Abstract
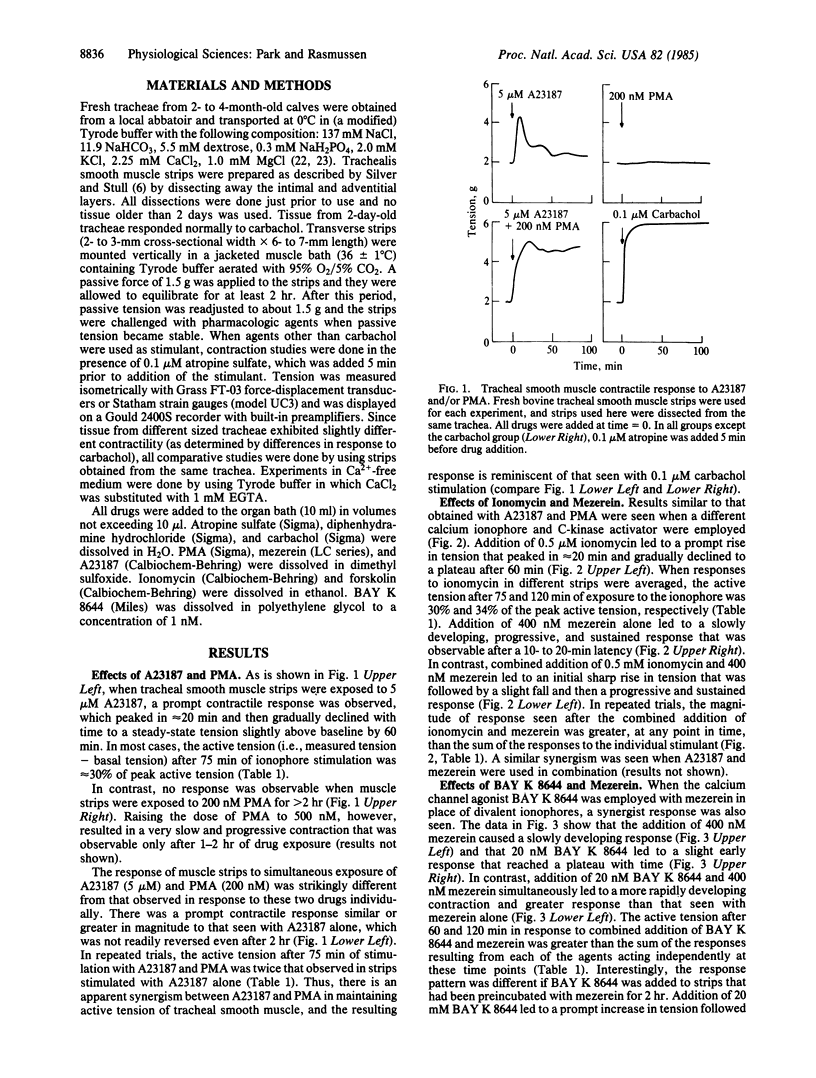
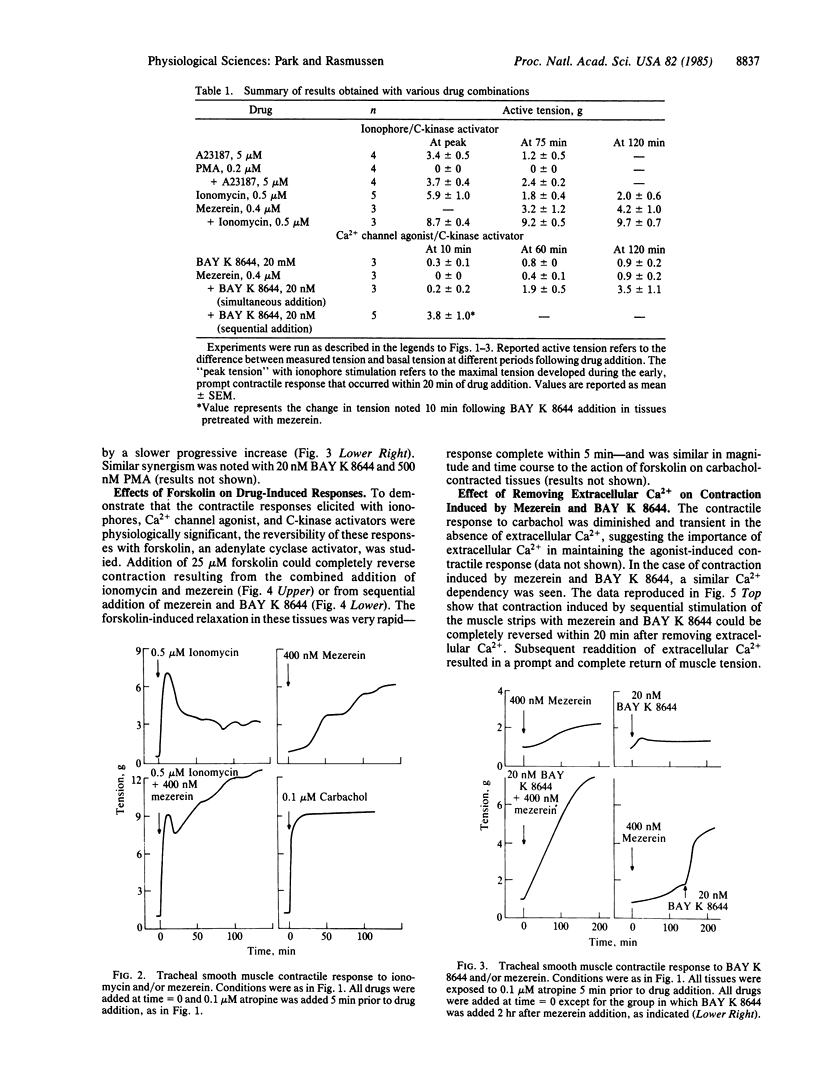
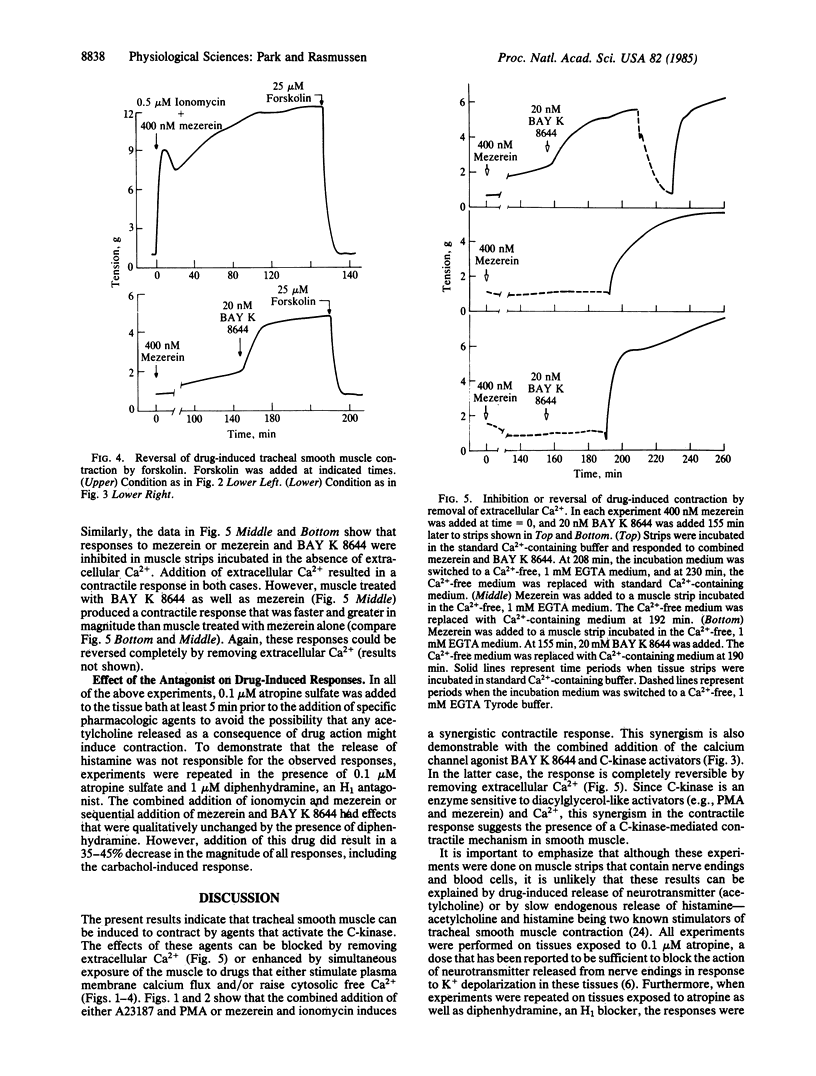
The effects of divalent ionophores (A23187 and ionomycin), Ca2+ channel agonist (BAY K 8644), and protein kinase C (C-kinase) activators [phorbol 12-myristate 13-acetate (PMA), mezerein] on bovine tracheal smooth muscle contraction were investigated. A23187 (5 microM) and ionomycin (0.5 microM) produced a prompt but transient contraction. C-kinase activators either produced no effect--e.g., PMA at 200 nM--or produced a rise in tension that was slow in onset but then gradually increased--e.g., mezerein at 400 nM. In contrast, ionophores and C-kinase activators, in combination, acted synergistically to produce a prompt and sustained contractile response that is reminiscent of that observed in response to carbachol, a cholinergic agonist. In addition, BAY K 8644 (20 nM), which has a minimal effect on tension by itself, could significantly enhance contraction induced by C-kinase activators. The contraction induced by all of these agents was quickly reversed either by removal of extracellular Ca2+ or upon addition of forskolin, an activator of adenylate cyclase. A similar reversal of carbachol-induced contraction by forskolin was observed with carbachol-induced contraction. These findings strongly suggest that C-kinase plays an important role in mediating tracheal smooth muscle contraction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Eisenberg E. Regulation and kinetics of the actin-myosin-ATP interaction. Annu Rev Biochem. 1980;49:921–956. doi: 10.1146/annurev.bi.49.070180.004421. [DOI] [PubMed] [Google Scholar]
