions in which hGH suppressed glucose metabolism. Thus, it is unlikely that extracellular IGFs mediate the effects of hGH on glucose metabolism in 3T3-F442A adipocytes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter-Su C., Schwartz J., Kikuchi G. Identification of a high affinity growth hormone receptor in rat adipocyte membranes. J Biol Chem. 1984 Jan 25;259(2):1099–1104. [PubMed] [Google Scholar]
- D'Ercole A. J., Stiles A. D., Underwood L. E. Tissue concentrations of somatomedin C: further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proc Natl Acad Sci U S A. 1984 Feb;81(3):935–939. doi: 10.1073/pnas.81.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch P. J., Rosen O. M., Rubin C. S. Identification and characterization of a latent pool of insulin receptors in 3T3-L1 adipocytes. J Biol Chem. 1982 May 25;257(10):5350–5358. [PubMed] [Google Scholar]
- Friesen H. G. Raben lecture 1980: a tale of stature. Endocr Rev. 1980 Fall;1(4):309–318. doi: 10.1210/edrv-1-4-309. [DOI] [PubMed] [Google Scholar]
- Gause I., Eden S., Jansson J. O., Isaksson O. Effects of in vivo administration of antiserum to rat growth hormone on body growth and insulin responsiveness in adipose tissue. Endocrinology. 1983 May;112(5):1559–1566. doi: 10.1210/endo-112-5-1559. [DOI] [PubMed] [Google Scholar]
- Goodman H. M. Growth hormone and the metabolism of carbohydrate and lipid in adipose tissue. Ann N Y Acad Sci. 1968 Feb 5;148(2):419–440. doi: 10.1111/j.1749-6632.1968.tb20367.x. [DOI] [PubMed] [Google Scholar]
- Gorin E., Goodman H. M. Covalent binding of growth hormone to surface receptors on rat adipocytes. Endocrinology. 1984 Apr;114(4):1279–1286. doi: 10.1210/endo-114-4-1279. [DOI] [PubMed] [Google Scholar]
- Grichting G., Levy L. K., Goodman H. M. Relationship between binding and biological effects of human growth hormone in rat adipocytes. Endocrinology. 1983 Sep;113(3):1111–1120. doi: 10.1210/endo-113-3-1111. [DOI] [PubMed] [Google Scholar]
- Isaksson O. G., Jansson J. O., Gause I. A. Growth hormone stimulates longitudinal bone growth directly. Science. 1982 Jun 11;216(4551):1237–1239. doi: 10.1126/science.7079756. [DOI] [PubMed] [Google Scholar]
- King G. L., Kahn C. R., Rechler M. M., Nissley S. P. Direct demonstration of separate receptors for growth and metabolic activities of insulin and multiplication-stimulating activity (an insulinlike growth factor) using antibodies to the insulin receptor. J Clin Invest. 1980 Jul;66(1):130–140. doi: 10.1172/JCI109826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyo J. L., Gennick S. E., Sauder S. E. Diabetogenic activity of native and biosynthetic human growth hormone in obese (ob/ob) mouse. Am J Physiol. 1984 Apr;246(4 Pt 1):E356–E360. doi: 10.1152/ajpendo.1984.246.4.E356. [DOI] [PubMed] [Google Scholar]
- MILMAN A. E., RUSSELL J. A. Some effects of purified pituitary growth hormone on carbohydrate metabolism in the rat. Endocrinology. 1950 Aug;47(2):114–128. doi: 10.1210/endo-47-2-114. [DOI] [PubMed] [Google Scholar]
- Maloff B. L., Levine J. H., Lockwood D. H. Direct effects of growth hormone on insulin action in rat adipose tissue maintained in vitro. Endocrinology. 1980 Aug;107(2):538–544. doi: 10.1210/endo-107-2-538. [DOI] [PubMed] [Google Scholar]
- Morikawa M., Green H., Lewis U. J. Activity of human growth hormone and related polypeptides on the adipose conversion of 3T3 cells. Mol Cell Biol. 1984 Feb;4(2):228–231. doi: 10.1128/mcb.4.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa M., Nixon T., Green H. Growth hormone and the adipose conversion of 3T3 cells. Cell. 1982 Jul;29(3):783–789. doi: 10.1016/0092-8674(82)90440-8. [DOI] [PubMed] [Google Scholar]
- Nixon B. T., Green H. Growth hormone promotes the differentiation of myoblasts and preadipocytes generated by azacytidine treatment of 10T1/2 cells. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3429–3432. doi: 10.1073/pnas.81.11.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg G., Smith U. Human adipose tissue in culture. VII. The long-term effect on growth hormone. Horm Metab Res. 1977 Jan;9(1):22–27. doi: 10.1055/s-0028-1093577. [DOI] [PubMed] [Google Scholar]
- Schoenle E., Zapf J., Froesch E. R. Regulation of rat adipocyte glucose transport by growth hormone: no mediation by insulin-like growth factors. Endocrinology. 1983 Jan;112(1):384–386. doi: 10.1210/endo-112-1-384. [DOI] [PubMed] [Google Scholar]
- Schoenle E., Zapf J., Humbel R. E., Froesch E. R. Insulin-like growth factor I stimulates growth in hypophysectomized rats. Nature. 1982 Mar 18;296(5854):252–253. doi: 10.1038/296252a0. [DOI] [PubMed] [Google Scholar]
- Schwartz J., Eden S. Acute growth hormone deficiency rapidly alters glucose metabolism in rat adipocytes. Relation to insulin responses and binding. Endocrinology. 1985 May;116(5):1806–1812. doi: 10.1210/endo-116-5-1806. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Enhanced sensitivity to insulin in rats treated with antibodies to rat growth hormone. Endocrinology. 1980 Oct;107(4):877–883. doi: 10.1210/endo-107-4-877. [DOI] [PubMed] [Google Scholar]
- Schwartz J., Goodman H. M. Comparison of the delayed actions of growth hormone and somatomedin on adipose tissue metabolism. Endocrinology. 1976 Mar;98(3):730–737. doi: 10.1210/endo-98-3-730. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Growth hormone directly alters glucose utilization in 3T3 adipocytes. Biochem Biophys Res Commun. 1984 Nov 30;125(1):237–243. doi: 10.1016/s0006-291x(84)80359-9. [DOI] [PubMed] [Google Scholar]


