Abstract
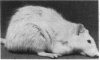
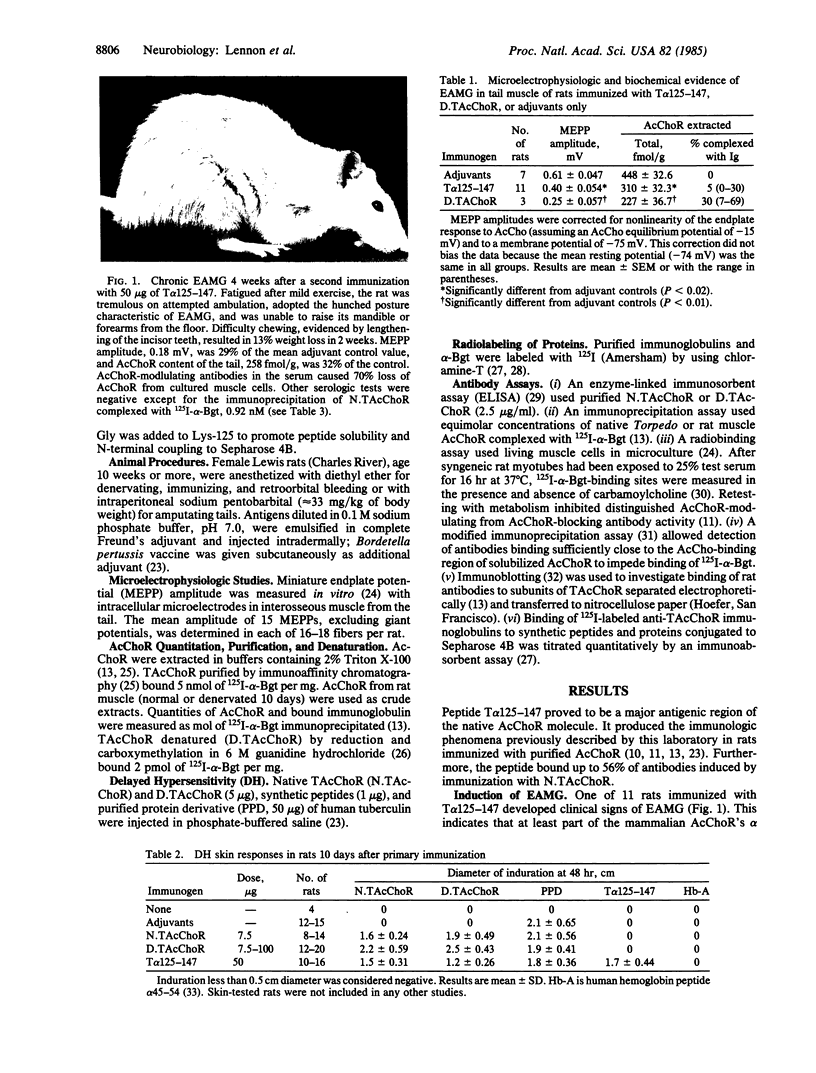
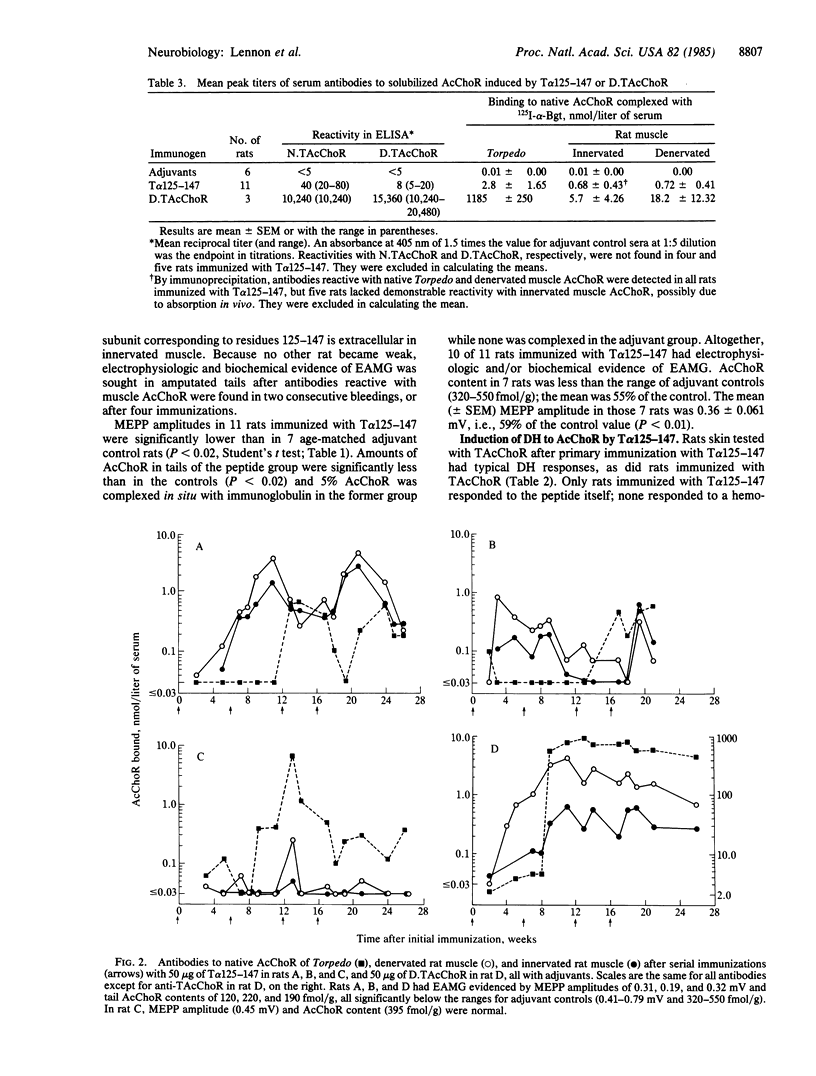
A major antigenic region of native nicotinic acetylcholine receptors (AcChoR) has been identified by using a synthetic disulfide-looped peptide corresponding to alpha-subunit residues 125-147 of Torpedo electric organ AcChoR: Lys-Ser-Tyr-Cys-Glu-Ile-Ile-Val-Thr-His-Phe- Pro-Phe-Asp-Gln-Gln-Asn-Cys-Thr-Met-Lys-Leu-Gly. The peptide bound 26-56% of polyclonal antibodies induced in rat, rabbit, and dog by immunization with native AcChoR. Rats inoculated with 50 micrograms of unconjugated peptide developed helper T-cell responses, delayed hypersensitivity, and antibodies to native AcChoR. Anti-peptide antibodies were more reactive with native than denatured AcChoR and bound to the alpha subunit. Some reacted exclusively with mammalian muscle AcChoR, some induced modulation of AcChoR on cultured myotubes, but none inhibited binding of alpha-bungarotoxin to solubilized or membrane-associated AcChoR. Repeated immunization induced experimental autoimmune myasthenia gravis: clinical signs in one rat and electrophysiologic and/or biochemical signs in 10 of 11 rats. Thus, at least part of the corresponding region of the mammalian AcChoR alpha subunit is extracellular at the neuromuscular junction and a potential target for pathogenic autoantibodies in patients with acquired myasthenia gravis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartfeld D., Fuchs S. Immunological characterization of an irreversibly denatured acetylcholine receptor. FEBS Lett. 1977 May 15;77(2):214–218. doi: 10.1016/0014-5793(77)80237-8. [DOI] [PubMed] [Google Scholar]
- Claudio T., Ballivet M., Patrick J., Heinemann S. Nucleotide and deduced amino acid sequences of Torpedo californica acetylcholine receptor gamma subunit. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1111–1115. doi: 10.1073/pnas.80.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti-Tronconi B. M., Hunkapiller M. W., Raftery M. A. Molecular weight and structural nonequivalence of the mature alpha subunits of Torpedo californica acetylcholine receptor. Proc Natl Acad Sci U S A. 1984 May;81(9):2631–2634. doi: 10.1073/pnas.81.9.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado M., Hochschwender S., Sarin V., Fox J. L., Lindstrom J. Evidence for unpredicted transmembrane domains in acetylcholine receptor subunits. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2004–2008. doi: 10.1073/pnas.82.7.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devillers-Thiery A., Giraudat J., Bentaboulet M., Changeux J. P. Complete mRNA coding sequence of the acetylcholine binding alpha-subunit of Torpedo marmorata acetylcholine receptor: a model for the transmembrane organization of the polypeptide chain. