Abstract
Autism spectrum disorder (ASD) and Fragile X syndrome (FXS) are relatively common childhood neurodevelopmental disorders with increasing incidence in recent years. They are currently accepted as disorders of the synapse with alterations in different forms of synaptic communication and neuronal network connectivity. The major excitatory neurotransmitter system in brain, the glutamatergic system, is implicated in learning and memory, synaptic plasticity, neuronal development. While much attention is attributed to the role of metabotropic glutamate receptors in ASD and FXS, studies indicate that the ionotropic glutamate receptors (iGluRs) and their regulatory proteins are also altered in several brain regions. Role of iGluRs in the neurobiology of ASD and FXS is supported by a weight of evidence that ranges from human genetics to in vitro cultured neurons. In this review we will discuss clinical, molecular, cellular and functional changes in NMDA, AMPA and kainate receptors and the synaptic proteins that regulate them in the context of ASD and FXS. We will also discuss the significance for the development of translational biomarkers and treatments for the core symptoms of ASD and FXS.
Keywords: AMPA receptor, Arc, autism spectrum disorder, Fragile X syndrome, GRIP1/2, kainate receptor, MAP1B, memantine, metabotropic glutamate receptor, neuroligin, NMDA receptor.
I. INTRODUCTION
The mechanisms that underlie the learning and memory, cognitive and social deficits associated with autism spectrum disorders (ASD) and Fragile X syndrome (FXS) are complex and depend to a large extent on glutamate receptors. Glutamate receptors are comprised of ionotropic glutamate receptors (iGluRs) and metabotropic glutamate receptors (mGluRs). Findings from various experimental systems implicate iGluR dysfunction in ASDs and FXS. They include human genetic studies, clinical drug trials, human neuro-imaging, and postmortem brain studies, animal models of ASD and FXS, and in vitro cell cultures, which will be discussed in the present review. The metabotropic glutamate receptors (mGluRs) are modulators of brain function and synaptic plasticity and have recently been shown to play a significant role in syndromic forms of ASD such as FXS through enhanced protein synthesis-dependent synaptic plasticity and perhaps peripheral mechanisms such as modulation of gastrointestinal functions. Therefore, mGluRs have been explored as therapeutic targets in FXS. However, clinical trials with group I mGluR (mGlu5) antagonists show that these drugs are not effective in all FXS subjects. For example, the Novartis mGlu5 antagonist AFQ056 improves behavioral symptoms in a fraction of FXS subjects (7 from 30) who have methylation of the FMR1 gene promoter and no detectable FMR1 messenger RNA, but not in individuals with partial promoter methylation. In addition to FXS, there are many other forms of ASD which have varied causes and neurobiology. Interestingly, different forms of ASD are associated with either hypo- or hyperfunction of the glutamatergic system despite presenting with similar symptoms, providing further evidence for involvement of different mechanisms and receptors. Early studies indicated that direct targeting of NMDA receptors with pharmacologic antagonists was associated with undesired side effects on cognition and neurotoxicity. However, it may be possible to target the functions of iGluRs in synaptic plasticity and ASD by modulating regulating proteins and/or signaling pathways, with less potent or more specific NMDAR antagonists, and with pharmacologic drugs targeting AMPA or kainate receptors. The purpose of this review is to describe key studies that implicate iGluRs and their interacting proteins and signaling pathways in ASD and FXS. Moreover, we will highlight mechanisms that may be important for development of new neuropharmacological treatments.
II. ASD AND FXS: DEFINITION, PREVALENCE, CAUSES. NEUROPHARMACOLOGICAL TREATMENTS
ASDs are neurodevelopmental disorders appearing first in early childhood usually before 3 years of age. Due to the great heterogeneity in the causes and presentation, the umbrella term ASD is accepted in the Diagnostic and Statistic Manual of Mental Disorders- Fifth Edition (DSM5) [1]. ASDs are characterized with impairments in two core symptoms domains: social/communication deficits and occurrence of repetitive behaviors, the symptoms being in a continuum from mild to severe in core and associated symptom domains. Within the social/communication domain there may be problems in social-emotional reciprocity, non-verbal communication behaviors, and developing and maintaining relationships. Within the area of restricted/repetitive behaviors, interests or activities there may be stereotyped, repetitive speech or motor movements, excessive adherence to routines or resistance to change, highly restricted, fixated interests, hypo- or hyper-reactivity to sensory input. There may be varying degree of intellectual disability, accompanying symptoms such as seizures, anxiety, mood swings, aggression, sleep problems, attention problems, hyperactivity, common with other psychiatric disorders, and gastrointestinal complaints [4]. ASD is relatively common occurring in 1 in 88 individuals, with a reported increase in incidence in recent years [2, 3]. ASD is about 4 times more common in boys than in girls. It is possible that the increased incidence is due to an ascertainment bias associated with greater awareness and more systematic screening of ASD, and perhaps also with changes in the diagnostic criteria [4].
FXS is a common monogenic cause of autism which has been invaluable in understanding the neurobiology of ASD and development of “targeted” drug treatments for the core symptoms [5-8]. FXS is caused by CGG repeats in the 5’ untranslated (UTR) region of the FMR1 gene which results in varying degree of loss of the Fragile X Mental Retardation Protein (FMRP), an RNA binding protein that regulates the synthesis and trafficking of many brain RNAs involved in synaptic plasticity and learning and memory [9]. Besides FXS, monogenic forms of ASD are tuberous sclerosis complex (TSC) [10], Rett syndrome [11, 12], neurofibromatosis (NF1)[13], Shank 2 and 3 deletion syndromes [14, 15]. Interestingly, recent study finds that genes causing syndromic forms of ASD such as TSC1/2 and SHANK3 can be implicated also in non-syndromic forms of ASD [16]. Genetic factors are important in the etiology of ASDs with monozygotic twins exhibiting a very high concordance rate [17]. Many neuronal genes are implicated in ASD and it is difficult to specify a shared genetic cause [18, 19]. Several excitatory and inhibitory neurotransmitter systems are implicated – glutamate [20-23], GABA [24], serotonin [25], norepinephrine [26, 27], dopamine [26, 28], acetylcholine [29, 30], endocannabinoids [31, 32] and neuropeptides [33-35]. A current understanding is that ASD and FXS are neurodevelopmental disorders of the synapse with abnormal synaptic connectivity, and that there is an imbalance in excitatory and inhibitory neurotransmission [36]. The main excitatory neurotransmitter system in the CNS, the glutamatergic system, plays a very important role in neuronal development and cognition. It should be noted that the pathophysiology of ASD is very complex and there are many interacting factors that may contribute to the disease manifestation and progression such as immunological, environmental factors, occurrence of neuronal loss, and glial factors. For example immunological factors may be caused by exposure of the mother to various viruses and environmental factors may be stress and toxins. The precise causal role of these factors, however, is heavily debated. Here, we restrict our review to role of iGluRs in ASD.
The treatments of ASD and FXS are complex and depend on the presenting symptoms. They are a combination of applied behavioral analysis, medications, occupational therapy, physical therapy and speech-language therapy (PubMed Health Information). Currently there are very few medications approved for treatment of ASD, none of which target the core symptoms. Two of the atypical antipsychotic drugs risperidone and aripiprazole are approved by the US Food and Drug Administration for treatment of aggression and irritability in children ages 5-16 with autism. These drugs are approved for treatment of schizophrenia which is also a neurodevelopmental disorder and has common features with ASD such as social deficits and neurobiological changes involving NMDA, GABA and dopamine receptors. Other medications used in clinical practice for treatment of patients with ASD are serotonin reuptake inhibitors such as fluoxetine approved for treatment of depression and obsessive-compulsive disorder (OCD) in children 7 years and older, divalproex sodium used to treat manic symptoms and epilepsy, and the psychostimulant drug methylphenidate used to treat attention-deficit hyperactivity disorder (ADHD). There are several reviews on the pharmacological treatments of ASD and FXS for further reading [37-41].
Due to research advances in understanding the neurobiology of FXS a new group of drugs which are antagonists of group I metabotropic glutamate receptors (gp I mGluR) are developed and have shown therapeutic efficacy in human FXS clinical trials [42, 43]. Importantly, studies in animal models show that drugs which reduce gp I mGluR-signaling in brain target the core symptoms of ASD [44-46]. Pharmacological drug enhancement of GABAergic neurotransmission has also shown potential to improve social function in FXS clinical trial [47]. In addition to mGlu and GABA receptors, there are pathological changes in FXS and ASD involving iGluRs. In some patients it may be more beneficial to target iGluRs due to the heterogeneous etiology, presentation, genetics and molecular neurobiology of ASD, individual drug sensitivity and other pharmacological factors [48]. There is evidence for this from human clinical trials and animal models. It is also possible that for treatment of ASD a combination of several drugs, acting at mGluR and iGluR targets may be beneficial. This notion is supported by evidence from clinical trials with mGlu5 antagonists which show partial therapeutic effectiveness in FXS [42, 43]. As mentioned, ASD are neurodevelopmental cognitive disorders and brain glutamate receptors are critical in regulation of neuronal development, synaptic plasticity and learning and memory. The changes in iGluRs in ASD and FXS may be result of altered gp I mGluR signaling as has been shown for AMPARs in FXS but they also may occur as a result of changes in other neuronal proteins and signaling pathways such as proteins regulating iGluR receptor expression and trafficking (Arc, MAP1B, GRIP1, STEP, Shanks, neuroligins). Therefore, it is important to understand the molecular and cellular changes in iGluR expression and signaling as this may provide additional drug targets.
III. STRUCTURE, EXPRESSION AND FUNCTIONS OF IGLURS
The actions of the major excitatory neurotransmitter glutamate in the mammalian central nervous system (CNS) are mediated by iGluRs and mGluRs.
1. Structure of iGluRs
IGluRs are ligand-gated ion channels classified into NMDA (N-methyl-D-aspartate), AMPA (a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) and kainate receptors based on structural, pharmacological and physiological properties [49-54]. IGluRs are tetramers encoded by 18 genes, several of which undergo alternative splicing or RNA editing, conferring different physiological properties to the receptor proteins. IGluRs have four distinct domains: an extracellular N-terminal domain, an extracellular ligand-binding domain, transmembrane domain and an intracellular carboxy-terminal domain [51]. NMDA receptors (NMDARs) are obligate heteromers formed as tetramers from co-assembly of GluN1, GluN2A-GluN2D, GluN3A or GluN3B subunits. Each NMDAR channel contains a combination of two GluN1 and two GluN2A-GluN2D subunits, or two GluN1 with one GluN2 and one GluN3 subunits. They form Ca2+ permeable ion channels, require both glutamate and glycine for activation, and are blocked by Mg2+ ions. The non-NMDA iGluRs are AMPA and kainate receptors. AMPARs may be homo- or hetero- tetramers formed from the GluA1-GluA4 subunits. They are Mg2+-insensitive. The GluA2 subunit confers impermeability to Ca2+ to the AMPAR channel as a result of RNA editing of the Q/R site of the GluA2 mRNA. AMPARs are further distinguished by having long-C-terminal tails – GluA1 and GluA4 or short C-terminal tails – GluA2 and GluA3. The C-termini of AMPARs have sites for phosphorylation by different enzymes such as protein kinase A (PKA), protein kinase C (PKC), and interact with PDZ-domain containing proteins such as protein 4.1N that interacts with GluA1, PKC alpha binding protein (PICK1) and glutamate receptor interacting proteins 1 and 2 (GRIP1/2) that interact with the GluA2 and 3 subunits and are important for AMPA receptor trafficking. AMPARs co-assemble with auxiliary subunits – trans-membrane AMPAR regulatory proteins (TARPs) such as stargazin, that are important for AMPAR expression, trafficking and functions. Kainate receptors are tetramers formed from combinations of the GluK1-GluK5 subunits. They have quite different synaptic roles in comparison with the other iGluRs despite structural and functional similarities, and they are mainly modulators of synaptic transmission and neuronal excitability [54]. The proteins PICK1 and GRIP that bind to AMPA receptors appear to play role in stabilizing kainate receptors at synapses. Another Kainate Receptor Interacting Protein, KIRP6, is important for modulation of receptor channel gating. IGluRs play key roles in basal synaptic transmission, different forms of synaptic plasticity, learning and memory, neuronal development and are involved in the neurobiology of many neurodevelopmental and neuropsychiatric disorders such as autism, FXS, schizophrenia, epilepsy, ADHD, Tourette syndrome, Alzheimer’s disease and Huntington’s disorder [54-56].
The functions of iGluRs are determined by their synaptic expression, trafficking, posttranslational modifications and signaling, and regulation by interacting proteins [57-61]. Different neuronal postsynaptic density (PSD) proteins, cell adhesion molecules, and cytoskeletal proteins interact with and regulate the expression and trafficking of iGluRs. GluA1 AMPARs interact with the PDZ-domain containing proteins synapse-associated protein-97 (SAP97) [62] and protein 4.1 [63], and GluA2/3 interact with PICK1 [64], GRIP1/2 proteins [65] and N-ethyl maleimide-sensitive factor (NSF) [66] which regulate the expression and trafficking of these receptors. The membrane-associated guanylate kinase (MAGUK) SAP97 has several splice isoforms and regulates the trafficking and localization of AMPA, NMDARs and potassium channels [62, 67, 68]. Postsynaptic density protein-95 (PSD-95) is important in the regulation of localization and trafficking of AMPA [69-72] and NMDARs [73, 74]. Regulation of the expression and trafficking of iGluRs is complex and some of the interacting PSD proteins have redundant functions. These mechanisms are important for synaptic plasticity and the neurobiology and treatment of ASD and FXS.