- Akhtar R. A., Abdel-Latif A. A. Carbachol causes rapid phosphodiesteratic cleavage of phosphatidylinositol 4,5-bisphosphate and accumulation of inositol phosphates in rabbit iris smooth muscle; prazosin inhibits noradrenaline- and ionophore A23187-stimulated accumulation of inositol phosphates. Biochem J. 1984 Nov 15;224(1):291–300. doi: 10.1042/bj2240291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksoy M. O., Mras S., Kamm K. E., Murphy R. A. Ca2+, cAMP, and changes in myosin phosphorylation during contraction of smooth muscle. Am J Physiol. 1983 Sep;245(3):C255–C270. doi: 10.1152/ajpcell.1983.245.3.C255. [DOI] [PubMed] [Google Scholar]
- Baraban J. M., Gould R. J., Peroutka S. J., Snyder S. H. Phorbol ester effects on neurotransmission: interaction with neurotransmitters and calcium in smooth muscle. Proc Natl Acad Sci U S A. 1985 Jan;82(2):604–607. doi: 10.1073/pnas.82.2.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danthuluri N. R., Deth R. C. Phorbol ester-induced contraction of arterial smooth muscle and inhibition of alpha-adrenergic response. Biochem Biophys Res Commun. 1984 Dec 28;125(3):1103–1109. doi: 10.1016/0006-291x(84)91397-4. [DOI] [PubMed] [Google Scholar]
- Delbeke D., Kojima I., Dannies P. S., Rasmussen H. Synergistic stimulation of prolactin release by phorbol ester, A23187 and forskolin. Biochem Biophys Res Commun. 1984 Sep 17;123(2):735–741. doi: 10.1016/0006-291x(84)90291-2. [DOI] [PubMed] [Google Scholar]
- Douglas J. S., Ridgway P., Brink C. Airway responses of the guinea pig in vivo and in vitro. J Pharmacol Exp Ther. 1977 Jul;202(1):116–124. [PubMed] [Google Scholar]
- Downes P., Michell R. H. Phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate: lipids in search of a function. Cell Calcium. 1982 Oct;3(4-5):467–502. doi: 10.1016/0143-4160(82)90031-8. [DOI] [PubMed] [Google Scholar]
- Freedman S. B., Miller R. J. Calcium channel activation: a different type of drug action. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5580–5583. doi: 10.1073/pnas.81.17.5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikebe M., Inagaki M., Kanamaru K., Hidaka H. Phosphorylation of smooth muscle myosin light chain kinase by Ca2+-activated, phospholipid-dependent protein kinase. J Biol Chem. 1985 Apr 25;260(8):4547–4550. [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Sawamura M., Hoshijima M., Fujikura T., Nishizuka Y. Synergistic functions of protein phosphorylation and calcium mobilization in platelet activation. J Biol Chem. 1983 Jun 10;258(11):6701–6704. [PubMed] [Google Scholar]
- Kawamoto S., Hidaka H. Ca2+-activated, phospholipid-dependent protein kinase catalyzes the phosphorylation of actin-binding proteins. Biochem Biophys Res Commun. 1984 Feb 14;118(3):736–742. doi: 10.1016/0006-291x(84)91456-6. [DOI] [PubMed] [Google Scholar]
- Kojima I., Kojima K., Kreutter D., Rasmussen H. The temporal integration of the aldosterone secretory response to angiotensin occurs via two intracellular pathways. J Biol Chem. 1984 Dec 10;259(23):14448–14457. [PubMed] [Google Scholar]
- Kojima I., Kojima K., Rasmussen H. Role of calcium fluxes in the sustained phase of angiotensin II-mediated aldosterone secretion from adrenal glomerulosa cells. J Biol Chem. 1985 Aug 5;260(16):9177–9184. [PubMed] [Google Scholar]
- Kojima I., Lippes H., Kojima K., Rasmussen H. Aldosterone secretion: effect of phorbol ester and A23187. Biochem Biophys Res Commun. 1983 Oct 31;116(2):555–562. doi: 10.1016/0006-291x(83)90559-4. [DOI] [PubMed] [Google Scholar]
- Kojima I., Lippes H., Kojima K., Rasmussen H. Aldosterone secretion: effect of phorbol ester and A23187. Biochem Biophys Res Commun. 1983 Oct 31;116(2):555–562. doi: 10.1016/0006-291x(83)90559-4. [DOI] [PubMed] [Google Scholar]
- Martin T. F., Kowalchyk J. A. Evidence for the role of calcium and diacylglycerol as dual second messengers in thyrotropin-releasing hormone action: involvement of Ca+2. Endocrinology. 1984 Oct;115(4):1527–1536. doi: 10.1210/endo-115-4-1527. [DOI] [PubMed] [Google Scholar]
- Martin T. F., Kowalchyk J. A. Evidence for the role of calcium and diacylglycerol as dual second messengers in thyrotropin-releasing hormone action: involvement of diacylglycerol. Endocrinology. 1984 Oct;115(4):1517–1526. doi: 10.1210/endo-115-4-1517. [DOI] [PubMed] [Google Scholar]