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2067–2071. doi: 10.1073/pnas.80.7.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez C. M., Richman D. P. Anti-acetylcholine receptor antibodies directed against the alpha-bungarotoxin binding site induce a unique form of experimental myasthenia. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4089–4093. doi: 10.1073/pnas.80.13.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao P. N., Dwork A. J., Kaldany R. R., Silver M. L., Wideman J., Stein S., Karlin A. Identification of the alpha subunit half-cystine specifically labeled by an affinity reagent for the acetylcholine receptor binding site. J Biol Chem. 1984 Oct 10;259(19):11662–11665. [PubMed] [Google Scholar]
- Kazim A. L., Atassi M. Z. Structurally inherent antigenic sites. Localization of the antigenic sites of the alpha-chain of human haemoglobin in three host species by a comprehensive synthetic approach. Biochem J. 1982 Apr 1;203(1):201–208. doi: 10.1042/bj2030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRochelle W. J., Wray B. E., Sealock R., Froehner S. C. Immunochemical demonstration that amino acids 360-377 of the acetylcholine receptor gamma-subunit are cytoplasmic. J Cell Biol. 1985 Mar;100(3):684–691. doi: 10.1083/jcb.100.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon V. A., Lambert E. H. Monoclonal autoantibodies to acetylcholine receptors: evidence for a dominant idiotype and requirement of complement for pathogenicity. Ann N Y Acad Sci. 1981;377:77–96. doi: 10.1111/j.1749-6632.1981.tb33725.x. [DOI] [PubMed] [Google Scholar]
- Lennon V. A., Lindstrom J. M., Seybold M. E. Experimental autoimmune myasthenia gravis: cellular and humoral immune responses. Ann N Y Acad Sci. 1976;274:283–299. doi: 10.1111/j.1749-6632.1976.tb47693.x. [DOI] [PubMed] [Google Scholar]
- Lennon V. A., Lindstrom J. M., Seybold M. E. Experimental autoimmune myasthenia: A model of myasthenia gravis in rats and guinea pigs. J Exp Med. 1975 Jun 1;141(6):1365–1375. doi: 10.1084/jem.141.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon V. A., Peterson S., Schubert D. Neurectoderm markers retained in phenotypical skeletal muscle cells arising from a glial cell line. Nature. 1979 Oct 18;281(5732):586–588. doi: 10.1038/281586a0. [DOI] [PubMed] [Google Scholar]
- Lennon V. A., Thompson M., Chen J. Properties of nicotinic acetylcholine receptors isolated by affinity chromatography on monoclonal antibodies. J Biol Chem. 1980 May 25;255(10):4395–4398. [PubMed] [Google Scholar]