2. Expression of iGluRs
Regulation of the expression and trafficking of AMPA, NMDA and kainate receptors are described in excellent reviews [58, 60, 75, 57, 76, 77, 78, 79, 80, 54] and will not presented in detail here. There is constitutive trafficking (cycling) of AMPA and NMDARs which is rapid, regulated trafficking during synaptic plasticity – various forms of long-term potentiation (LTP) or long-term depression (LTD), and trafficking during homeostatic plasticity [59]. The subunit composition of AMPA and NMDARs may change during development, in response to synaptic activity and in response to pathological processes in the central nervous system (CNS). The molecular mechanisms of iGluR receptor expression and trafficking are complex and may be cell-type-specific. AMPARs are highly dynamic and undergo constant trafficking in and out of synapses by a combination of endo/exocytosis and lateral diffusion [81]. AMPARs can diffuse at such high rates within the PSD that their surface trafficking is presumed to participate not only in setting receptor numbers at individual synapses but also in fine- tuning synaptic transmission during short-term plasticity. How these events are altered in ASD and neurodevelopmental disorders is not well understood. NMDARs, once considered not as mobile as AMPARs, also undergo trafficking during development, in response to neuronal activity and sensory experience by interaction with PSD proteins such as PSD-95 and SAP-102 [56]. Besides receptor protein trafficking, protein synthesis and proteasomal degradation, alternative splicing, and mRNA trafficking are important for expression of iGluRs and their synaptic function. iGluRs play roles in different forms of synaptic plasticity: Hebbian forms of plasticity-LTD, LTP which are considered as cellular models of learning and memory, and homeostatic plasticity. In general, during LTP AMPARs are delivered to the synapse by exocytosis whereas during LTD AMPARs are trafficked away from the synapse by endocytosis. The mechanisms of trafficking are different during different forms of LTP and
LTD [82]. One form of synaptic plasticity enhanced in mouse models of FXS and ASD is hippocampal gp I mGluR-LTD triggered by activation of gp I mGluR, dependent on rapid dendritic protein synthesis, and expressed with AMPAR internalization [83]. It is notable that stress which is important in the pathogenesis of ASD and FXS plays role in AMPAR trafficking [84]. Some molecules implicated in AMPAR trafficking during homeostatic plasticity are implicated in ASD and FXS, among them being tumor necrosis factor alpha (TNFα) [85], retinoic acid [86], PICK1 [87], activity-regulated cytoskeletal gene and protein (Arc/Arg3.1) [88] and phosphatidylinositide-3 kinase (PI3K) signaling [89].
3. Roles of iGluRs in neuronal development
The maturation of glutamatergic synapses is associated with changes in the composition and functional properties of iGluRs and synaptic morphological changes. The expression of NMDA and AMPARs follows a specific sequence during development; immature synapses have only NMDARs, followed by appearance of AMPARs and “unsilencing” of synapses with appearance of LTP [90, 91]. Early in development neurons express GluN2B receptors which are later substituted GluN2A receptors [92]. The precise mechanisms of synapse unsilencing with neuronal activity and maturation are somewhat contradictory. Earlier studies establish that NMDAR blockade in hippocampal neurons decreases AMPARs and increases silent synapses [93]. AMPAR blockade increases the appearance of AMPARs [93]. However, another study finds that the primary role of NMDARs during neuronal development appears to be limiting the number of functional synaptic inputs and synapse maturation [94]. Thus, postnatal GluN1 deletion in hippocampus increases the numbers of synapses containing AMPARs [94]. The roles of NMDARs in the structural properties of synapses are also a matter of debate. It is reported that NMDARs may influence generation of new spines [95] but another study does not find that deletion of GluN1 has an effect on spine number [94]. These differences may be attributed to the method of NMDAR blockade –NMDAR antagonists [94], siRNA [96] or mosaic genetic deletion in single neurons [94]. Moreover, it is hypothesized that the role of NMDARs in regulation of AMPARs depends on the maturational state of the circuit. Thus, early in neuronal development NMDARs negatively regulate AMPARs, whereas in the adult neuronal circuits NMDARs have positive effect on AMPAR numbers and synaptic function [96]. It is not well established if the expression of iGluRs during development is altered in ASD and FXS but there is some experimental evidence for this in the Fmr1 knockout (KO) mouse which will be discussed here. The expression changes of iGluRs may cause altered synaptic development, plasticity and neuronal connectivity which are important for ASD and FXS.
4. Role of iGluRs in learning and memory and cognitive processes
IGluRs are involved in different forms of synaptic plasticity - LTP, LTD and homeostatic plasticity (synaptic scaling) [82, 97, 59]. LTP and LTD are cellular mechanisms of learning and memory and are important for information storage in the brain and cognitive processes. Homeostatic plasticity is believed to maintain neuronal activity within a physiological range in the presence of changes and participates in the refinement of neuronal circuits [98]. Activation of NMDARs may trigger LTP or LTD, both of which may be accompanied with trafficking of postsynaptic AMPA and NMDARs. Generally, during NMDAR-dependent LTP, AMPARs are inserted into synapses by exocytosis whereas during NMDAR-LTD, AMPARs are trafficked out of synapses by endocytosis [79, 99, 80, 100]. Activation of gp I mGluR may trigger LTD which may be expressed with endocytosis of AMPARs, similarly to NMDAR-LTD [101-103]. A key distinction between NMDAR-LTD and mGluR-LTD at CA1 hippocampal synapses is that mGluR-LTD and its expression in the form of AMPAR endocytosis depend on rapid dendritic protein synthesis [83]. Another important postsynaptic expression mechanism of LTP and LTD is phosphorylation of AMPARs during LTP [104, 105] and dephosphorylation during LTD [104, 106]. For example, phosphorylation of GluA1 is observed during CA1 hippocampal LTP [107, 108] and phosphorylation at S831 and S845 is sufficient to lower the threshold for LTP induction, increasing the probability of synaptic plasticity [105]. NMDAR-LTD at CA1 hippocampal synapses is associated with dephosphorylation of the GluA1 subunit at serine 845, a cAMP-dependent PKA substrate [106]. In addition, presynaptic expression mechanisms may contribute to LTP and LTD involving changes in neurotransmitter release and cleft glutamate concentration [109, 82,110]. LTP and LTD are usually accompanied by structural changes such as synaptic growth during LTP and synapse elimination as a result of LTD. The classical forms of LTP and LTD triggered by activation of NMDARs or mGluRs are expanded with additional mechanisms. For example, activation of muscarinic acetylcholine receptors [111, 112] may also trigger LTD in hippocampus that is expressed with AMPAR endocytosis. These types of synaptic plasticity may also have relevance for ASD since muscarinic cholinergic receptors are implicated in FXS [112]. Hebbian forms of plasticity (LTP and LTD) and homeostatic plasticity appear to involve different molecular mechanisms of AMPAR trafficking [57, 98, 87, 113, 98]. The molecular and cellular mechanisms of several forms of synaptic plasticity involving iGluRs are studied in brain in animal models of ASD and FXS with hope to understand the neurobiological mechanisms responsible for the cognitive deficits, and they will be summarized in this review.
IV. HUMAN STUDIES IMPLICATING IGLURS IN ASD AND FXS
Human studies provide contrasting evidence that autism may be both hypo- [114] and hyper-glutamatergic [115] disorder. Evidence for hypoglutamatergic state in autism is provided by the therapeutic effects of piracetam, a positive AMPAR modulator [116]. In contrast, significantly higher concentrations of glutamate are reported in the serum of ASD patients in comparison with normal controls [22]. Memantine which is an uncompetitive NMDAR antagonist [117] has shown efficacy in autism [118]. These contradictory findings may be due to the heterogeneity of ASD, the patient population (e.g., pediatric versus adult), and to differences in the ontogenetic period investigated, brain regions studied, experimental methods, and types of glutamate receptors involved. For example, increased activity of NMDARs may be associated with increased NMDA-LTD and increased AMPAR internalization which is expressed with decreased AMPAR activity. Therefore, both and NMDAR antagonist (memantine) and AMPAR potentiator (piracetam) may be have therapeutic effects. Interestingly, recent analysis of gene expression patterns in autistic postmortem brain at two developmental time points finds strikingly different patterns of gene expression between young and more mature brains of autistic individuals [119]. Among several groups of genes with different functions, genes related to glutamate receptors in brain which are found to be expressed aberrantly are protein synthesis genes such as mTOR in young autistic prefrontal cortex (PFC), and signaling genes involved in glutamate regulation of D1 signaling in mature autistic PFC.
1. Defects in Genes Encoding iGluRs and their Interacting/Regulating Proteins in ASD
Genetic studies strongly support involvement of iGluRs in ASD, the most frequent reports so far on changes affecting NMDARs. Sequencing of candidate genes in ASD probands identifies disruptive mutations in the GluN2B gene (GRIN2B) which may contribute, together with other genes such as CHD8, DYRK1A, PTEN and TBR1 to 1% of sporadic ASDs [120]. There are reports for association between haplotypes in the GRIN2B gene and ASDs in Korean families [121], and between the GRIN2A gene and autism [122]. Support for role of NMDARs in autism is provided by a study which identifies de novo mutations in GRIN2A and GRIN2B in patients with sporadic schizophrenia and autism, respectively [123]. Interestingly, these NMDAR subunits have differential expression during development with GluN2B expressed early in development, followed by GluN2A later in development and as synapses mature [61]. This sequence in expression of NR2 receptor subunits seems to be reflected in the genetic changes found in this study. Support for NMDAR involvement in autism is provided by a study which finds de novo mutations in GRIN2B in individuals with mental retardation: a frame shift, a missense and two splice-site mutations [124]. In a cohort of subjects with idiopathic epilepsy and/or mental retardation, a GRIN2A nonsense mutation is identified in a three-generation family. It is speculated that mutations disrupting different GluN2 subunits may have differential effects on the physiological properties of the receptors which affect the Mg2(+) block and Ca2(+) permeability of the receptor channel [124].
There are isolated reports for genetic alterations in AMPA and kainate receptors in human ASD. Genetic study finds that a child with autism has an interstitial deletion of chromosome 4q which results in hemizygocity for the GluA2 AMPAR, hemizygocity for the glycine receptors GLRA3, GLRB, and the neuropeptide receptors NPY1R and NPY5R [125]. Another study identifies chromosome 6q21 as a candidate region for autism, and the kainate subtype glutamate receptor 6 (GluR6 or GRIK2) gene within this region as a functional candidate [126]. Studies report that the GluK2 gene is in linkage disequilibrium with ASD [126, 127]. A complex mutation in the GluK2 gene is described which cosegregates with moderate-to-severe nonsyndromic autosomal recessive mental retardation in a large, consanguineous Iranian family [128], and results in loss of function of the GluK2 protein.
An association is found between autism and single nucleotide polymorphisms within the mitochondrial aspartate/glutamate carrier SLC25A12 gene located on the autism susceptibility locus chromosome region 2q24-q33 [129]. However, an association of the SLC25A12 gene with autism is not established in another set of 327 families with autistic offspring, pointing out the genetic heterogeneity in ASD. Genetic changes are reported in synaptic proteins regulating the expression and functions of iGluRs. In a very large genetic study of 1181 autism families with at least 2 affected individuals, linkage and copy number variation analyses implicate chromosome 11p12-p13 and neurexins, respectively, among other candidate loci. Neurexins are their presynaptic interacting partners neuroligins (NLGN) are implicated in glutamatergic synaptogenesis and expression of AMPA and NMDARs, highlighting glutamate-related genes as promising candidates that contribute to ASDs [130]. Mutations are identified in human GRIP1 [131], Shank3 [132, 133], Shank 2 [15, 134], and E3 ubiquitin ligase (Ube3A) [135, 136], which may have effects on the expression and functions of AMPA and NMDARs in ASD. Mutations are reported in the NLGN1-4 genes in human ASD, making them very strong candidate genes for these disorders [137-144]. Interestingly, mutations in the X-linked NLGN4 may be associated with a wide range of neuro-psychiatric conditions such as autism, Asperger’s syndrome, mental retardation, Tourette syndrome, attention deficit hyperactivity disorder, depression and anxiety [141].
2. Clinical Pharmacological Studies
Clinical trials with pharmacological drugs provide invaluable evidence for involvement of iGluRs in ASD and FXS. Oxytocin has shown some efficacy in humans with ASD, including enhancement of social cognition [145, 34, 146]. Electrophysiological studies indicate that the molecular and cellular mechanisms of oxytocin in the infralimbic medial prefrontal cortex (IL-mPFC), region important for social cognition, are through synaptic plasticity and glutamatergic neurotransmission [147]. In oxytocin-treated brain slices suppression of basal glutamatergic neuro-transmission in the IL-mPFC layer V pyramidal neurons is observed, which may be mediated by a reduction in glutamate release. Treatment of brain slices with oxytocin for 1 hour converts long-lasting depression into long-lasting potentiation of glutamatergic neurotransmission. It is concluded that the suppression of basal glutamatergic neurotransmission and facilitation of activity-dependent synaptic plasticity in the IL-mPFC might be critical for the effect of oxytocin on social cognition [147].
