Abstract
Traumatic brain injury (TBI) is characterized by an abrupt blow or exchange of force against the head and can be categorized as mild, moderate, and severe. The secondary cell death after TBI displays ischemic-like patterns including neuroinflammation. The scavenger receptor cluster of differentiation (CD) 36 is a lipid-associated protein capable of transducing intracellular signals to promote inflammatory mechanisms within different cell types. Expression and activation of CD36 is closely related to dyslipidemia secondary to diabetes. Diabetes mellitus (DM) has been documented as a co-morbidity factor in TBI, in that patients with a history of diabetes present with more severe brain damage and slower recovery from TBI than non-diabetic patients. Indeed, a strict regulation of blood serum glucose by the use of insulin promotes a better outcome for TBI patients. Based on these recent findings, we now advance the hypothesis that CD36 via DM insulin-associated pathways is closely involved in TBI chronic pathology.
Societal Impact of Traumatic Brain Injury
Every year in the US, 1.7 million people bear a Traumatic Brain Injury (TBI); from which 275,000 are hospitalized and 52,000 die (1). Overall incidence of TBI in the US is estimated to be 506.4 cases for every 100,000 (2). Of note, armed troops deployed in Afghanistan and Iraq, are exposed to blast-induced TBI. This type of TBI has been commonly said to be a “signature wound” continuously increasing among the military population (3,4). Scarce data about the impact of TBI in low and middle-income countries from the Latin American & Caribbean region suggest high incidence ratios of TBI caused mainly by violence and traffic accidents which may even overcome those in developed countries (5).
CD36-mediated neuroinflammation in TBI
The secondary cell death triggered by TBI displays ischemic-like patterns including neuroinflammation (6). Neuroinflammation is said to be a “double-edged sword”, capable of eliciting both damaging and reparative effects (7). The physiologic goal of inflammation is to draft diverse immune cell types into the site of the injury to remove damaged tissue and cellular debris allowing further creation of scar tissue. Microglia is the main immune cell type within the central nervous system (CNS). Activation of microglia varies according to stimuli, promoting three phenotypes: classical activation, alternative activation, and acquired deactivation. While the last two have been identified as promoters of anti-inflammatory mechanisms, the first is related to pro-inflammatory mechanisms (8).
Thirty minutes after a TBI, microglia respond and migrate to the injured area through chemokines and damage-associated molecular patterns, and may remain activated for many years promoting a chronic inflammatory process (9,10). In addition, blood brain barrier disruption following brain injury stimulates the infiltration of inflammatory cells including leukocytes and macrophages to the brain parenchyma, which further contribute to secondary brain injury (11). Several immune receptors are responsible for this pro-inflammatory effect, including toll-like receptors and scavenger receptors (12, 13).
The CD36 is an 88-kDa heavily glycosylated scavenger receptor with a large extracellular domain capable of interacting with several ligands and Src family kinases (SFK) (14,15). In mice, it has been identified as a key sensing protein that regulates chylomicron synthesis when stimulated by luminal long chain fatty acids (16). Action of the CD36 in the peripheral tissue has been linked to that of lipoprotein lipase and contributes to the uptake of free fatty acids (17). Nevertheless functions of the CD36 go further than those of lipid metabolism, as it interacts with toll-like receptors (TLR) 4 and 6 to form a heterotrimeric complex capable of transducing intracellular responses (18). Advanced glycation-end products, Amyloid β (Aβ) and oxidized low-density lipoproteins (OxLDL) are all ligands of the CD36; the last one specifically binds to a domain within amino acids 155-183 of the protein (19). Interestingly, OxLDL has been shown to share molecular similarities with a bacterial pathogen Streptococcus pneumoniae (20,21). Parallel to this, Aβ is a pathogenic endogenous peptide that is linked to the pathology of Alzheimer disease by the formation of amyloid plaques with further induction of chronic inflammatory damage in the brain. Indeed the capacity of the CD36 to recognize pathogen associated molecular patterns has been known for some time, for the CD36 promotes phagocytosis of Plasmodium falciparum infected erythrocytes and Staphylococcus aureus. This evidence suggests that although CD36 is capable of regulating the absorption of lipids within the peripheral tissue, oxidation of low-density lipoproteins (LDL) into OxLDL and Aβ may promote a different pro-inflammatory effect within the same receptor.