- Mathé A. A., Aström A., Persson N. A. Some bronchoconstricting and bronchodilating responses of human isolated bronchi: evidence for the existence of -adrenoceptors. J Pharm Pharmacol. 1971 Dec;23(12):905–910. doi: 10.1111/j.2042-7158.1971.tb09891.x. [DOI] [PubMed] [Google Scholar]
- Miyake R., Tanaka Y., Tsuda T., Kaibuchi K., Kikkawa U., Nishizuka Y. Activation of protein kinase C by non-phorbol tumor promoter, mezerein. Biochem Biophys Res Commun. 1984 Jun 15;121(2):649–656. doi: 10.1016/0006-291x(84)90231-6. [DOI] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Stimulus-specific patterns of intracellular calcium levels in smooth muscle of ferret portal vein. J Physiol. 1984 Jun;351:155–167. doi: 10.1113/jphysiol.1984.sp015239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Vascular smooth muscle: the first recorded Ca2+ transients. Pflugers Arch. 1982 Oct;395(1):75–77. doi: 10.1007/BF00584972. [DOI] [PubMed] [Google Scholar]
- Nishikawa M., Hidaka H., Adelstein R. S. Phosphorylation of smooth muscle heavy meromyosin by calcium-activated, phospholipid-dependent protein kinase. The effect on actin-activated MgATPase activity. J Biol Chem. 1983 Dec 10;258(23):14069–14072. [PubMed] [Google Scholar]
- Rasmussen H., Barrett P. Q. Calcium messenger system: an integrated view. Physiol Rev. 1984 Jul;64(3):938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Kojima I., Kojima K., Zawalich W., Apfeldorf W. Calcium as intracellular messenger: sensitivity modulation, C-kinase pathway, and sustained cellular response. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;18:159–193. [PubMed] [Google Scholar]