Evidence for a role of iGluRs in ASD is provided by the therapeutic effectiveness of topiramate, which antagonizes AMPA/kainate receptors, in children with pervasive developmental disorder [148], autism [149, 150] and adults with OCD [151]. Clinical studies using the NMDAR antagonist memantine in ASD show that it has efficacy in improving social withdrawal and inattention [152] and memory and hyperactivity, lethargy, and irritability [153, 154], providing support for role of NMDARs in ASD. In one study, significant improvements in language function, social behavior, and self-stimulatory behaviors are observed in ASD subjects treated with memantine [154]. It should be noted that memantine, besides being a noncompetitive NMDAR antagonist, is an antagonist at 5-HT3 [155, 156], nicotinic acetylcholine receptors [157], and agonist of D2 receptors [158]. The clinical studies with memantine so far are valuable but need to be expanded in a larger ASD patient population. Further support for involvement of NMDARs in ASD is obtained from the modest effect of amantadine, another NMDAR antagonist [159], in a placebo-controlled clinical trial of 39 children with ASD [160]. When assessed on the basis of parent-rated Aberrant Behavior Checklist – Community Version (ABC-CV), amantadine does not show improvement of irritability and hyperactivity over the placebo response. However, in the amantadine-treated group there are statistically significant improvements in absolute changes in clinician-rated ABC-CVs for hyperactivity and inappropriate speech. Clinical Global Impression (CGI) scale ratings are higher in the amantadine group, suggesting that further studies with this or other drugs acting on the glutamatergic system are warranted.
Another drug supporting role of NMDARs in autism is acamprosate, which is believed to attenuate hyper-glutamatergic states by antagonizing of NMDA and mGlu5 receptors, and by modulating intracellular calcium release [161-163]. In a small study acamprosate is effective in improving social impairment in youth with autism [164]. In another study, acamprosate use is associated with significant improvement in social behavior and reduction in inattention/hyperactivity in 9/12 youth FXS subjects [165]. D-cycloserine, which is an NMDAR glycine site partial agonist and is effective in treatment of schizophrenia [166] has shown effectiveness in improving the social withdrawal, a core symptom in children with ASD [167].
3. Postmortem Brain Studies
Postmortem brain studies find that humans with ASD have specific abnormalities in AMPA receptors (AMPARs) and glutamate transporters in the cerebellum that may be directly involved in the disease pathogenesis [168]. Gene expression patterns from postmortem cerebella from autism subjects and healthy controls are tested using cDNA microarrays followed by PCR validation, Western blotting and receptor autoradiography. The mRNA levels of several genes are significantly increased in the autism subjects, including excitatory amino acid transporter 1 and glutamate receptor AMPA1 (GluA1). Abnormalities in the protein or mRNA levels of several additional molecules in the glutamate system are identified, including glutamate receptor binding proteins. AMPAR density is decreased in ASD cerebellum.
4. Human Neuroimaging Studies
Human neuroimaging studies provide evidence for abnormal glutamate levels in ASD. Concentrations glutamate+glutamine (Glx) are determined by 3T proton magnetic resonance spectroscopy imaging (1H MRS) in high-functioning medication-free adults with ASD and age- and Intelligence Quotient (IQ)-matched healthy controls (HC) in the anterior cingulate cortex (ACC), thalamus, temporoparietal junction (TPJ), and areas near or along the intraparietal sulcus (IPS), which are associated with networks subserving alerting, orienting, and executive control of attention in ASD. Compared to HC group, the ASD group shows significantly lower Glx concentration in right ACC [169]. In another study use of in vivo single-voxel (1H MRS) and proton magnetic resonance spectroscopic imaging (1H MRSI) shows that there is hyperglutamatergia in the pregenual anterior cingulate cortex (pACC) in middle to late childhood and adolescence in ASD [115]. These findings are interpreted to be in correspondence with abnormal increase of excitation relative to inhibition in key neural systems in autism [170].
V. NMDAR ALTERATIONS IN ANIMAL MODELS OF ASD AND FXS, AND IN VITRO IN CELL CULTURE
In FXS there are changes involving mGluRs, AMPARs and NMDARs which may be brain-region-specific [171-173] and may be expressed differently in ontogeny [174-175]. In Fmr1 KO mice there is enhanced mGlu5 receptor signaling and enhanced CA1 hippocampal mGluR-LTD that can be corrected using mGlu5 pharmacological inhibitors [176-180] which restore the normal behavioral, biochemical and neuroanatomical phenotype. However, in FXS and ASD there are additional alterations in iGluRs and proteins regulating glutamate receptors. This may be a reason why in some FXS and ASD studies mGlu5 antagonists have limited efficacy in reversing abnormal changes [181, 182]. Here we will review the significant findings in the scientific literature on changes in NMDAR expression, signaling and functions in animal models of ASD and FXS and cell culture. Some studies detect changes in the function of iGluRs and autistic-like behaviors and do not measure directly the expression levels of receptors.
The neurobiological effects of the NMDAR antagonist memantine have been explored in cultured cerebellar granule cells (cGC) from Fmr1 KO mouse as a model of monogenic autism [183]. In the Fmr1 KO there is delayed maturation of dendritic spines and fewer excitatory synapses. Memantine treatment of cGC has a stimulatory effect on dendritic spine maturation and excitatory synapse formation, and promotes the adhesion of cGC [183]. These processes are important for proper neuronal development and connectivity and have been linked to NMDARs. NMDARs inhibit the expression of AMPARs during development [94]. Early expression of GluN2A in organotypic hippocampal slices reduces the number of synapses formed, decreases the spine density and frequency of AMPAR mEPSC [184]. This is attributed to the low-affinity interaction of GluN2A with calcium-calmodulin kinase II (CaM KII) and the effect of GluN2A to reduce LTP. In contrast, overexpression of GluN2B does not affect synapse number and growth; however, it increases spine motility, adding and retracting spines at a higher rate. The C terminus of GluN2B and its ability to bind CaMKII is sufficient to allow proper synapse formation and maturation. The switch from GluN2B to GluN2A content in synaptic NMDARs observed in hippocampus during development may contribute to reduced plasticity by controlling the binding of active CaMKII [185]. These studies provide a likely explanation for the effects of memantine on excitatory synapses in cCG, and suggest that there is aberrant NMDAR expression and functions in the Fmr1 KO mouse resulting in fewer and less mature excitatory synapses and less AMPARs.
Several studies describe alterations in NMDA-receptor dependent synaptic plasticity, NMDAR levels, phosphorylation and signaling in mouse models of FXS or ASD which are brain region specific (Table 1). In the prefrontal cortex (PFC) of the Fmr1 KO mouse, a region important for cognitive processes, there is decreased LTP due to deficient D1 receptor facilitation that is accompanied with decreased insertion of GluA1 and deficient GluN2B phosphorylation [186, 187]. Expression of mGlu5, GluN2A and GluN2B subunits are not different in the PFC between WT and Fmr1 KO mice. LTP in PFC, GluN2B phosphorylation and insertion of GluA1 can be restored in Fmr1 KO by administration of a D1 agonist SKF81297 in combination with a gp I mGluR-antagonist DL-AP3, but not by treatment with either drug alone. Behavioral tests indicate that the simultaneous treatment with D1 agonist and gp I mGluR antagonist inhibits hyperactivity and improves the learning in the Fmr1 KO mice. Thus, a combination of D1 agonist and gp I mGluR antagonist influences the properties of iGluRs and is a potential drug therapy for the learning and memory deficits in FXS.
Table 1.
Ionotropic Glutamate Receptor Alterations in Animal Models of ASD and FXS
| Animal Model/ Targeted Gene-protein |
Brain Region/s Studied | iGluR Alterations |
Functional Alterations | Behavioral Characteristics | Drug Treatment and Effects | References |
|---|---|---|---|---|---|---|
| Fmr1 KO/FMRP | PFC | ↓synaptic insertion of GluA1, ↓GluN2B phosophorylation |
↓D1R facilitation of LTP in PFC, ↑gpI mGluR activity |
hyperactivity, learning deficits |
combined SKF81297 and DL-AP3 rescue deficient GluN2B phosphorylation, reduce hyperactivity and improve learning | [186,187] |
| Fmr1 KO/FMRP | PFC | ↓GluN1,↓GluN2A, ↓GluN2B, ↓PSD-95, ↓SAPAP3,↓Arc |
cognitive impairment in acquisition of visual- spatial discrimination task | [188] | ||
| Fmr1 KO/FMRP | hippocampus | ↔ in GluN1, ↔GluN2A, ↔GluN2B by Western blot; 3’UTR translation assay suggests ↑GluN2A at 1-2 weeks | [192] | |||
| Fmr1 KO2/FMRP | CA1 hippocampus | ↓synaptic GluA1 and ↓GluA2 at 14 days, ↑synaptic GluN1 at 14 days and not at 6-7 weeks |
↓AMPA/ NMDA ratio due to ↓AMPA and ↑NMDA currents at 14 days but not at 6-7 weeks, ↑NMDAR-LTP ↔NMDAR-LTD |
MPEP does not have a blocking effect on enhanced NMDAR-LTP at 14 days | [174] | |
| Fmr1 KO/FMRP | CA1 hippocampus | ↓synaptic delivery of GluA1 | ↓LTP, ↓Ras-PI3K-PKB signaling |
Ras overexpression restores GluA1 delivery and LTP; PI3K inhibitor blocks LTP |
[194] | |
| Fmr1 KO/FMRP | CA1 hippocampus | DHPG induces ↓GluA1 and ↓GluA2/3 | ↑mGluR-LTD | [234] | ||
| Fmr1 KO/FMRP | CA1 hippocampus | ↓GluA2 | ↑mGluR-LTD | ↑audiogenic seizures, abnormal social and non-social anxiety-related behaviors | Genetic decrease of STEP diminishes audiogenic seizures and restores social and anxiety-related behaviors | [237, 242] |
| Fmr1 KO/FMRP | CA1 hippocampus | Absent retinoic-acid (RA)-dependent translation of GluA1 in dendrites | RA-mediated synaptic scaling is abolished | Postsynaptic expression of FMRP with lentivirus in Fmr1 KO neurons restores synaptic scaling | [86] | |
| Fmr1 KO/FMRP | Anterior piriform cortex | ↓ synaptic NMDA receptors | ↓LTP | [173] | ||
| Fmr1 KO/FMRP | Amygdala | ↓GluA1 | ↓mGluR-LTP at thalamic afferents to lateral amygdala (LA) | MPEP fails to rescue LTP deficit in LA but restores deficits in presynaptic release | [182] | |
| MeCP2 KO (Rett syndrome) | Nucleus tractus solitarius (nTS), mPFC, cingulate cortex | ↑hyperexcitability in nTS, enhanced spontaneous, evoked excitatory mEPSCs in nTS; hypoactivity in mPFC and cingulate cortex |
altered Fos expression – ↑ in nTS, ↓ in mPFC and cingulate cortex; abnormal PPI of acoustic startle |
ketamine (NMDAR antagonist) rescues abnormal PPI of acoustic startle and normalizes Fos expression | [198] | |
| MeCP2 KO (Rett syndrome) | Entire brain and hippocampus | ↓synaptic GluA2/3, ↓GluN2A receptors and other synaptic proteins (↓Vglut1, ↓Synapsin1, ↓CamKIIα and CamKIIβ), ↓GABABR2 | ↓activity, ↓forelimb strength with shorter fall latency, ↓motor coordination, symptoms worse in female mice |
[199] | ||
| Tsc1 KO (using viral delivery of Cre recombinase in select neurons) | hippocampus CA1 | ↑evoked AMPA and ↑evoked NMDA synaptic currents, abolished mGluR-LTD, ↔ NMDAR-LTD |
[200] | |||
| Rats bred for low rates of play-induced pro-social USV | ↓social contact time, ↓play-induced pro-social USV, ↑monotonous USV |
NMDAR glycine partial agonist GLYX-13 rescues deficits in play-induced USV and ↓ monotonous USV | [22] | |||
| Balb/c mice (inbred) Swiss-Webster mice (outbred) |
impaired measures of sociability in 4 and 8-week old mice | D-cycloserine (NMDAR glycine site agonist) improves sociability in both mouse strains; MPEP impairs measures of sociability in both strains |
[203,204] | |||
| Constitutive GluN1 KO mice | cortex | 85‰ ↓ GluN1 | neuronal hypexcitability and ↑E/I balance; ↓gamma signal-to-noise ratio (SNR) |
↓ social preference, impaired spatial memory | GABAB agonist baclofen improves E/I balance, gamma SNR, reverses behavioral deficits | [207] |
| Parvalbumin (PV) interneuron-selective GluN1KO mice | cortex | selective ↓GluN1 in PV interneurons | ↓N1 latency ↓sociability ↓premating USV power |
[208] | ||
| Islet Brain-2 protein lacking mice (IB2-/-) | cerebellum | no change in GluA2, GluN1, GluN2A and GluN2B, altered Purkinje cell morphology with thinner dendrites | ↓AMPAR and ↑NMDAR-mediated transmission at cerebellar mossy fiber-granule cell synapses,↓ delayed AMPA/kainate neuro- transmission at cerebellar fiber to Purkinje cell synapses |
↓ social interactions, impaired exploration of novel environment, motor performance and learning deficit on rotarod | [225] | |
| Ube3A KO mice (Angelman syndrome) | hippocampus | ↓synaptic GluA1 ↑ GluA1 endocytosis ↑Arc |
Altered AMPAR function - ↓mEPSC frequency; ↓AMPA/NMDA ratio |
High frequency of seizures, ataxia, abnormal EEGs, poor performance on learning and memory tests | Decreasing Arc using shRNA restores normal synaptic GluA1 levels | [245] |
| GluK2 KO mice | Hippocampus (mossy fiber-CA3 synapses) |
↓GluA1 | Delayed functional maturation of mf-CA3 synapses; ↓amplitude of AMPA EPSC |
[251] |
The table summarizes studies of animal models of ASD and FXS that are discussed in the text involving changes in iGluRs, and presents the associated molecular/neuroanatomical, functional and behavioral alterations. The numbers in the “References” column refer to the citation numbers in the text. ↔ - no change; ↓ - decrease; ↑- increase. For ease of comparison of the results between the studies, each study is presented separately in a row, even if the same animal model is used (e.g., the Fmr1 KO mouse). It is evident that in one animal model changes may affect more than one iGluR subtype such as AMPA and NMDA receptors. If a treatment approach (pharmacological drug or genetic approach) is used in the study to correct the iGluR levels, functional and/or behavioral changes, it is indicated in the table. In the Fmr1 KO mouse, administration of mGlu5 receptor antagonists such as MPEP, MTEP, fenobam and CTEP in animal models has shown therapeutic promise in reversing biochemical, neuroanatomical, synaptic plasticity and behavioral aberrations associated with FXS, but these studies are not indicated here because the focus of the review is on iGluRs.