- Lindstrom J. M., Einarson B. L., Lennon V. A., Seybold M. E. Pathological mechanisms in experimental autoimmune myasthenia gravis. I. Immunogenicity of syngeneic muscle acetylcholine receptor and quantitative extraction of receptor and antibody-receptor complexes from muscles of rats with experimental automimmune myasthenia gravis. J Exp Med. 1976 Sep 1;144(3):726–738. doi: 10.1084/jem.144.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J. M., Lennon V. A., Seybold M. E., Whittingham S. Experimental autoimmune myasthenia gravis and myasthenia gravis: biochemical and immunochemical aspects. Ann N Y Acad Sci. 1976;274:254–274. doi: 10.1111/j.1749-6632.1976.tb47691.x. [DOI] [PubMed] [Google Scholar]
- Lindstrom J., Criado M., Hochschwender S., Fox J. L., Sarin V. Immunochemical tests of acetylcholine receptor subunit models. Nature. 1984 Oct 11;311(5986):573–575. doi: 10.1038/311573a0. [DOI] [PubMed] [Google Scholar]
- McCormick D. J., Atassi M. Z. Localization and synthesis of the acetylcholine-binding site in the alpha-chain of the Torpedo californica acetylcholine receptor. Biochem J. 1984 Dec 15;224(3):995–1000. doi: 10.1042/bj2240995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. J., Lennon V. A., Atassi M. Z. Synthesis of an antigenic site of native acetylcholine receptor peptide 159-169 of Torpedo acetylcholine receptor alpha-chain. Biochem J. 1985 Feb 15;226(1):193–197. doi: 10.1042/bj2260193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina M., Tobimatsu T., Imoto K., Tanaka K., Fujita Y., Fukuda K., Kurasaki M., Takahashi H., Morimoto Y., Hirose T. Location of functional regions of acetylcholine receptor alpha-subunit by site-directed mutagenesis. 1985 Jan 31-Feb 6Nature. 313(6001):364–369. doi: 10.1038/313364a0. [DOI] [PubMed] [Google Scholar]
- Momoi M. Y., Lennon V. A. Evidence for structural dissimilarity in the neurotransmitter binding region of purified acetylcholine receptors from human muscle and Torpedo electric organ. J Neurochem. 1986 Jan;46(1):76–81. doi: 10.1111/j.1471-4159.1986.tb12927.x. [DOI] [PubMed] [Google Scholar]
- Momoi M. Y., Lennon V. A. Purification and biochemical characterization of nicotinic acetylcholine receptors of human muscle. J Biol Chem. 1982 Nov 10;257(21):12757–12764. [PubMed] [Google Scholar]