- Silver P. J., Stull J. T. Phosphorylation of myosin light chain and phosphorylase in tracheal smooth muscle in response to KCl and carbachol. Mol Pharmacol. 1984 Mar;25(2):267–274. [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Stull J. T. Phosphorylation of contractile proteins in relation to muscle function. Adv Cyclic Nucleotide Res. 1980;13:39–93. [PubMed] [Google Scholar]
- Suematsu E., Hirata M., Hashimoto T., Kuriyama H. Inositol 1,4,5-trisphosphate releases Ca2+ from intracellular store sites in skinned single cells of porcine coronary artery. Biochem Biophys Res Commun. 1984 Apr 30;120(2):481–485. doi: 10.1016/0006-291x(84)91279-8. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Kawahara Y., Minakuchi R., Sano K., Kikkawa U., Mori T., Yu B., Kaibuchi K., Nishizuka Y. Calcium and phosphatidylinositol turnover as signalling for transmembrane control of protein phosphorylation. Adv Cyclic Nucleotide Res. 1981;14:301–313. [PubMed] [Google Scholar]
- Villalobos-Molina R., Uc M., Hong E., García-Sáinz J. A. Correlation between phosphatidylinositol labeling and contraction in rabbit aorta: effect of alpha-1 adrenergic activation. J Pharmacol Exp Ther. 1982 Jul;222(1):258–261. [PubMed] [Google Scholar]
- Yu B. Calcium-activated, phospholipid-dependent protein kinase in smooth muscle and its possible relation to phosphatidylinositol turnover. Kobe J Med Sci. 1981 Dec;27(6):225–237. [PubMed] [Google Scholar]
- Zawalich W., Brown C., Rasmussen H. Insulin secretion: combined effects of phorbol ester and A23187. Biochem Biophys Res Commun. 1983 Dec 16;117(2):448–455. doi: 10.1016/0006-291x(83)91221-4. [DOI] [PubMed] [Google Scholar]
- Zawalich W., Zawalich K., Rasmussen H. Insulin secretion: combined tolbutamide, forskolin and TPA mimic action of glucose. Cell Calcium. 1984 Dec;5(6):551–558. doi: 10.1016/0143-4160(84)90031-9. [DOI] [PubMed] [Google Scholar]
- de Pont J. J., Fleuren-Jakobs A. M. Synergistic effect of A23187 and a phorbol ester on amylase secretion from rabbit pancreatic acini. FEBS Lett. 1984 May 7;170(1):64–68. doi: 10.1016/0014-5793(84)81369-1. [DOI] [PubMed] [Google Scholar]