Diabetes promotes a cyclic neuroinflammatory process in the brain by OxLDL crosstalk with CD36
Diabetes mellitus (DM) is a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action or both (22). In North American countries, the prevalence for DM has been estimated to be 8.7%, 9.2% and 11.3% of all the Canadians, Mexicans and U.S. Citizens over 20 years of age respectively. The pathology is considered a significant health issue (23–25). “Diabetic dyslipidemia” is a term used to refer to the abnormal levels of lipids and lipoproteins under the pathology of diabetes (17). Diabetic dyslipidemia positively correlates with levels of triglycerides and cholesterol hyperlipidemia, LDL hyperlipoproteinemia and perhaps HDL hypolipoproteinemia (17). The relevance of increased LDL levels relies on the ease with which LDLs may be oxidized, for even minimal oxidation of lipids within LDLs is enough to trigger pro-inflammatory mechanisms that lead to further oxidation of these lipoproteins and deleterious pathways within the organism (26). Diabetes and TBI both increase oxidative pathways of LDL through hyperglycemia and inflammation (6,27–29). Recognition of OxLDL by the CD36 is known to promote pro-inflammatory mechanisms in atherosclerotic plaques and worsen outcome for cerebral ischemia murine models (30–32).
The metabolic failure present after TBI results in the depletion of ATP levels, which in turn leads to the failure of Na+/K+ ATPase. Subsequently, the loss of membrane potential induces sodium and calcium influx into the cell and release of neurotransmitters (33,34). Production of reactive oxygen species (ROS) in the mitochondria is closely related to calcium levels inside the cell, meaning that higher levels of calcium induce ROS production that in turn stimulate further calcium release creating a feedback loop that can be deleterious in situations where there is a calcium overload in the cell (29). Mitochondrial uncouplers can inhibit mitochondrial production of ROS by promoting H+ leakage; this has been shown to reduce the damage after both stroke and TBI (35,36). Furthermore, cell stress activates the membrane bound enzyme NADPH oxidase (37), which catalyzes the production of ROS, specifically O2- and peroxynitrite (ONOO−) (38,39). Oxidative stress occurs when the capacity of the cells to detoxify ROS is exceeded by production of ROS, and under oxidative stress the process of lipid peroxidation through which LDLs are oxidized into OxLDL is increased (40). Aβ is produced when the BACE1 and γ–secretase cleave the amyloid precursor protein (APP) within an endosome (41). BACE1 is upregulated by the effects of oxidative stress secondary to hyperglycemia, hypercholesteremia and hypoxia after acute neurological conditions (42).
Interaction of CD36 with OxLDL exacerbates inflammation in atherosclerotic plaques (43). Nevertheless this has been a thoroughly debated subject, in that increasing evidence suggests the lack of CD36 may actually worsen the outcome of atherosclerotic models (44). A pathophysiologic event advances the notion of a frustrated phagocytosis whereby the uptake of OxLDL by macrophages is a normal physiologic process but under western diet such process becomes pathologic (45). The result is the build-up of macrophages that “eat more than they can chew” eventually turning into foam cells and triggering chronic inflammatory mechanisms (46). Phagocytosis of OxLDL may lead to the formation of a secondary lysosome in which the insoluble molecules of cholesterol are capable of forming intracellular cholesterol crystals (30). Inflammatory mechanisms by which cholesterol crystals promote inflammation have been documented (47,49).
The interaction of the CD36 microglia with a prionic protein results in the expression of the pro-inflammatory cytokines, including CD36 mRNA, and SFK phosphorylation (50). The crosstalk between CD36 and Aβ can stimulate the production of ROS in microglia and macrophages, resulting in CNS recruitment of inflammatory cells (51,52). Signaling pathways for these microglial and macrophagic neuroinflammatory effects have been identified (53) involving precipitation of the CD36 with cholesterol crystal formation by Aβ via SFK (30). This evidence suggests that the constant LDL hyperlipoproteinemia within diabetic patients may promote an excessive activation of CD36 mediated inflammatory pathways after oxidation of such lipoproteins, promoting cyclic microglial activation that worsens the long-term outcome after a TBI.