Individuals with FXS have deficits in attentional function, inhibitory control, and cognitive flexibility which are thought to be associated with the PFC. In the Fmr1 KO mice a robust cognitive impairment is identified which may correspond to the deficits in cognitive flexibility in individuals with FXS. Importantly, the levels of proteins involved in synaptic function, including the NMDAR subunits GluN1, GluN2A, and GluN2B, the scaffolding proteins PSD-95 and SAPAP3, and the plasticity-related gene Arc, are decreased in the prefrontal cortex of Fmr1 KO mice and are partly correlated with behavioral performance [188].
The clinical hallmarks of autism include excessive adherence to patterns and impaired detection of socially important patterns, and the dentate gyrus (DG) region of the hippocampus has a putative role in pattern separation (for time, space, and features) and pattern completion. There is diminished medial perforant path-granule cell LTP in the DG of Fmr1 KO mice [189]. In addition, a smaller peak amplitude of NMDAR-mediated excitatory postsynaptic currents (EPSCs) is observed, whereas AMPAR-mediated EPSCs are comparable, yielding a lower NMDA/AMPA ratio.
The Fmr1 KO2 mice are a newer generation of Fmr1 KO mice created by deletion of both the promoter and exon 1 of the Fmr1 gene as a result of which they are deficient in both the Fmr1 mRNA and FMRP [190], unlike the previous Fmr1 KO which has remaining levels of Fmr1 mRNA and FMRP [191]. In acute hippocampal slices from Fmr1 KO2 mice early in development (14 days) in CA1 neurons there are reduced AMPAR-mediated currents and increased NMDAR-mediated responses, which reduce the AMPA/NMDA ratio. The reduction in AMPA/NMDA ratio is not observed at 6-7 weeks. The changes in iGluR currents are accompanied by corresponding decreases in the synaptic GluA1 and GluA2 and increase in the synaptic GluN1 receptors in hippocampal synaptoneurosomes at 14 days, but not at 6-7 weeks [174]. In correspondence, NMDAR-LTP induced by low-frequency stimulation (LFS) is significantly enhanced in the Fmr1 KO2 early in development at 14 days and not at 6-7 weeks. NMDAR-LTD or short-term depression are normal in the Fmr1 KO2. Interestingly, MPEP (2-methyl-6-phenylethynyl-pyridine) does not have an effect on the enhanced NMDAR-LTP. The authors propose that these hippocampal changes in iGluRs and synaptic dysfunction early in development cause learning and memory deficits that contribute to the Fragile X phenotype, together with parallel developmental irregularities in the cortex. They speculate whether NMDAR antagonists will be a suitable therapeutic option for FXS early in development which will depend on the nature of the NMDAR increase. For example, if it is compensatory, NMDAR antagonists may have a worsening effect.
Another study does not find significant differences in the expression of GluN1, GluN2A, GluN2B, and GluA1 in hippocampal slices from 1 or 2 week old Fmr1 KO and WT mice using Western blot [192]. However, upon further analysis in vitro it is found that knockdown of FMRP (which resembles the Fmr1 KO condition) strongly stimulates the translation of GluN2A 3’UTR reporter but not the GluN1 or GluN2B 3’ UTR reporters [192]. Therefore, using this in vitro assay the authors determine somewhat indirectly that there is increased hippocampal expression of GluN2A in Fmr1KO neurons, which may be obscured in vivo by different factors such as compensatory mechanisms during development or assay sensitivity. In another study, immuno-fluorescent immunostaining of primary hippocampal neurons from Fmr1 KO mice finds significant increase in dendritic GluN2A early in development, 7 days in vitro (DIV) in comparison with neurons from WT mice, which corresponds approximately to mouse age 14 days [193]. These findings are similar to the ones in the Fmr1 KO2 mouse in that there is increased NMDAR expression early in development although the GluN2A subunit is characterized instead of the obligatory GluN1 subunit [174]. What effects these expression changes will have on the NMDAR channel properties and synaptic plasticity remains to be determined. However, they are different than the results obtained in the Fmr1 KO mouse using Western blot [192]. The difference may be observed because indirect immunofluorescent assays are more powerful in detecting small local differences in receptor expression in dendrites and at synapses than Western blotting using total protein extracts; therefore sometimes discrepancies in studies may occur due to the experimental technique used. This is why it may be good to ascertain changes in receptors using several techniques. Nevertheless, the elevated levels of GluN2A early in development in the Fmr1 KO may have effects on synaptic development and plasticity, and may be attributed in part to regulation by microRNAs such as miR-125b [192], which bind to FMRP, and have inhibiting effects on synapse development and synaptic plasticity.
Another study does not find differences in the AMPA/NMDAR ratios in CA1 hippocampal slices from 2-week old Fmr1 KO mice [194], similarly to earlier studies that do not report changes in LTP in hippocampus from Fmr1 KO mice 3-4 weeks old [172, 83]. The investigators find reduced LTP due to selective decrease in the synaptic delivery of GluA1 due to deficient Ras-PI3K-Protein kinase B (PKB) signal transduction [195]. This discrepancy between the different studies in AMPA and NMDA responses may be due in part to the remaining levels of Fmr1 mRNA and FMRP in the Fmr1 KO. In human FXS there are varying degrees of changes in the levels of FMR1 mRNA and FMRP which may result in varying changes in iGluR expression and functions. Thus, FXS subjects with premutation in the 5’ region of the FMR1 gene (~55-200 CGG repeats) have decreased levels of FMRP and milder cognitive impairment than individuals with full mutation alleles (>200 CGG repeats) in which the FMRP is absent [185, 196]. The individuals with premutation are also less likely to develop ASD than those with full mutation [196]. It should be noted that in humans the FMR1 premutation may result in FXS, ASD and cognitive impairment through two mechanisms: decrease in the FMRP [197] and increase in FMR1 RNA which may result in RNA toxicity, effects on the miRNA pathway, cell death and mitochondrial pathways [196]. This may have implications for using the Fmr1 KO mouse, which still has residual levels of FMRP, as a model for FXS. Levels of FMRP and Fmr1 mRNA should be measured when determining changes in glutamate receptors and synaptic plasticity. Additional reason for the observed discrepancy in NMDAR expression in different studies may be the difference in maturation of neurons in cell culture [193] and in vivo [192] which may result in differences in receptor expression.
The Fmr1 KO mouse exhibits age-dependent deficits in LTP at association (ASSN) synapses in the anterior piriform cortex (APC). To investigate the mechanisms for this, whole-cell voltage-clamp recordings of ASSN stimulation-evoked synaptic currents are made in APC of slices from adult Fmr1 KO and WT mice, using the competitive NMDAR antagonist, 3-(2-Carboxypiperazin-4-yl)propyl-1-phosphonic acid (CPP), to distinguish currents mediated by NMDA and AMPARs. NMDA/AMPA ratios are lower in Fmr1 KO than in WT mice, at ages ranging from 3-18 months. Since the amplitude and frequency of AMPAR mEPSCs are not found to be different in Fmr1 KO and WT mice at these ages, the results suggest that NMDAR-mediated currents are selectively reduced in the Fmr1 KO [173]. Analyses of voltage-dependence and decay kinetics of NMDAR-mediated currents do not reveal differences between Fmr1 KO and WT mice, suggesting that reduced NMDA currents in Fmr1 KO mice are due to fewer synaptic receptors rather than differences in receptor subunit composition. Evoked currents and mEPSCs are also examined in senescent Fmr1 KO and WT mice at 24-28 months of age. NMDA/AMPA ratios are similar in senescent Fmr1 KO and WT mice, due to a decrease in the ratio in the WT mice, without significant change in AMPAR-mediated mEPSCs. These findings of age-dependent changes in NMDARs suggest that pharmacological treatments in FXS may have age-specific effects.
Mutations in the X-linked gene Methyl CpG binding protein 2 (MeCP2) are linked to Rett syndrome, a severe form of autism, and it is believed that MeCP2 is involved in brain maturation. Differences in NMDAR expression and functions are noted in studies using Rett syndrome mouse models. However, the findings are inconsistent, maybe due to the different animal models and brain regions studied. Mapping expression of the immediate-early gene Fos as a marker of neuronal activation in the brains of WT and MeCP2 Null mice before and after the appearance of overt symptoms (3 and 6 weeks of age, respectively) in one study reveals significantly less Fos labeling at 6 weeks in Null in comparison with WT mice in limbic cortices and subcortical structures [199]. In contrast, Null mice have significantly more Fos labeling than WT mice in hindbrain, most evident in cardiorespiratory regions of the nucleus tractus solitarius (nTS). Using nTS as a model, whole-cell recordings demonstrate that increased Fos expression in Nulls at 6 weeks is associated with synaptic hyperexcitability, including increased frequency of spontaneous and miniature EPSCs and increased amplitude of evoked EPSCs. No such effect of genotype on Fos or synaptic function is seen at 3 weeks. In the mutant forebrain, reduced Fos expression, as well as abnormal sensorimotor function is reversed by the NMDAR antagonist ketamine, which upregulates Fos expression in limbic forebrain of mice [198]. In another study, removing MeCP2 from mouse brains at the early developmental stage that coincides with Rett development, late juvenile or adult stages results in active shrinking of the brain and higher than normal neuronal cell density. Deletion of MeCP2 in juvenile or adult mice results in Rett-like behavioral deficits. The mature dendritic arbors of pyramidal neurons are severely retracted and dendritic spine density is dramatically reduced. Hippocampal astrocytes have significantly less complex ramified processes. There is a striking reduction in the levels of several synaptic proteins, including CaMKII α/β, AMPA, and NMDARs, and the synaptic vesicle proteins vesicular glutamate transporter (Vglut) and synapsin, which represent critical modifiers of synaptic function and dendritic arbor structure. Since the mRNA levels of these proteins remain unchanged, it is likely that MeCP2 regulates these synaptic proteins post-transcriptionally, directly or indirectly. It is concluded that genetic changes in MeCP2 lead to changes in glutamatergic synaptic receptors and proteins which influence neuronal development and networks [199].
Mutations in the tuberous sclerosis complex 1 or 2 genes (TSC1 or TSC2) cause the disease tuberous sclerosis complex (TSC) in humans that is characterized with multiple benign tumors, neurological deficits, autism, cognitive dysfunction, and epilepsy. Deletion of the TSC1 or TSC2 genes disrupts the TSC1/2 complex, results in aberrant activation of the mammalian target of rapamycin complex 1 (mTORC1), and upregulation of protein translation. Deletion of Tsc1 in mouse CA1 hippocampal neurons causes enhancement of glutamatergic synaptic function evidenced by larger evoked AMPAR and NMDAR currents and increased frequency of mEPSCs [200]. Protein translation-dependent mGluR-LTD is absent in Tsc1 KO neurons, whereas NMDAR-LTD is not affected [200]. These changes occur in the absence of changes in dendritic spine number, morphology, or presynaptic release probability. They suggest that loss of Tsc1 in hippocampal neurons impairs the ability to activate the signaling pathways necessary for mGluR-LTD and causes enhanced excitatory drive which may have consequences for circuit information processing and network excitability. This in contrast to FXS in which there is upregulation of basal protein translation and enhancement of hippocampal mGluR-LTD [83, 201]. These studies imply that synapses are the neuroanatomical substrates in FXS and ASD and that the nature of the changes involving excitatory neurotransmission is disease-specific.
Another study uses rats selectively bred for low rates of play-induced pro-social ultrasonic vocalizations (USVs) to model certain core symptoms of autism and to understand the role of NMDARs in autism [22]. Low-line animals exhibit autism-like behaviors: they engage in less social contact time with conspecifics, show lower rates of play induced pro-social ultrasonic vocalizations (USVs), and show an increased proportion of non-frequency modulated (i.e. monotonous) USVs compared to non-selectively bred random-line animals. Gene expression patterns in the low-line animals have significant enrichment in autism-associated genes and particularly the NMDAR family. Treatment of low-line animals with the NMDAR glycine site partial agonist GLYX-13 rescues the deficits in play-induced pro-social 50-kHz and reduced monotonous USVs. Thus, the NMDAR is shown to play a functional role in autism, and enhancement of NMDAR activity with GLYX-13 shows promise for the treatment of autism in this particular model [22].
The role of NMDARs in ASD is shown in Balb/c mice which are a model of impaired sociability and social motivation relevant to ASDs [202, 203]. Impaired sociability of 8- or 4-week old Balb/c mice is attenuated by agonists of the glycine(B) site on the NMDAR, such as d-cycloserine. Because stereotypies can compete with the salience of social stimuli, the investigators compare Balb/c and Swiss Webster mice on several spontaneous stereotypic behaviors emerging during social interaction with a social stimulus mouse. Similarly to 8-week old mice, spontaneous stereotypic behaviors during social interaction are more intense in the 4-week old Swiss Webster mice; furthermore, d-cycloserine reduces their intensity. Thus, d-cycloserine improves both sociability and stereotypic behaviors and these effects may lack strain-selectivity. The data suggest that targeting the NMDAR can have promising therapeutic effects on two prominent domains of psychopathology in ASDs: impaired sociability and spontaneous stereotypic behaviors.