- Noda M., Furutani Y., Takahashi H., Toyosato M., Tanabe T., Shimizu S., Kikyotani S., Kayano T., Hirose T., Inayama S. Cloning and sequence analysis of calf cDNA and human genomic DNA encoding alpha-subunit precursor of muscle acetylcholine receptor. 1983 Oct 27-Nov 2Nature. 305(5937):818–823. doi: 10.1038/305818a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Furutani Y., Hirose T., Asai M., Inayama S., Miyata T., Numa S. Primary structure of alpha-subunit precursor of Torpedo californica acetylcholine receptor deduced from cDNA sequence. Nature. 1982 Oct 28;299(5886):793–797. doi: 10.1038/299793a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Hirose T., Asai M., Takashima H., Inayama S., Miyata T. Primary structures of beta- and delta-subunit precursors of Torpedo californica acetylcholine receptor deduced from cDNA sequences. Nature. 1983 Jan 20;301(5897):251–255. doi: 10.1038/301251a0. [DOI] [PubMed] [Google Scholar]
- Norcross N. L., Griffith I. J., Lettieri J. A. Measurement of acetylcholine receptor and anti-receptor antibodies by ELISA. Muscle Nerve. 1980 Jul-Aug;3(4):345–349. doi: 10.1002/mus.880030412. [DOI] [PubMed] [Google Scholar]
- Plümer R., Fels G., Maelicke A. Antibodies against preselected peptides to map functional sites on the acetylcholine receptor. FEBS Lett. 1984 Dec 10;178(2):204–208. doi: 10.1016/0014-5793(84)80601-8. [DOI] [PubMed] [Google Scholar]
- Reiter M. J., Cowburn D. A., Prives J. M., Karlin A. Affinity labeling of the acetylcholine receptor in the electroplax: electrophoretic separtion in sodium dodecyl sulfate. Proc Natl Acad Sci U S A. 1972 May;69(5):1168–1172. doi: 10.1073/pnas.69.5.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibahara S., Kubo T., Perski H. J., Takahashi H., Noda M., Numa S. Cloning and sequence analysis of human genomic DNA encoding gamma subunit precursor of muscle acetylcholine receptor. Eur J Biochem. 1985 Jan 2;146(1):15–22. doi: 10.1111/j.1432-1033.1985.tb08614.x. [DOI] [PubMed] [Google Scholar]
- Takai T., Noda M., Furutani Y., Takahashi H., Notake M., Shimizu S., Kayano T., Tanabe T., Tanaka K., Hirose T. Primary structure of gamma subunit precursor of calf-muscle acetylcholine receptor deduced from the cDNA sequence. Eur J Biochem. 1984 Aug 15;143(1):109–115. doi: 10.1111/j.1432-1033.1984.tb08348.x. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Noda M., Furutani Y., Takai T., Takahashi H., Tanaka K., Hirose T., Inayama S., Numa S. Primary structure of beta subunit precursor of calf muscle acetylcholine receptor deduced from cDNA sequence. Eur J Biochem. 1984 Oct 1;144(1):11–17. doi: 10.1111/j.1432-1033.1984.tb08424.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E. F., Ralston E., Blake J., Ramachandran J., Hall Z. W., Stroud R. M. Topological mapping of acetylcholine receptor: evidence for a model with five transmembrane segments and a cytoplasmic COOH-terminal peptide. Proc Natl Acad Sci U S A. 1985 Jan;82(2):626–630. doi: 10.1073/pnas.82.2.626. [DOI] [PMC free article] [PubMed] [Google Scholar]