Hypothesis
DM patients diagnosed with TBI have a higher mortality along with a longer hospital stay, lower Glasgow comma scale values, and higher injury severity scores (54). Changes in glucose levels are well known to occur in the CNS following TBI (55) and indeed secondary brain injury management requires aggressive control of the metabolic supply into the brain (56). Although many of these parameters have been related to the progression of TBI, much of the interactions between these values are yet to be described (56). The use of insulin to maintain blood glucose levels less than 110 mg/dl significantly diminished mortality rates after severe TBI, but the exact mechanism for this protective effect remains to be determined (57). To this end, we advance the hypothesis that hyperlipidemia and hyperglycemia, which are hallmark features of diabetes, may exacerbate TBI in the long-term via the CD36-mediated neuroinflammation.
DM and TBI may be pathologically linked via pro-inflammatory by-products of CD36, which have been implicated as comorbidity factors for chronic neurological conditions including Alzheimer’s disease and Parkinson’s disease (2,58,59), all of which have been documented as TBI-associated disease symptoms. Accordingly, CD36 can be used as TBI biomarker for the long-term assessment of the pathology. If CD36 is closely associated with neuroinflammation then studying the levels of CD36 will provide an excellent approximation of long-term TBI pathology, as well as a sensitive outcome measure of novel treatments for TBI. The complexity of CD36 mediated neuroinflammatory pathways leads us to believe that even slight elevations in CD36 levels of activity could initiate massive processes of self-propagating neuroinflammation. Determining a threshold of CD36 values, which if exceeded may potentiate such self-propagating neuroinflammation, will definitely help clinicians in the management of glucose and lipid levels of TBI patients in the long-term. The use of adjacent biomarkers for the management of blood glucose levels in diabetic patients is not a novelty. Glycosylated hemoglobin (HbA1c) levels are widely used in the daily clinical practice of many physicians to evaluate the different blood glucose levels throughout the past three months allowing them to adjust the treatment accordingly (60). Hyperlipidemic ApoE−/−, CD36 −/− double KO mice have been used to determine the role played by CD36 in the pathophysiology of stroke (61). Similar studies using both hyperglycemic and hyperlipidemic comparative animal TBI models will definitely help acquire further understanding of the mechanisms and impact CD36 plays on TBI pathology. Management of DM may be indicated for TBI. Both tight and conventional tendencies prevail regarding glycemic control with levels ranging from 80–110 mg/dl and 180–215 mg/dl respectively (62). Given the frailty of the CD36-mediated neuroinflammatory pathways, we believe that glucose management based on CD36 levels will emulate that of tight glycemic control. Additionally, DM drugs may have therapeutic benefits in TBI as recently demonstrated by an improved long-term outcome of TBI murine models following the administration of Exendin-4 (63), a glucagon-like peptide hormone known to stimulate glucose-dependent insulin secretion. Similarly, metformin, another drug used to treat DM, has shown to promote neurogenesis and spatial memory through the activation of signaling pathways that enhance neural stem cell differentiation and recruitment (64), which could similarly abrogate the cell loss associated with TBI. The present hypothesis implicates CD36 pathway DM and TBI, and warrants preclinical studies to reveal the safety and efficacy of DM drugs for long-term management of TBI patients.
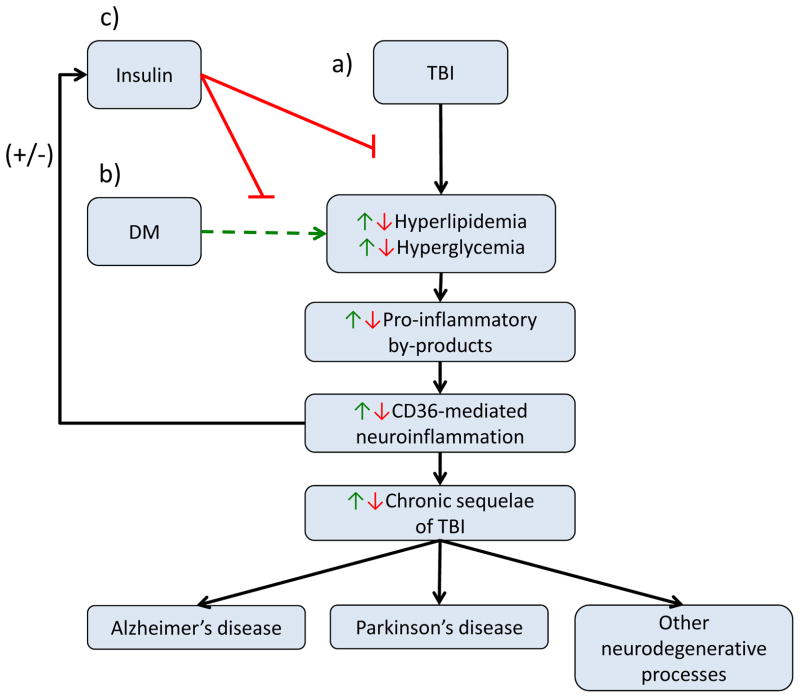
Figure 1.