Based on the effectiveness of mGlu5 antagonists in FXS, examination of the effects of the mGlu5 antagonist 2-methyl-6-(phenylethynyl) pyridine MPEP on sociability and stereotypic behaviors in Balb/c and Swiss Webster mice shows a mixed situation; MPEP has complex effects on sociability, impairing some measures of sociability in both strains, while it reduces the intensity of some spontaneous measures of stereotypic behaviors emerging during free social interaction in Swiss Webster mice [204]. Conceivably, mGlu5 antagonism exacerbates diminished endogenous tone of NMDAR-mediated neurotransmission in neural circuits relevant to some measures of sociability in Balb/c mice; the mGlu5 receptor contributes to regulation of the phosphorylation state of the NMDAR. It is concluded that medication strategies aimed to attenuate the severity of stereotypies in ASDs via antagonism of mGlu5 receptors must be pursued cautiously because of their potential to worsen some measures of sociability, providing rationale to develop drugs targeting NMDARs, in addition to mGluRs, in ASD.
Mouse models with NMDAR hypofunction as a result of the administration of the NMDAR antagonist MK801 [205] or constitutive [206,207] or selective deletion in parvalbumin interneurons [208] of the obligatory GluN1 subunit reproduce behavioral, cellular and electrophysiological abnormalities observed clinically in ASD. Adult constitutive GluN1 KO mice show behavioral deficits relevant to all core ASD symptoms, including decreased social interactions, altered ultrasonic vocalizations and increased repetitive behaviors. NMDAR disruption recapitulates clinical endophenotypes such as reduced prepulse inhibition (PPI), auditory-evoked response N1 latency delay and reduced gamma synchrony. Auditory electrophysiological abnormalities closely resemble those seen in clinical studies of autism. The γ-aminobutyric acid type B (GABAB)-receptor agonist baclofen improves excitatory/inhibitory balance, the auditory-evoked gamma-signal to noise ratio and broadly reverses the behavioral deficits in the constitutive GluN1 KO mouse [207]. Therefore, a molecular defect in NMDARs results in increase in intrinsic pyramidal cell excitability and selective disruption of parvalbumin-expressing GABAergic interneurons which may provide useful translational biomarker for ASD (Fig. 1). These ASD mouse models are characterized with NMDAR hypofunction, unlike the Tsc1 KO mouse which has increased hippocampal NMDAR excitatory neurotransmission. However, there may be brain-region, species-specific and developmental differences in NMDARs in different subtypes of ASD.
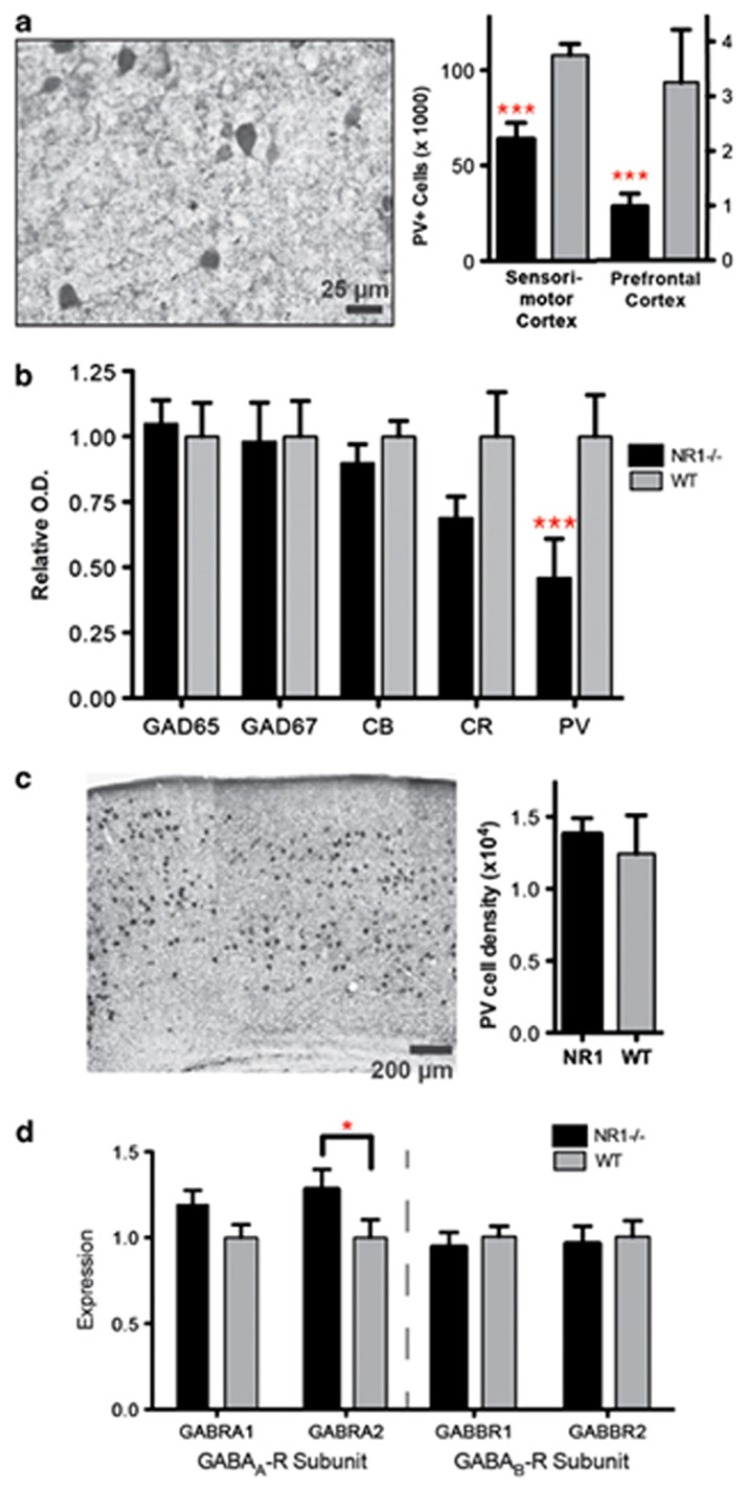
Fig. (1).
Constitutive NMDA-receptor hypofunction causes selective disruption of parvalbumin-expressing (PV+), fast-spiking GABAergic interneurons (FSI). (a) Immunoreactivity for FSI (parvalbumin) is significantly reduced in NR1neo-/- mice. (b) Protein expression for markers of GABA interneuron populations assessed by Western blot. Calbindin and calretinin are markers for non-FSI, whereas GAD65 and GAD67 are expressed in all GABAergic interneurons. PV expression is significantly reduced in NR1neo-/- mice, whereas other proteins are unaffected. (c) No group differences are observed following in situ hybridization for PV mRNA, indicating that FSI are present, but disrupted, in NR1neo-/- mice. (d) Expression of postsynaptic GABAA- and GABAB-receptor subunits is measured by quantitative PCR (qPCR). The GABAA-receptor alpha-2 subunit is significantly upregulated in NR1neo-/- mice as seen in schizophrenia,55 whereas other subunits are unaffected. Graphs show mean +/- s.e.m., *P<0.05, **P<0.01, ***P<0.001.
Reprinted by permission from Macmillan Publishers Ltd: Translational Psychiatry, GABAB-mediated rescue of altered excitatory–inhibitory balance, gamma synchrony and behavioral deficits following constitutive NMDAR-hypofunction, M J Gandal, J Sisti, K Klook, P I Ortinski, V Leitman, Y Liang, T Thieu, R Anderson, R C Pierce, G Jonak, R E Gur, G Carlson and S J Siegel, copyright – 2012. http://www.nature. com/tp/journal/v2/n7/full/tp201269a.html
The connection between GABAB receptors and iGluRs seen in the GluN1 KO mouse is established also in the Fmr1 KO mouse [209]. Pharmacologic activation of the GABAB receptor in neurons from Fmr1 KO mice with the selective GABABR agonist STX209 (arbaclofen, R-baclofen) reduces elevated basal protein synthesis and elevated AMPAR internalization. Acute administration of STX209 in vivo, at doses that modify behavior, decreases mRNA translation in the cortex of Fmr1 KO mice. Chronic administration of STX209 in juvenile mice corrects the increased spine density in Fmr1 KO mice without affecting spine density in WT mice. Thus, activation of the GABAB receptor corrects synaptic abnormalities central to FXS pathophysiology, suggesting that STX209 is a potentially effective therapy for the core symptoms in FXS and ASD. In a clinical trial with human FXS subjects arbaclofen has shown promising effects in improving social function and behavior but not on the primary endpoint measure of irritability, as determined using the Aberrant Behavior Checklist-Irritability subscale [47, 210]. Moreover the results from clinical trials with STX209 in FXS and ASD subjects indicate that it is overall well-tolerated and may be effective in amelioration of some symptoms of FXS and ASD in a fraction of the patients. Future challenges to the field are to choose suitable primary outcome measures for ASD and FXS clinical trials and predict which patients will respond favorably to the drug.
VI. AMPAR ALTERATIONS IN ANIMAL MODELS OF ASD AND FXS AND IN VITRO IN CELL CULTURE
There are many studies in animal models and primary neurons suggesting that AMPARs are important in the neurobiology of ASD and FXS (Table 1). AMPARs mediate the expression of several forms synaptic plasticity related to cognitive processes such as LTP, LTD and homeostatic plasticity. AMPAR alterations in animal models of ASD and FXS are usually manifested as changes in the expression and trafficking of receptors. Other parallel mechanisms are AMPAR phosphorylation/dephosphorylation, alterations in the trafficking of AMPAR mRNAs and synthesis/degradation of the receptor proteins. Another mechanism by which AMPARs are involved in the neurobiology of ASD and FXS is the role of AMPARs in stress that is known to exacerbate neuropsychiatric disorders including ASD [211, 212] and FXS [213-216], and has been shown to have effects on hippocampal synaptic plasticity, AMPAR trafficking and phosphorylation [217, 218, 84, 219, 220, 221]. AMPAR involvement in ASD and FXS affects mainly the cerebellum, hippocampus, amygdala, and prefrontal cortex.
The cerebellum is a brain region frequently implicated in the neurobiology of ASD and FXS [222], which is important for cognition and learning during development [223]. AMPARs have significant role in synaptic plasticity in this brain region. As pointed out, human postmortem brain studies report decreased AMPAR expression in cerebellum in ASD [168]. Correspondingly, in the Fmr1 KO mouse synaptic plasticity (mGluR-dependent LTD) in cerebellum is altered (enhanced) resulting in deficits in learning [224]. One study using a mouse model with deletion of the neuronal Islet Brain-2 protein (IB2) which is integral part of the PSD, is positioned near Shank 3, and is lacking often in Phelan- McDermid syndrome, a cause of autism, finds significant decrease in AMPAR-mediated and increase in NMDAR-mediated glutamatergic transmission in cerebellar mossy fiber to granule cell synapses [225]. This is accompanied by motor and cognitive deficits in IB2 KO mice suggestive of an autism phenotype. However, it should be noted that in addition to glutamate, endocannabinoids may be involved in synaptic plasticity and learning and memory in cerebellum [226, 227].
Another brain region important for ASD and FXS that exhibits changes in iGluRs is the hippocampus. The Fmr1 KO mouse has enhanced gp I mGluR-signaling and enhanced hippocampal gp I mGluR-LTD [83] that have provided basis for the mGluR theory of FXS [228]. This theory has guided development of mGlu5 antagonists for treatment of FXS, which have shown effectiveness in animal models and human clinical trials [42, 43, 176-178]. In the Fmr1 KO mouse enhanced hippocampal gp I mGluR-LTD is characterized with enhanced internalization of AMPARs [229-232]. Unlike the normal hippocampal mGluR-LTD [101, 103], in the Fmr1 KO mGluR-LTD does not require new protein synthesis due to basally elevated proteins regulating AMPAR trafficking, as a result of absence of translational repression by FMRP. Proteins regulating AMPAR endocytosis such as Arc, microtubule-associated protein 1B (MAP1B), STrial-Enriched protein Tyrosine Phosphatase (STEP), amyloid precursor protein (APP), termed “LTD” proteins, are basally upregulated in neuronal dendrites from Fmr1 KO mice, and do not increase further during mGluR-LTD. The role of FMRP in excessive mGluR-dependent internalization of AMPARs is shown in normal rat neuronal hippocampal cultures using FMRP siRNA [233]. In the Fmr1 KO mouse, in addition to decreased AMPA receptors during hippocampal mGluR-LTD [229, 230, 234], the expression of AMPARs in hippocampus is decreased at basal state during early development, 7-12 DIV [235]. This is likely due to the basally elevated levels of LTD proteins. At early developmental time points (7-12 days in vitro hippocampal neurons) decrease in GluA2 is reported [235] whereas at later developmental time points, decreases in both GluA1 [234, 229] and GluA2 [234] are reported. Although both GluA1 and GluA2 levels may be measured to determine AMPAR internalization in hippocampus, the leading model suggests that the GluA2 AMPAR subunit controls endocytosis during mGluR-LTD [80, 236]. The molecular mechanisms of AMPAR endocytosis in the Fmr1 KO mouse during hippocampal mGluR-LTD have been studied in detail [234, 229, 237, 231]. The regulation of dendritic protein synthesis and therefore, AMPAR internalization, during hippocampal gp I mGluR-LTD is achieved through the eukaryotic elongation factor 2 (eEF2 kinase) pathway [229, 103] and this mechanism is altered in the Fmr1 KO mouse [229]. The neurodevelopmental cytoskeletal protein MAP1B is synthesized during hippocampal mGluR-LTD in neuronal dendrites [103, 238], and is elevated in hippocampus of Fmr1 KO mice [239]. MAP1B plays role in AMPAR endocytosis during mGluR-LTD through interaction with GRIP1 by keeping internalized GluA2 away from the synaptic surface [103], and this mechanism is likely enhanced in the Fmr1 KO mouse. Since MAP1B siRNA blocks the endocytosis of AMPARs during normal hippocampal mGluR-LTD [103], it may be possible to block the enhanced mGluR-LTD and restore the decreased levels of AMPARs in FXS by decreasing MAP1B protein levels using small interfering RNA (siRNA). STEP is a brain-enriched tyrosine phosphatase that normally opposes synaptic strengthening by dephosphorylating key neuronal signaling molecules including NMDARs, AMPARs, extracellular signal-regulated kinase 1 and 2 (ERK1/2), stress-activated protein kinase p38 (p38), and the tyrosine kinase Fyn [240, 241]. STEP regulates AMPAR internalization by modulating dephosphorylation of the GluA2 subunit. Reducing STEP in the Fmr1 KO mouse diminishes seizures and restores select social and nonsocial anxiety-related behaviors [242]. Taken together, these studies suggest that restoring normal levels of AMPARs in hippocampal neurons has therapeutic potential in FXS. Interestingly, one study does not find decrease of GluA1 protein in hippocampus and cerebellum from Fmr1 KO mice but only in PFC which is accompanying to reduced cortical LTP [171]. This difference may be due to the experimental approaches used which may not detect subtle changes in AMPAR endocytosis, and the age of the mice (8-10 weeks), underscoring the importance of the experimental approaches used. This study does not find changes in the expression of NR2 subunits in PFC, cerebellum or hippocampus from Fmr1 KO mice [171].
