Abstract
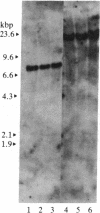
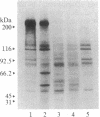
Acute nonlymphocytic leukemia associated with the chromosomal translocation t(6;9)(p23;q34) is an entity that is frequently associated with basophilia, which it shares with chronic myelogenous leukemia. The breakpoint on chromosome 9, q34, appears to be cytogenetically identical in both malignancies and is the site of the cellular oncogene c-abl. We investigated the role of c-abl in cells from two patients with the t(6;9) using in situ chromosomal hybridization, Southern hybridization, and in vitro phosphorylation. We showed that c-abl is not translocated from chromosome 9, resulting in a breakpoint that is on the 3' side of this gene. The t(6;9) translocation does not appear to result in the production of an aberrantly sized protein product or in the acquisition of in vitro tyrosine kinase activity. This is in direct contrast to the findings in chronic myelogenous leukemia, in which c-abl is translocated, leading to the production of a structurally altered c-abl protein with activated tyrosine kinase. Lastly, we demonstrated that the cells of one patient contain sequences from chromosome 9 inserted at the junction of a reciprocal translocation between chromosomes 4 and 10 on the 4q+ chromosome. This insertion, which is at least 100 kilobase pairs in length, represents a duplication and translocation of the protein coding region of c-abl.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besmer P., Hardy W. D., Jr, Zuckerman E. E., Bergold P., Lederman L., Snyder H. W., Jr The Hardy-Zuckerman 2-FeSV, a new feline retrovirus with oncogene homology to Abelson-MuLV. Nature. 1983 Jun 30;303(5920):825–828. doi: 10.1038/303825a0. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Kubonishi I., Miyoshi I., Groudine M. T. Altered transcription of the c-abl oncogene in K-562 and other chronic myelogenous leukemia cells. Science. 1984 Jul 6;225(4657):72–74. doi: 10.1126/science.6587568. [DOI] [PubMed] [Google Scholar]
- Croce C. M., Thierfelder W., Erikson J., Nishikura K., Finan J., Lenoir G. M., Nowell P. C. Transcriptional activation of an unrearranged and untranslocated c-myc oncogene by translocation of a C lambda locus in Burkitt. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6922–6926. doi: 10.1073/pnas.80.22.6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. L., Konopka J. B., Witte O. N. Activation of the c-abl oncogene by viral transduction or chromosomal translocation generates altered c-abl proteins with similar in vitro kinase properties. Mol Cell Biol. 1985 Jan;5(1):204–213. doi: 10.1128/mcb.5.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabkin H. A., Diaz M., Bradley C. M., Le Beau M. M., Rowley J. D., Patterson D. Isolation and analysis of the 21q+ chromosome in the acute myelogenous leukemia 8;21 translocation: evidence that c-mos is not translocated. Proc Natl Acad Sci U S A. 1985 Jan;82(2):464–468. doi: 10.1073/pnas.82.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson J., Nishikura K., ar-Rushdi A., Finan J., Emanuel B., Lenoir G., Nowell P. C., Croce C. M. Translocation of an immunoglobulin kappa locus to a region 3' of an unrearranged c-myc oncogene enhances c-myc transcription. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7581–7585. doi: 10.1073/pnas.80.24.7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson J., ar-Rushdi A., Drwinga H. L., Nowell P. C., Croce C. M. Transcriptional activation of the translocated c-myc oncogene in burkitt lymphoma. Proc Natl Acad Sci U S A. 1983 Feb;80(3):820–824. doi: 10.1073/pnas.80.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale R. P., Canaani E. An 8-kilobase abl RNA transcript in chronic myelogenous leukemia. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5648–5652. doi: 10.1073/pnas.81.18.5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groffen J., Stephenson J. R., Heisterkamp N., de Klein A., Bartram C. R., Grosveld G. Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell. 1984 Jan;36(1):93–99. doi: 10.1016/0092-8674(84)90077-1. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N., Stephenson J. R., Groffen J., Hansen P. F., de Klein A., Bartram C. R., Grosveld G. Localization of the c-ab1 oncogene adjacent to a translocation break point in chronic myelocytic leukaemia. Nature. 1983 Nov 17;306(5940):239–242. doi: 10.1038/306239a0. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Konopka J. B., Watanabe S. M., Singer J. W., Collins S. J., Witte O. N. Cell lines and clinical isolates derived from Ph1-positive chronic myelogenous leukemia patients express c-abl proteins with a common structural alteration. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1810–1814. doi: 10.1073/pnas.82.6.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka J. B., Watanabe S. M., Witte O. N. An alteration of the human c-abl protein in K562 leukemia cells unmasks associated tyrosine kinase activity. Cell. 1984 Jul;37(3):1035–1042. doi: 10.1016/0092-8674(84)90438-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Beau M. M., Westbrook C. A., Diaz M. O., Rowley J. D. c-src is consistently conserved in the chromosomal deletion (20q) observed in myeloid disorders. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6692–6696. doi: 10.1073/pnas.82.19.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitelman F. Restricted number of chromosomal regions implicated in aetiology of human cancer and leukaemia. 1984 Jul 26-Aug 1Nature. 310(5975):325–327. doi: 10.1038/310325a0. [DOI] [PubMed] [Google Scholar]
- Pearson M. G., Vardiman J. W., Le Beau M. M., Rowley J. D., Schwartz S., Kerman S. L., Cohen M. M., Fleischman E. W., Prigogina E. L. Increased numbers of marrow basophils may be associated with a t(6;9) in ANLL. Am J Hematol. 1985 Apr;18(4):393–403. doi: 10.1002/ajh.2830180409. [DOI] [PubMed] [Google Scholar]
- Pegoraro L., Matera L., Ritz J., Levis A., Palumbo A., Biagini G. Establishment of a Ph1-positive human cell line (BV173). J Natl Cancer Inst. 1983 Mar;70(3):447–453. [PubMed] [Google Scholar]
- Prywes R., Foulkes J. G., Rosenberg N., Baltimore D. Sequences of the A-MuLV protein needed for fibroblast and lymphoid cell transformation. Cell. 1983 Sep;34(2):569–579. doi: 10.1016/0092-8674(83)90389-6. [DOI] [PubMed] [Google Scholar]
- Rowley J. D. Biological implications of consistent chromosome rearrangements in leukemia and lymphoma. Cancer Res. 1984 Aug;44(8):3159–3168. [PubMed] [Google Scholar]
- Rowley J. D. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973 Jun 1;243(5405):290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- Rowley J. D., Potter D. Chromosomal banding patterns in acute nonlymphocytic leukemia. Blood. 1976 May;47(5):705–721. [PubMed] [Google Scholar]
- Shtivelman E., Lifshitz B., Gale R. P., Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985 Jun 13;315(6020):550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- Vermaelen K., Michaux J. L., Louwagie A., Van den Berghe H. Reciprocal translocation t(6;9)(p21;q33): a new characteristic chromosome anomaly in myeloid leukemias. Cancer Genet Cytogenet. 1983 Oct;10(2):125–131. doi: 10.1016/0165-4608(83)90115-2. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Baltimore D. Cellular RNA homologous to the Abelson murine leukemia virus transforming gene: expression and relationship to the viral sequence. Mol Cell Biol. 1983 May;3(5):773–779. doi: 10.1128/mcb.3.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunis J. J. Recurrent chromosomal defects are found in most patients with acute nonlymphocytic leukemia. Cancer Genet Cytogenet. 1984 Feb;11(2):125–137. doi: 10.1016/0165-4608(84)90106-7. [DOI] [PubMed] [Google Scholar]
- de Klein A., van Kessel A. G., Grosveld G., Bartram C. R., Hagemeijer A., Bootsma D., Spurr N. K., Heisterkamp N., Groffen J., Stephenson J. R. A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1982 Dec 23;300(5894):765–767. doi: 10.1038/300765a0. [DOI] [PubMed] [Google Scholar]