a) Traumatic brain injury (TBI) pathophysiology involves hyperlipidemia and hyperglycemia, which promotes a cascade of events leading to CD36 mediated neuroinflammation, causing chronic sequelae of TBI including Alzheimer’s disease, Parkinson’s disease and other neurodegenerative processes. b) Diabetes mellitus (DM) increases the levels of hyperglycemia and hyperlipidemia following TBI, which in turn upregulates the pathological mechanism of TBI. c) Insulin decreases the levels of hyperglycemia and hyperlipidemia following TBI, resulting in a down regulation of the pathological mechanisms of TBI.
Acknowledgments
CVB is supported by NIH NINDS 1R01NS071956-01, Department of Defense W81XWH1110634, James and Esther King Foundation for Biomedical Research Program 1KG01-33966, SanBio Inc., Celgene Cellular Therapeutics, KMPHC and NeuralStem Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Faul M, Xu L, Wald MM, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Atlanta, GA: Centers for Disease Control and Prevention; National Center for Injury Prevention and Control; 2010. [Google Scholar]
- 2.Corrigan JD, Selassie AW, Orman JA. The Epidemiology of Traumatic Brain Injury. J Head Trauma Rehabil. 2010;25(2) doi: 10.1097/HTR.0b013e3181ccc8b4. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharjee Y. Neuroscience. Shell shock revisited: solving the puzzle of blast trauma. Science. 2008 Jan 25;319(5862):406–408. doi: 10.1126/science.319.5862.406. [DOI] [PubMed] [Google Scholar]
- 4.Gubata ME, Packnett ER, Blandford CD, Piccirillo AL, Niebuhr DW, Cowan DN. Trends in the Epidemiology of Disability Related to Traumatic Brain Injury in the US Army and Marine Corps: 2005 to 2010. J Head Trauma Rehabil. 9000 doi: 10.1097/HTR.0b013e318295f590. Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 5.Puvanachandra P, Hyder AA. Traumatic brain injury in Latin America and the Caribbean: a call for research. Salud Publica Mex. 2008;50:s3–s5. doi: 10.1590/s0036-36342008000700002. [DOI] [PubMed] [Google Scholar]
- 6.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007 Jul;99(1):4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 7.Doyle KP, Buckwalter MS. The double-edged sword of inflammation after stroke: What sharpens each edge? Ann Neurol. 2012;71(6):729–731. doi: 10.1002/ana.23579. [DOI] [PubMed] [Google Scholar]
- 8.Luo X, Chen S. The changing phenotype of microglia from homeostasis to disease. Translational Neurodegeneration. 2012;1(1):9. doi: 10.1186/2047-9158-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koshinaga M, Katayama Y, Fukushima M, Oshima H, Suma T, Takahata T. Rapid and widespread microglial activation induced by traumatic brain injury in rat brain slices. J Neurotrauma. 2000 Mar;17(3):185–192. doi: 10.1089/neu.2000.17.185. [DOI] [PubMed] [Google Scholar]
- 10.Bachstetter AD, Rowe RK, Kaneko M, Goulding D, Lifshitz J, Van Eldik LJ. The p38alpha MAPK regulates microglial responsiveness to diffuse traumatic brain injury. J Neurosci. 2013 Apr 3;33(14):6143–6153. doi: 10.1523/JNEUROSCI.5399-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finnie JW. Neuroinflammation: beneficial and detrimental effects after traumatic brain injury. Inflammopharmacology. 2013 Aug;21(4):309–320. doi: 10.1007/s10787-012-0164-2. [DOI] [PubMed] [Google Scholar]
- 12.Laird MD, Shields JS, Sukumari-Ramesh S, Kimbler DE, David Fessler R, Shakir B. High mobility group box protein-1 promotes cerebral edema after traumatic brain injury via activation of toll-like receptor 4. Glia. 2013 Oct 28; doi: 10.1002/glia.22581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z, Zhang ZY, Wu Y, Schlusener HJ. Immunolocalization of Toll-like receptors 2 and 4 as well as their endogenous ligand, heat shock protein 70, in rat traumatic brain injury. Neuroimmunomodulation. 2012;19(1):10–9. doi: 10.1159/000326771. [DOI] [PubMed] [Google Scholar]