Another molecular mechanism by which FMRP may regulate the levels of synaptic AMPARs in neurons is through control of the local synthesis of AMPAR subunits, PSD-95, and CaMKII alpha downstream of mGluR-activation [243]. Besides activation of gp I mGluRs, activation of other G-protein coupled receptors such as Gq-coupled, M1 muscarinic acetylcholine receptors (mAChRs) can trigger LTD that shares similar expression mechanisms with mGluR-LTD as it also requires protein synthesis, activation of the ERK and mammalian target of rapamycin (mTOR) translational pathways, stimulation of translation of FMRP and FMRP-binding mRNAs, and is expressed with AMPAR internalization. Both mGluR- and mAChR-dependent protein synthesis and LTD are enhanced in Fmr1 KO mice [112]. Therefore, mAChR antagonists may have therapeutic potential in FXS, in addition to gp I mGluR antagonists. Additional regulation of AMPAR levels and synaptic plasticity in FXS may be achieved through regulation of FMRP ubiquitination and degradation [244, 238]. For example, during hippocampal mGluR-LTD there is brief increase in synthesis of FMRP which is degraded rapidly by the ubiquitin-proteasome pathway [238]. This mechanism is lacking in the Fmr1 KO mouse and further contributes to the reduced AMPARs.
Another form of hippocampal synaptic plasticity altered in Fmr1 KO mice is homeostatic plasticity dependent on retinoic acid (RA) [86]. Suppression of synaptic activity increases synaptic strength by inducing synthesis of RA, which activates postsynaptic synthesis of AMPARs in dendrites and promotes synaptic insertion of newly synthesized AMPARs. FMRP is essential for this process and RA-dependent dendritic translation of GluA1 is impaired in Fmr1 KO mice.
The amygdala are implicated by several studies in FXS and ASD, and the strong emotional symptoms of FXS likely involve the amygdala [197]. Synaptic plasticity in the amygdala is investigated using whole-cell recordings in brain slices from adult Fmr1 KO mice [182]. MGluR-dependent LTP at thalamic inputs to principal neurons in the lateral amygdala is impaired resulting in reduced surface expression of GluA1 AMPARs in the Fmr1 KO mice. Additionally, there is lower presynaptic release manifested by a decrease in the frequency of spontaneous miniature excitatory postsynaptic currents (mEPSCs), increased paired-pulse ratio, and slower use-dependent block of NMDAR currents. Surprisingly, pharmacological inactivation of mGlu5 with MPEP fails to rescue either the deficit in LTP or surface GluA1. However, the same acute MPEP treatment reverses the decrease in mEPSC frequency, a finding of potential therapeutic relevance.
As discussed earlier, in PFC facilitation of synaptic LTP by D1 receptor is impaired in Fmr1 KO mice, and this correlates with decreased surface GluA1 [187]. Surface GluA1 insertion in Fmr1 KO is increased by administration of the D1 agonist SKF81297 in combination with gp I mGluR-antagonist, DL-AP3. This treatment inhibits hyperactivity and improves the learning ability of the Fmr1 KO mice. Interestingly, this study does not find consistent D1 modulation of basal AMPAR transmission in adult slice recordings, while in cultured PFC neurons D1 receptor activation causes GluA1 subunit surface expression and synaptic insertion. This inconsistency between adult slices and cultured neurons may be explained by developmental differences (cultured neurons may express proteins not expressed in vivo) or the differences between in vivo and in vitro neuronal networks. The investigators use PFC slices from mice, 5-6 weeks of age, and cultures prepared from Spague-Dawley rats, E18 at 10-14 DIV. This study emphasizes the need for careful comparison of animal and cell culture studies.
Alterations involving AMPARs in other animal ASD models besides the Fmr1 KO mouse are largely due to defects in proteins regulating the expression and trafficking of AMPARs and will be discussed in a following section of this review. A study using a KO mouse model of Angelman syndrome shows that there is dysfunction in AMPAR trafficking, enhanced AMPAR endocytosis and decreased hippocampal synaptic AMPARs [245]. Mutations of the E3 ubiquitin ligase Ube3A, located within chromosome 15q11-q13, are reported in individuals with Angelman syndrome. This neurological disorder manifests with autism, motor dysfunction, mental retardation, speech impairment, and seizures. Ube3A KO mice lacking the Ube3A ligase gene display a number of features of Angelman syndrome such as high frequency of seizures, general ataxia, abnormal EEGs, and poor performance on tests of learning and memory [245]. Analysis of the synaptic expression of AMPAR in cultured hippocampal neurons from Ube3A KO mice and their WT littermates reveals that Ube3A KO neurons have significantly reduced synaptic and surface GluA1 expression compared to WT neurons, without any changes in the surface expression of NMDARs. The reduced GluA1 expression is due to elevated levels of Arc in the KO neurons since small hairpin RNA (shRNA) targeting Arc in Ube3A KO restores surface GluA1 expression. These experiments suggest that the excessive internalization of AMPARs in Ube3A knockout neurons is likely a result of failure to ubiquitinate and degrade Arc, and may be a mechanism through which Ube3A deficiency is responsible for cognitive deficits in Angelman syndrome. However, the defect in synaptic GluA1 AMPARs is not the only thing that has gone awry in Angelman syndrome. It is possible that Ube3A substrates, in addition to Arc, play roles in nervous system development and contribute to development of the neurological and psychiatric disturbances.
Postnatal Tsc1 loss in mouse hippocampal cultures is associated with increased levels of Arc protein, significantly decreased GluA1 and GluA2 receptors and functional reduction of glutamatergic synaptic strength. This is a homeostatic compensatory mechanism due to suppression of hippocampal inhibitory neurotransmission associated with loss of Tsc1, which is insufficient and is accompanied with excitatory-inhibitory synaptic imbalance [246]. These changes are accompanied by hypexcitability in Tsc1 KO mice, are expressed through the mTOR pathway and are ameliorated by chronic treatment with rapamycin.
Another mechanism through which AMPARs are involved in ASD and FXS is through their role in excitotoxicity and cell death [247]. This is determined by the permeability of the receptor to Ca 2+ ions, and is dependent on the expression of the GluA2 subunit which limits Ca 2+ permeability [248]. Epilepsy is common in ASD and FXS, and AMPARs are implicated in its pathophysiology and treatments [249, 250]. This is a broad topic that is a subject of a review on its own.
VII. KAINATE RECEPTOR ALTERATIONS IN ANIMAL MODELS OF ASD AND FXS AND IN VITRO IN CELL CULTURE
Due to a report of a complex mutation in GluK2 that cosegregates with nonsyndromic autosomal recessive mental retardation [128], studies are carried out in mice to understand its significance for ASD. Electrophysiological data show that this mutation causes loss-of-function of the GluK2 protein. Subsequently, a study in GluK2(-/-) KO mice shows that the GluK2 receptor plays a role in ASD by regulating the maturation of synaptic circuits involved in learning and memory [251]. It is shown that the functional and morphological maturation of hippocampal mossy fiber to CA3 pyramidal cell synapses is delayed in GluK2 (-/-) KO mice, and this deficit is manifested by a transient reduction in the amplitude of AMPA EPSCs at a critical time point of postnatal development, whereas the NMDA component is spared. A decrease in GluA1 expression is observed. Analysis of the time-course of structural maturation of CA3 synapses by confocal imaging of yellow fluorescent protein (YFP)-expressing cells shows that major changes in synaptic structures occur subsequently to the sharp increase in synaptic transmission, and that the course of structural maturation of synaptic elements is impaired in GluK2(-/-) KO mice [251] (Table 1). In another study, the mutation M8361 of the GluK2 gene which is linked to autism is studied in cell culture - oocytes and COS-7 cells. It is established that this is a gain-of-function mutation leading to enhanced plasma membrane expression and increased current amplitudes of GluK2(M8361) compared to wild-type GluK2 that seems to be regulated by Rab11 [252]. These studies show that kainate receptor mutations found in ASD affect synaptic maturation and may affect simultaneously expression and functions of kainate and AMPARs.
VIII. ALTERATIONS IN IGLUR RECEPTOR-INTERACTING PROTEINS IN ANIMAL MODELS OF ASD AND FXS AND IN VITRO IN CELL CULTURE
As emphasized, mutations in neuronal synaptic proteins which interact with and regulate the expression and functions of iGluRs are found in human subjects with ASD. These proteins include postsynaptic neuroligins (NLGN) 1-4 and their presynaptic binding partners neurexins (NRXN) [253], Shank 1-3 proteins [134, 254], and GRIP1 [131]. Animal models have been created with the affected genes knocked out or mutants knocked in [255-257]. These mice reliably reproduce certain molecular, cellular and behavioral characteristics of ASD and have been instrumental in increasing our understanding of the disease mechanisms, and hopefully will aid in development of new drug treatments.
The NLGNs are a family of transmembrane cell-adhesion molecules expressed mainly postsynaptically which regulate excitatory and inhibitory synapse development, validation, maturation and functions [142, 258] (Fig. 2). NLGNs regulate synapse formation and synaptic transmission by interactions with NRXNs, PSD-95, AMPA and NMDARs, Shanks, and cooperate with leucine-rich repeat transmembrane neuronal proteins (LRRTM) in their interaction with NRXNs and excitatory synapse-regulating functions [259-261]. NLGN1 is associated with excitatory glutamatergic synapses, NLGN2 is associated with inhibitory GABAergic synapses [262], NLGN3 is found both at glutamatergic and GABAergic synapses [263], and NLGN4 is associated with glutamatergic and inhibitory glycinergic synapses [264]. Studies in KO mice suggest that similar synaptic plasticity and behavioral mechanisms involve NLGNs in nonsyndromic forms of ASD and in syndromic forms such as FXS [265].
Fig. (2).
Architecture of the trans-synaptic neurexin-neuroligin complex. Shown is: a) a synapse with presynaptic neurexins (NRXNs) and their postsynaptic binding partners neuroligins (NLGNs); receptors at the synapse. b) magnified view of the excitatory synapse with localization of NRXNs, NLGNs, PSD-95 which interacts with NLGNs through its third PDZ domain, the postsynaptic localization of AMPA receptors which are regulated by NLGNs and directly interact with them through an N-terminal interaction, and with PSD-95 (perhaps through stragazin?), and the location of SHANKs. Reprinted by permission from Macmillan Publishers Ltd: Nature, Neuroligins and neurexins link synaptic function to cognitive disease, Thomas C SÜdhof, 455, 903-911; copyright – 2008. http://www.nature.com/nature/ journal/v455/n7215/full/nature07456.html
The molecular and cellular mechanisms by which NLGNs are involved in the pathogenesis of ASD and FXS and bring about changes in iGluRs are complex. Overall, they involve alterations in synapse development, expression of iGluRs and resulting alterations in synapse formation and synaptic plasticity, leading to deficits in learning and memory and neuronal communication. It is shown in vitro that interaction of NRXN1b and NLGN1 is important for the formation of excitatory synapses and the recruitment of PSD-95 and NMDAR scaffolds and subsequently, recruitment of GluA2 AMPARs [266, 267]. The levels of AMPARs and AMPAR-mediated synaptic transmission are decreased in hippocampal slices from NLGN1 KO mice, confirming an important role of NLGN1 in driving AMPARs to nascent synapses through a diffusion trap mechanism, involving PSD-95 scaffolds (NLGN1/PSD-95 clusters assembled by NRXN-1b multimers) in competition with existing synapses [268]. NLGN1 KO mice also display alterations in NMDAR-mediated transmission. A study characterizes the effects of NLGN1 on synapse formation and AMPAR recruitment in primary cortical cultures from NLGN1 KO mice and WT littermates using live imaging of fluorescently tagged PSD-95, GluA2 and the presynaptic vesicle molecule SV2A to follow the constancy of the contents of these molecules at individual synapses over time. It is shown that loss of NLGN1 is associated with larger fluctuations in the synaptic contents of these molecules and a poorer preservation of their contents at individual synapses [269]. In turn, AMPARs may also play a role in the stabilization of synapses and elimination of inappropriate connections [270]. Thus, removal of surface AMPARs leads to a decrease in the number and stability of excitatory presynaptic inputs, whereas overexpression increases synapse number and stability. Overexpression of GluA2 AMPARs along with NLGN1 in 293T cells is sufficient to stabilize presynaptic inputs from cortical neurons onto heterologous cells which is not dependent on receptor-mediated current and instead relies on structural interactions mediated by the N-terminal domain of GluA2 [270]. Therefore, in ASD, loss of AMPARs may impair synapse stabilization and appropriate formation of neuronal connections.
The extracellular domains of NLGNs may also be important in the recruitment of AMPA and NMDARs at synapses and synaptic plasticity [271, 272]. Recently, NLGN1 isoform-specific cis interaction of the extracellular domain with the GluN1 NMDAR subunit is reported [272], which regulates the postsynaptic expression of NMDARs. The regulatory effects of NLGN1 on NMDA and AMPAR expression at excitatory synapses may explain in part the altered expression of NMDA and AMPAR subunits in mouse models FXS discussed earlier. NLGN1 deficiency is reported in Fmr1 KO mice and overexpressing hemagglutinin (HA)-neuroligin1 in Fmr1 KO mice improves social behavior but does not have a positive effect on learning and memory [273,274]. However, a more significant mechanism for the decrease AMPARs in the Fmr1 KO mouse is likely the enhanced mGluR-LTD and the associated increased rates of AMPAR internalization due to increased basal levels of “LTD proteins” [232]. It is also possible that the mechanisms of AMPAR expression vary during development with NLGN-dependent mechanisms having greater significance during early development.
NLGN1 KO mice display deficits in spatial learning and memory that correlate with impaired hippocampal LTP [275]. NLGN1 KO mice also exhibit a dramatic increase in repetitive, stereotyped grooming behavior, a potential autism-relevant abnormality. This repetitive grooming abnormality is associated with a reduced NMDA/AMPA ratio at corticostriatal synapses. The increased repetitive grooming phenotype can be rescued in adult mice by administration of the NMDAR partial coagonist d-cycloserine [275]. Interestingly, the NLGN1 KO mice do not exhibit changes in the expression of NMDAR subunits in brain as measured by Western blot. However, there is about 30% compensatory increase in the expression of NLGN3 and about 20% decrease in the expression of NRXN-a and b. Furthermore, there may be local changes not detected by Western blot with total brain proteins but with a more sensitive technique such as indirect immunofluorescent staining. In turn, NMDAR activity may regulate the structure and function of NLGN1 and synaptic transmission [276]. NMDAR activation is shown to trigger NLGN1 cleavage at single spines which requires Ca2+ /calmodulin-dependent protein kinase, and is mediated by proteolytic activity of matrix metalloprotease 9 (MMP9). Cleavage of NLGN1 causes rapid destabilization of its presynaptic partner neurexin-1β and depresses synaptic transmission by abruptly reducing presynaptic release probability.
Different mutations in NLGNs are found in human autism that may have brain region-specific effects on iGluR receptors and synaptic transmission. These mutations have been reproduced in mice to understand their effects. For example, the R451C substitution in NLGN3 in knock-in mice results in behaviors consistent with shift of synaptic transmission to inhibition – impaired social behaviors, enhanced learning in the water maze test and increased synaptic inhibition in somatosensory cortex [257]. However, in the hippocampus this mutation causes about 1.5 fold increase in AMPA-mediated excitatory synaptic transmission, induces upregulation of GluN2B and increase in LTP. The NLGN3 KO mice do not exhibit any of these changes. Another original approach is to introduce the R704C mutation that targets a conserved arginine residue in the cytoplasmic tail of all NLGNs, and is associated with autism in human genetic studies (the genetic defect affects NLGN4 in humans with ASD), into mouse NLGN3, and examine its effect on synapses in vitro and in vivo [256]. Electro-physiological and morphological studies reveal that the NLGN3 R704C mutation does not significantly alter synapse formation, but causes a marked selective decrease in AMPAR-mediated synaptic transmission in pyramidal neurons of the hippocampus, without similarly changing NMDA or GABA receptor-mediated synaptic transmission, and without altering presynaptic neurotransmitter release. These results suggest that the cytoplasmic tail of NLGN3 has a central role in synaptic transmission by modulating the recruitment of AMPARs to postsynaptic sites at excitatory synapses. Another line of NLGN3 KO mice show lack of mGluR-LTD in parallel fiber synapses in cerebellum, which is a result of occlusion of gp I mGluR-LTD due to constitutive activation and increased basal GluA2 S880 phosphorylation and increased expression of mGlu1α [ 265]. These deficits can be rescued by overexpression of NLGN3 suggesting that they are reversible and possible to modify with pharmacologic treatments.
ProSAP/Shanks are a family of multi-domain scaffolding proteins enriched in the PSD of excitatory synapses that play important role in the formation, maturation, and maintenance of synapses through linking postsynaptic mGluRs, NMDARs, AMPARs, NLGNs and the actin cytoskeleton [277, 278]. Genetic studies have identified mutations in human SHANK1, 2 and 3 genes in ASD [254]. Studies in KO mouse models shed light on the roles of Shanks in ASD and how they affect glutamatergic neurotransmission and iGluRs. Genetic deletion of Shank2 in mice results in an early, brain-region-specific upregulation of iGluRs at the synapse and increased levels of Shank3 [279]. Shank2 homozygous KO mice exhibit fewer dendritic spines and show reduced basal synaptic transmission, reduced frequency of mEPSCs and enhanced NMDAR-mediated excitatory currents at the physiological level. The mutants are hyperactive and display profound autistic-like behaviors including repetitive grooming and abnormalities in vocal and social behaviors [279].
In another study, Shank2 homozygous KO mice carrying a mutation identical to the ASD-associated microdeletion in the human SHANK2 gene exhibit ASD-like behaviors such as reduced social interaction, reduced social communication by ultrasonic vocalizations, and repetitive jumping [280]. These mice show a marked decrease in NMDAR function. Direct stimulation of NMDARs with D-cycloserine, a partial agonist of NMDARs, normalizes NMDAR function and improves the social interactions in Shank2 KO mice. Treatment of Shank2 KO mice with a positive allosteric modulator of mGlu5, which enhances NMDAR function via mGlu5 activation, also normalizes NMDAR function and enhances social interaction. These results suggest that reduced NMDAR function contributes to the development of ASD-like phenotypes in Shank2 KO mice, and that both NMDAR and mGlu5 modulation of NMDARs are potential therapeutic strategies in ASD [280].
Loss of a functional copy of the SHANK3 gene leads to the neurobehavioral manifestations of 22q13 deletion syndrome and ASD [281, 282]. Study of Shank3 haploinsufficient mice supports a role for this protein in glutamate receptor function and spine morphology [283]. In Shank 3 heterozygous mice there is reduced basal hippocampal synaptic transmission due to reduced AMPAR transmission, reduced GluA1 immuno-reactive puncta in CA1 stratum radiatum, reduced LTP, and no significant change in LTD. Male Shank3 heterozygotes displayed less social sniffing and fewer ultrasonic vocalizations during interactions with estrus female mice, as compared to wild-type littermate controls. This study suggests that modulation of glutamatergic neurotransmission to increase GluA1 AMPARs is a therapeutic option in this form of ASD.
Similar effects of Shank 3 deletion on GluA1 receptors and synaptic plasticity in vivo is obtained by another group [284]. The major isoforms of the gene are disrupted in mice by deleting exons 4-9. Shank3 (e4-9) homozygous KO mice display abnormal social behaviors, communication patterns, repetitive behaviors and learning and memory. Male KO mice display more severe impairments than females in motor coordination. Shank 3 KO mice have reduced levels of GluA1 and show attenuated activity-dependent redistribution of GluA1-receptors, morphological alterations in dendritic spines, normal CA1 hippocampal synaptic transmission, and deficient LTP [284]. In addition, the ability of Shank3 to interact with the cytoplasmic tail of NLGNs coordinates pre- and postsynaptic signaling through the NRXN-NLGN complex in hippocampal neurons [278]. Synaptic levels of Shank3 regulate AMPA and NMDAR-mediated synaptic transmission through NRXN-NLGN signaling, and ASD-associated mutations in Shank3 disrupt not only postsynaptic AMPA and NMDAR signaling but also interfere with the ability of Shank3 to signal across the synapse to alter presynaptic structure and function [278]. This is a mechanism through which Shank 3 mutations can disrupt neuronal connectivity during development. Due to similarities of the Shank 3 KO mice with human ASD, they may be useful for development of new drugs to evaluate the effects on AMPA, NMDARs and LTP.
GRIP1 is a multi-PDZ domain protein that interacts through PDZ domains 4-6 with the C-terminus of GluA2/3 AMPARs and regulates their synaptic clustering and trafficking [65, 285]. In addition, GRIP1 interacts with other postsynaptic molecules such as MAP1B to regulate AMPAR trafficking [103], and regulates the trafficking of GABA receptors [286]. GRIP1 is mapped to chromosomal 12q14.3 region, which is associated with autism [287]. Screening of autistic families identifies several GRIP1 single-nucleotide polymorphism variants, which have been further studied in vitro in cultured hippocampal neurons using pH-sensitive pHluorin-GluA2 fusion protein (pH-GluA2) [131]. Some of the GRIP1 mutations associated with autism correlate with more severe social impairment, result in gain-of-function and increased interaction of GRIP1 with GluA2/3 receptors, higher rates of recycling of GluA2/3 and higher surface levels of GluA2 [131]. These findings are correlated with behavioral studies of Grip1/2 double KO mice which exhibit increased sociability, impaired PPI, and object recognition, suggesting a role for GRIP proteins in the modulation of social behavior and possibly cognitive or memory function. GRIP1 may also regulate the excitation/inhibition balance in brain due to its ability to regulate the trafficking of both GluA2/3 and GABA receptors and is therefore an attractive molecule to investigate further in subjects with autism.
CONCLUSIONS. DIRECTIONS FOR FUTURE NEUROPHARMACOLOGICAL RESEARCH
It is evident that ASD and FXS are disorders affecting the synapse and synaptic plasticity. Human and animal model studies indicate that iGluR expression, trafficking and functions are altered, resulting in altered synapse development and plasticity in a brain region- and disorder-specific manner. The studies point to complex molecular and cellular neuro-biological changes involving iGluRs and their associated proteins in ASD and FXS, and indicate that several pharma-cological drugs may be used therapeutically. It is notable that brain changes in iGluRs may be reversed using drugs acting at other receptors such as mGlu5, D1, M1 muscarinic acetylcholine, and GABAB receptors. Further clinical, animal and molecular/cellular studies are necessary to understand in greater detail the changes of iGluRs in various ASD subtypes. For example, NLGN3 genetic changes in various mouse models can cause increase or decrease in AMPAR-mediated transmission in hippocampus, or enhanced GluA2 S800 phosphorylation. Similarly, deletion of Shank 2 in mice is associated with decreased or enhanced function of NMDARs. Animal model studies suggest that there are reduced levels of AMPARs in several brain regions in FXS and Shank 3 deletion syndrome, while in GRIP1/2 KO mice there are increased levels of AMPARs. It is necessary to understand well the iGluR-dependent alterations and glutamatergic hypo- vs. hyper-function in ASD brain in order to select appropriate pharmacologic treatments. Importantly, the iGluRs and associated proteins and neuronal signaling pathways which are altered may provide translational biomarkers for ASD and FXS. In future, it is necessary to characterize better the timing of the changes affecting iGluRs during development which will be useful for selection of optimal therapeutic window. Overall, it can be concluded that animal models and in vitro cellular models are very useful for understanding neurobiological pathways affecting iGluRs in ASD and FXS with the caveat that they cannot reproduce all characteristics of the human disorders. This may be due to species-specific differences in neuroreceptor and neuro-transmitter systems in brain and complex behaviors. It is important to note that the majority of the existing animal models of ASD are models of monogenic forms of ASD such as FXS, TSC, Rett and Angelman syndromes, and single-gene knockout or knock-in animal models. In humans, ASD may be the result of defects in many genes, which interact with environmental factors, although the nature of the genetic changes is not well understood. Known environmental factors that may affect iGluRs in ASD include stress which exacerbates ASD and FXS and has effects on synaptic plasticity and AMPA receptor trafficking, and cytokine production due to infection. Since iGluRs are exquisitely regulated by experience, many environmental factors may modulate their functions including nutrition, environmental toxins and infections. Future studies should address the significance of other factors which regulate the expression and functions iGluRs. Lastly, ultimately the studies on ASD neurobiology involving iGluRs and development of new pharmacologic treatments have to be validated in humans.
ACKNOWLEDGEMENTS
Dr. Jason Shepherd is supported by the NINDS (4R00NS076364-03).
CONFLICT OF INTEREST
Dr. Eric Hollander has consulted for Roche.