- 14.Tandon NN, Lipsky RH, Burgess WH, Jamieson GA. Isolation and characterization of platelet glycoprotein IV (CD36) Journal of Biological Chemistry. 1989 May 05;264(13):7570–7575. [PubMed] [Google Scholar]
- 15.Collot-Teixeira S, Martin J, McDermott-Roe C, Poston R, McGregor JL. CD36 and macrophages in atherosclerosis. Cardiovascular Research. 2007 Aug 01;75(3):468–477. doi: 10.1016/j.cardiores.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Tran TTT, Poirier H, Clément L, Nassir F, Pelsers MMAL, Petit V, et al. Luminal Lipid Regulates CD36 Levels and Downstream Signaling to Stimulate Chylomicron Synthesis. Journal of Biological Chemistry. 2011 Jul 15;286(28):25201–25210. doi: 10.1074/jbc.M111.233551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg IJ, Eckel RH, Abumrad NA. Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CD36-mediated pathways. Journal of Lipid Research. 2009 Apr 01;50(Supplement):S86–S90. doi: 10.1194/jlr.R800085-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010 Feb;11(2):155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Navazo MDP, Daviet L, Ninio E, McGregor JL. Identification on Human CD36 of a Domain (155-183) Implicated in Binding Oxidized Low-Density Lipoproteins (Ox-LDL) Arteriosclerosis, Thrombosis, and Vascular Biology. 1996 Aug 01;16(8):1033–1039. doi: 10.1161/01.atv.16.8.1033. [DOI] [PubMed] [Google Scholar]
- 20.Binder CJ, Horkko S, Dewan A, Chang MK, Kieu EP, Goodyear CS, et al. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med. 2003 Jun;9(6):736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 21.Vale AM, Kapoor P, Skibinski GA, Elgavish A, Mahmoud TI, Zemlin C, et al. The link between antibodies to OxLDL and natural protection against pneumococci depends on D(H) gene conservation. J Exp Med. 2013 May 6;210(5):875–890. doi: 10.1084/jem.20121861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2004 Jan 01;27(suppl 1):s5–s10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 24.Hernández-Ávila M, Gutiérrez JP. Diabetes mellitus: la urgencia de reforzar la respuesta en políticas públicas para su prevernción y control [Internet] Mexico: Instituto Nacional de Salud Publica; 2012. [cited 2013 Nov 9]. Available from: http://ensanut.insp.mx/doctos/analiticos/DiabetesMellitus.pdf. [Google Scholar]
- 25.Public Health Agency of Canada. Diabetes in Canada: Facts and figures from a public health perspective. Ottawa: Public Health Agency of Canada; 2011. [Google Scholar]
- 26.Yoshida H, Kisugi R. Mechanisms of LDL oxidation. Clinica Chimica Acta. 2010 Dec 14;411(23–24):1875–1882. doi: 10.1016/j.cca.2010.08.038. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan M, Aviram M, Hayek T. Oxidative stress and macrophage foam cell formation during diabetes mellitus-induced atherogenesis: role of insulin therapy. Pharmacol Ther. 2012 Nov;136(2):175–185. doi: 10.1016/j.pharmthera.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Green K, Brand MD, Murphy MP. Prevention of Mitochondrial Oxidative Damage as a Therapeutic Strategy in Diabetes. Diabetes. 2004 Feb 01;53(suppl 1):S110–S118. doi: 10.2337/diabetes.53.2007.s110. [DOI] [PubMed] [Google Scholar]
- 29.Feissner RF, Skalska J, Gaum WE, Sheu SS. Crosstalk signaling between mitochondrial Ca2+ and ROS. Front Biosci. 2009 Jan 1;14:1197–1218. doi: 10.2741/3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013 Jun 30; doi: 10.1038/ni.2639. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho S, Kim E. CD36: a multi-modal target for acute stroke therapy. J Neurochem. 2009 May;109( Suppl 1):126–132. doi: 10.1111/j.1471-4159.2009.05801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snipelisky D, Ziajka P. Diabetes and hyperlipidemia: A direct quantitative analysis—A direct analysis of the effects of insulin resistance on lipid levels in relation to atherosclerotic coronary artery disease. World Journal of Cardiovascular Diseases. 2012;2(1):20–25. [Google Scholar]
- 33.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. British Journal of Anaesthesia. 2007 Jul 01;99(1):4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 34.Santos MS, Moreno AJ, Carvalho AP. Relationships Between ATP Depletion, Membrane Potential, and the Release of Neurotransmitters in Rat Nerve Terminals: An In Vitro Study Under Conditions That Mimic Anoxia, Hypoglycemia, and Ischemia. Stroke. 1996 May 01;27(5):941–950. doi: 10.1161/01.str.27.5.941. [DOI] [PubMed] [Google Scholar]
- 35.Korde AS, Pettigrew LC, Craddock SD, Maragos WF. The mitochondrial uncoupler 2,4-dinitrophenol attenuates tissue damage and improves mitochondrial homeostasis following transient focal cerebral ischemia. J Neurochem. 2005;94(6):1676–1684. doi: 10.1111/j.1471-4159.2005.03328.x. [DOI] [PubMed] [Google Scholar]
- 36.Pandya JD, Pauly JR, Nukala VN, Sebastian AH, Day KM, Korde AS, et al. Post-Injury Administration of Mitochondrial Uncouplers Increases Tissue Sparing and Improves Behavioral Outcome following Traumatic Brain Injury in Rodents. J Neurotrauma. 2007 May;24(5):798–811. doi: 10.1089/neu.2006.3673. [DOI] [PubMed] [Google Scholar]
- 37.Sorce S, Krause KH. NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal. 2009 Oct;11(10):2481–2504. doi: 10.1089/ars.2009.2578. [DOI] [PubMed] [Google Scholar]
- 38.Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, et al. Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal. 2011 Apr 15;14(8):1505–1517. doi: 10.1089/ars.2010.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yurdakan G, Tekin IO, Comert M, Acikgoz S, Sipahi EY. The presence of oxidized low-density lipoprotein and inducible nitric oxide synthase expression in renal damage after intestinal ischemia reperfusion. Kaohsiung J Med Sci. 2012 Jan;28(1):16–22. doi: 10.1016/j.kjms.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Brien RJ, Wong PC. Amyloid Precursor Protein Processing and Alzheimer’s Disease. Annu Rev Neurosci 2011. 2013 Jul 21;34(1):185–204. doi: 10.1146/annurev-neuro-061010-113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamagno E, Guglielmotto M, Monteleone D, Tabaton M. Amyloid-β Production: Major Link Between Oxidative Stress and BACE1. Neurotoxicity Research. 2012 Oct 01;22(3):208–219. doi: 10.1007/s12640-011-9283-6. [DOI] [PubMed] [Google Scholar]
- 43.Cho S, Kim E. CD36: a multi-modal target for acute stroke therapy. J Neurochem. 2009 May;109( Suppl 1):126–132. doi: 10.1111/j.1471-4159.2009.05801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore KJ, Freeman MW. Scavenger Receptors in Atherosclerosis: Beyond Lipid Uptake. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006 Aug 01;26(8):1702–1711. doi: 10.1161/01.ATV.0000229218.97976.43. [DOI] [PubMed] [Google Scholar]
- 45.Silverstein RL, Li W, Park YM, Rahaman SO. Mechanisms of cell signaling by the scavenger receptor CD36: implications in atherosclerosis and thrombosis. Trans Am Clin Climatol Assoc. 2010;121:206–220. [PMC free article] [PubMed] [Google Scholar]
- 46.Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006 Sep;4(3):211–221. doi: 10.1016/j.cmet.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grebe A, Latz E. Cholesterol crystals and inflammation. Curr Rheumatol Rep. 2013 Mar;15(3):313–012-0313-z. doi: 10.1007/s11926-012-0313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freigang S, Ampenberger F, Spohn G, Heer S, Shamshiev AT, Kisielow J, et al. Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis. Eur J Immunol. 2011 Jul;41(7):2040–2051. doi: 10.1002/eji.201041316. [DOI] [PubMed] [Google Scholar]
- 49.Grebe A, Latz E. Cholesterol crystals and inflammation. Curr Rheumatol Rep. 2013 Mar;15(3):313–012-0313-z. doi: 10.1007/s11926-012-0313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kouadir M, Yang L, Tan R, Shi F, Lu Y, Zhang S, et al. CD36 Participates in PrP 106–126 Induced Activation of Microglia. PLoS ONE. 2012 Jan 26;7(1):e30756. doi: 10.1371/journal.pone.0030756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coraci IS, Husemann J, Berman JW, Hulette C, Dufour JH, Campanella GK, et al. CD36, a Class B Scavenger Receptor, Is Expressed on Microglia in Alzheimer’s Disease Brains and Can Mediate Production of Reactive Oxygen Species in Response to β-Amyloid Fibrils. The American Journal of Pathology. 2002;1;160(1):101–112. doi: 10.1016/s0002-9440(10)64354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.El Khoury JB, Moore KJ, Means TK, Leung J, Terada K, Toft M, et al. CD36 Mediates the Innate Host Response to β-Amyloid. The Journal of Experimental Medicine. 2003 Jun 16;197(12):1657–1666. doi: 10.1084/jem.20021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moore KJ, El Khoury J, Medeiros LA, Terada K, Geula C, Luster AD, et al. A CD36-initiated Signaling Cascade Mediates Inflammatory Effects of β-Amyloid. Journal of Biological Chemistry. 2002 Dec 06;277(49):47373–47379. doi: 10.1074/jbc.M208788200. [DOI] [PubMed] [Google Scholar]
- 54.Ley EJ, Srour MK, Clond MA, Barnajian M, Tillou A, Mirocha J, et al. Diabetic patients with traumatic brain injury: insulin deficiency is associated with increased mortality. J Trauma. 2011 May;70(5):1141–1144. doi: 10.1097/TA.0b013e3182146d66. [DOI] [PubMed] [Google Scholar]
- 55.Yoshino A, Hovda DA, Kawamata T, Katayama Y, Becker DP. Dynamic changes in local cerebral glucose utilization following cerebral concussions in rats: evidence of a hyper- and subsequent hypometabolic state. Brain Res. 1991 Oct 4;561(1):106–119. doi: 10.1016/0006-8993(91)90755-k. [DOI] [PubMed] [Google Scholar]
- 56.Hemphill JC, Andrews P, De Georgia M. Multimodal monitoring and neurocritical care bioinformatics. Nat Rev Neurol. 2011 Jul 12;7(8):451–460. doi: 10.1038/nrneurol.2011.101. [DOI] [PubMed] [Google Scholar]
- 57.Van dB, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive Insulin Therapy in Critically Ill Patients. N Engl J Med. 2001 Nov 08;345(19):1359–1367. doi: 10.1056/NEJMoa011300. 2013/07. [DOI] [PubMed] [Google Scholar]
- 58.Campdelacreu J. Parkinson disease and Alzheimer disease: environmental risk factors. Neurologia. 2012 Jun 13; doi: 10.1016/j.nrl.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 59.Lee PC, Bordelon Y, Bronstein J, Ritz B. Traumatic brain injury, paraquat exposure, and their relationship to Parkinson disease. Neurology. 2012 Nov 13;79(20):2061–2066. doi: 10.1212/WNL.0b013e3182749f28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weykamp C. HbA1c: A Review of Analytical and Clinical Aspects. Ann Lab Med. 2013 Nov;33(6):393–400. doi: 10.3343/alm.2013.33.6.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim E, Tolhurst AT, Qin LY, Chen XY, Febbraio M, Cho S. CD36/fatty acid translocase, an inflammatory mediator, is involved in hyperlipidemia-induced exacerbation in ischemic brain injury. J Neurosci. 2008 Apr 30;28(18):4661–70. doi: 10.1523/JNEUROSCI.0982-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Green DM, O’Phelan KH, Bassin SL, Chang CW, Stern TS, Asai SM. Intensive versus conventional insulin therapy in critically ill neurologic patients. Neurocrit Care. 2010 Dec;13(3):299–306. doi: 10.1007/s12028-010-9417-3. [DOI] [PubMed] [Google Scholar]
- 63.Rachmany L, Tweedie D, Li Y, Rubovitch V, Holloway H, Miller J, et al. Exendin-4 induced glucagon-like peptide-1 receptor activation reverses behavioral impairments of mild traumatic brain injury in mice. Age. 2012 Aug 15;:1–16. doi: 10.1007/s11357-012-9464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang J, Gallagher D, DeVito LM, Cancino GI, Tsui D, He L, et al. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell. 2012 Jul 6;11(1):23–35. doi: 10.1016/j.stem.2012.03.016. [DOI] [PubMed] [Google Scholar]