REFERENCES
- 1.Diagnostic and Statistic Manual of Mental Disorders . 5th Edition. Vol. 991. Washington D C: American Psychiatric Publishing; 2013. American Psychiatric Association. [Google Scholar]
- 2.Autism Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators Center for Disease Control and Prevention Prevalence of autism spectrum disorders--Autism and Developmental Disabilities Monitoring Network 14 sites. United States 2008. 2012;61(3):1–19. [PubMed] [Google Scholar]
- 3.Thurm A, Swedo SE. The importance of autism research. Dialog. Clin. Neurosci. 2012;14(3):219–22. doi: 10.31887/DCNS.2012.14.3/athurm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hollander E, Kolevzon A, Coyle JT, editors. Vol. 603. Washington D C: American Psychiatric Publishing.; 2011. Texbook of Autsim Spectrum Disorders. [Google Scholar]
- 5.Bagni C, Tassone F, Neri G, Hagerman R. Fragile X syndrome: causes diagnosis mechanisms and therapeutics. J. Clin. Invest. 2012;22(12):4314–22. doi: 10.1172/JCI63141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gurkan CK, Hagerman RJ. Targeted treatments in autism and Fragile X syndrome. Res. Autism Spectr Disord. 2012;6(4):1311–20. doi: 10.1016/j.rasd.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry-Kravis E, Kno A, Hervey C. Targeted treatments for fragile X syndrome. J. Neurodev. Disord. 2011;3(3):193–210. doi: 10.1007/s11689-011-9074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dolen G, Carpenter RL, Ocain TD, Bear MF. Mechanism-based approaches to treating fragile X. Pharmacol. Ther. 2010;127(1):78–93. doi: 10.1016/j.pharmthera.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Bassell GJ, Gross C. Reducing glutamate signaling pays off in fragile X. Nat. Med. 2008;14(3):249–250. doi: 10.1038/nm0308-249. [DOI] [PubMed] [Google Scholar]
- 10.Khwaja OS, Sahin M. Translational research: Rett syndrome and tuberous sclerosis complex. Curr. Opin. Pediatr. 2011;23(6):633–639. doi: 10.1097/MOP.0b013e32834c9251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neul JL. The relationship of Rett syndrome and MECP2 disorders to autism. Dialog. Clin. Neurosci. 2012;14(3):253–262. doi: 10.31887/DCNS.2012.14.3/jneul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moretti P, Zoghbi HY. MeCP2 dysfunction in Rett syndrome and related disorders. Curr. Opin. Genet. Develop. 2006;16(3):276–281. doi: 10.1016/j.gde.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Walsh KS, Velez JI, Kardel PG, Imas DM, Muenke M, Packer RJ, Castellanos FX, Acosta MT. Symptomatology of autism spectrum disorder in a population with neurofibromatosis type 1. Dev. Med. Child Neurol. 2013;55(2):131–138. doi: 10.1111/dmcn.12038. [DOI] [PubMed] [Google Scholar]
- 14.Guo W, Murthy AC, Zhang L, Johnson EB, Schaller EG, Allan AM, Zhao X. Inhibition of GSK3beta improves hippocampus-dependent learning and rescues neurogenesis in a mouse model of fragile X syndrome. Hum. Mol. Genet. 2012;21(3):681–691. doi: 10.1093/hmg/ddr501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berkel S, Marshall CR, Weiss B, Howe J, Roeth R, Moog U, Endris V, Roberts W, Szatmari P, Pinto D, Bonin M, Riess A, Engels H, Sprengel R, Scherer SW, Rappold GA. Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat. Genet. 2010;42(6):489–491. doi: 10.1038/ng.589. [DOI] [PubMed] [Google Scholar]
- 16.Kelleher RJ, 3rd; Geigenmuller U, Hovhannisyan H, Trautman E, Pinard R, Rathmell B, Carpenter R, Margulies D. High-throughput sequencing of mGluR signaling pathway genes reveals enrichment of rare variants in autism. PloS One. 2012;7(4):e35003. doi: 10.1371/journal.pone.0035003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113(5):e472–86. doi: 10.1542/peds.113.5.e472. [DOI] [PubMed] [Google Scholar]
- 18.O'Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, Levy R, Ko A, Lee C, Smith JD, Turner EH, Stanaway IB, Vernot B, Malig M, Baker C, Reilly B, Akey JM, Borenstein E, Rieder MJ, Nickerson DA, Bernier R, Shendure J, Eichler EE. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485(7393):246–250. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Betancur C. Etiological heterogeneity in autism spectrum disorders: more than 100 genetic and genomic disorders and still counting. Brain Res. 2011;1380:42–77. doi: 10.1016/j.brainres.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 20.Essa MM, Braidy N, Vijayan KR, Subash S, Guillemin GJ. Excitotoxicity in the pathogenesis of autism. Neurotox. Res. 2013;23(4):393–400. doi: 10.1007/s12640-012-9354-3. [DOI] [PubMed] [Google Scholar]
- 21.Bhakar AL, Dolen G, Bear MF. The pathophysiology of fragile X (and what it teaches us about synapses). Ann. Rev. Neurosci. 2012;35:417–443. doi: 10.1146/annurev-neuro-060909-153138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moskal JR, Burgdorf J, Kroes RA, Brudzynski SM, Panksepp J. A novel NMDA receptor glycine-site partial agonist, GLYX-13, has therapeutic potential for the treatment of autism. Neurosci. Biobehav. Rev. 2011;35(9):1982–1988. doi: 10.1016/j.neubiorev.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Shinohe A, Hashimoto K, Nakamura K, Tsujii M, Iwata Y, Tsuchiya KJ, Sekine Y, Suda S, Suzuki K, Sugihara G, Matszukai H, Minabi Y, Sugiyama T, Kawai M, Iyo M, Takei N, Mori N. Increased serum levels of glutamate in adult patients with autism. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2006;30(8):1472–1477. doi: 10.1016/j.pnpbp.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Chao HT, Chen H, Samaco R.C. Xue, M. Chahrour, M. Yoo, J. Neul, J.L. Gong, S. Lu, H.C. Heintz, N. Ekker, M. Rubenstein JL, Neobels JL, Rosenmund C, Zoghbi HY. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468(7321):263–269. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrington RA, Lee LC, Crum RM, Zimmerman AW, Hertz-Picciotto I. Serotonin hypothesis of autism: implications for selective serotonin reuptake inhibitor use during pregnancy. Autism Res. 2013;6(3):149–168. doi: 10.1002/aur.1288. [DOI] [PubMed] [Google Scholar]
- 26.Karam RA, Rezk NA, Abdelrahman HM, Hassan TH, Mohammad D, Hashim HM, Fattah NR. Catechol-O-methyltransferase Val158Met polymorphism and hyperactivity symptoms in Egyptian children with autism spectrum disorder. Res. Develop. Disabil. 2013;34(7):2092–2097. doi: 10.1016/j.ridd.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Bodner KE, Beversdorf DQ, Saklayen SS, Christ SE. Noradrenergic moderation of working memory impairments in adults with autism spectrum disorder. J. Int. Neuropsychol. Soc. 2012;18(3):556–564. doi: 10.1017/S1355617712000070. [DOI] [PubMed] [Google Scholar]
- 28.Dichter GS, Damiano CA, Allen JA. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. J. Neurodev. Dis. 2012;4(1):19. doi: 10.1186/1866-1955-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnold LE, Aman MG, Hollway J, Hurt E, Bates B, Li X, Farmer C, Anand R, Thompson S, Ramadan Y, Williams C. Placebo-controlled pilot trial of mecamylamine for treatment of autism spectrum disorders. J. Child Adolesc. Psychopharmacol. 2012;22(3):198–205. doi: 10.1089/cap.2011.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palma E, Conti L, Roseti C, Limatola C. Novel approaches to study the involvement of alpha7-nAChR in human diseases. Curr. Drug Targets. 2012;13(5):579–586. doi: 10.2174/138945012800398838. [DOI] [PubMed] [Google Scholar]
- 31.Jung KM, Sepers M, Henstridge CM, Lassalle O, Neuhofer D, Martin H, Ginger M, Frick A, DiPatricio NV, Mackie K, Katona I, Piomelli D, Manzoni OJ. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nat. Communic. 2012;3:1080. doi: 10.1038/ncomms2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Busquets-Garcia A, Gomis-Gonzalez M, Guegan T, Agustin-Pavon C, Pastor A, Mato S, Perez-Samartin A, Matute C, de la Torre R, Dierrsen M, Maldonaldo R, Ozaita A. Targeting the endocannabinoid system in the treatment of fragile X syndrome. Nat. Med. 2013;19(5):603–607. doi: 10.1038/nm.3127. [DOI] [PubMed] [Google Scholar]
- 33.Zink CF, Meyer-Lindenberg A. Human neuroimaging of oxytocin and vasopressin in social cognition. Horm. Behav. 2012;61(3):400–409. doi: 10.1016/j.yhbeh.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green JJ, Hollander E. Autism and oxytocin: new developments in translational approaches to therapeutics. Neurotherapeutics. 2010;7(3):250–257. doi: 10.1016/j.nurt.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartz JA, Hollander E. The neuroscience of affiliation: forging links between basic and clinical research on neuropeptides and social behavior. Horm. Behav. 2006;50(4):518–528. doi: 10.1016/j.yhbeh.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 36.Spooren W, Lindemann L, Ghosh A, Santarelli L. Synapse dysfunction in autism: a molecular medicine approach to drug discovery in neurodevelopmental disorders. Trends Pharmacol. Sci. 2012;33(12):669–684. doi: 10.1016/j.tips.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Soorya L, Kiarashi J, Hollander E. Psychopharmacologic interventions for repetitive behaviors in autism spectrum disorders. Child Adoles. Psychiatr. Clin. N. Am. 2008;17(4):753–771. doi: 10.1016/j.chc.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Hollander E, Phillips AT, Yeh CC. Targeted treatments for symptom domains in child and adolescent autism. Lancet. 2003;362(9385):732–734. doi: 10.1016/S0140-6736(03)14236-5. [DOI] [PubMed] [Google Scholar]
- 39.Doyle CA, McDougle CJ. Pharmacotherapy to control behavioral symptoms in children with autism. Expert Opin. Pharmacother. 2012;13(11):1615–1629. doi: 10.1517/14656566.2012.674110. [DOI] [PubMed] [Google Scholar]
- 40.Tranfaglia MR. Fragile X syndrome: a psychiatric perspective. Results Probl. Cell Differ. 2012;54:281–295. doi: 10.1007/978-3-642-21649-7_16. [DOI] [PubMed] [Google Scholar]
- 41.Hagerman RJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Lachiewicz A. Kronk, R. Delahunty, C. Hessl, D. Visootsak, J.;Picker J, Gane L, Tranfaglia M. Advances in the treatment of fragile X syndrome. Pediatrics. 2009;123(1):378–390. doi: 10.1542/peds.2008-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berry-Kravis E, Hessl D, Coffey S, Hervey C, Schneider A, Yuhas J, Hutchison J, Snape M. single dose trial of fenobam in adults with fragile X syndrome. J. Med. Genet. . 2009;46(4):266–271. doi: 10.1136/jmg.2008.063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacquemont S, Curie A, des Portes V, Torrioli MG, Berry-Kravis E, Hagerman RJ, Ramos FJ, Cornish K, He Y, Paulding C, Neri G, Chen F. Hadjikhani, N. Martinet, D. Meyer J, Beckmann JS, Delange K, Brun A, Bussy G. Gasparini F, Hilse T, Floesser A, Branson J, Bilbe , Johns D, Gomez-Mancilla B. Epigenetic modification of the FMR1 gene in fragile X syndrome is associated with differential response to the mGluR5 antagonist. AFQ056. Sci. Transl. Med. 2011;3(64):64ra1. doi: 10.1126/scitranslmed.3001708. [DOI] [PubMed] [Google Scholar]
- 44.Gandal MJ, Edgar JC, Ehrlichman RS, Mehta M, Roberts TP, Siegel SJ. Validating gamma oscillations and delayed auditory responses as translational biomarkers of autism. Biol. Psychiatry. 2010;68(12):1100–1106. doi: 10.1016/j.biopsych.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mehta MV, Gandal MJ, Siegel SJ. mGluR5-antagonist mediated reversal of elevated stereotyped repetitive behaviors in the VPA model of autism. PloS One. 2011;6(10):e26077. doi: 10.1371/journal.pone.0026077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silverman JL, Smith DG, Rizzo SJ, Karras MN, Turner SM, Tolu SS, Bryce DK, Smith DL, Fonseca K, Ring RH, Crawley JN. Negative allosteric modulation of the mGluR5 receptor reduces repetitive behaviors and rescues social deficits in mouse models of autism. Sci. Transl. Med. 2012;4(131):131ra51. doi: 10.1126/scitranslmed.3003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berry-Kravis E, Hessl D, Rathmell B, Zarevics P. Effects of STX209 (arbaclofen) on neurobehavioral function in children and adults with fragile X syndrome a randomized controlled phase 2 trial. Sci. Transl. Med. 2012;4(152):152ra127. doi: 10.1126/scitranslmed.3004214. [DOI] [PubMed] [Google Scholar]
- 48.Burket JA, Herndon AL, Winebarger EE, Jacome LF, Deutsch SI. Complex effects of mGluR5 antagonism on sociability and stereotypic behaviors in mice: possible implications for the pharmacotherapy of autism spectrum disorders. Brain Res. Bull. 2011;86(3-4):152–158. doi: 10.1016/j.brainresbull.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 49.Mayer ML. Emerging models of glutamate receptor ion channel structure and function. Structure. 2011;19(10):1370–1380. doi: 10.1016/j.str.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mayer ML. Structure and mechanism of glutamate receptor ion channel assembly, activation and modulation. Curr. Opin. Neurobiol. 2011;21(2):283–290. doi: 10.1016/j.conb.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure regulation and function. Pharmacol. Rev. 2010;62(3):405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Collingridge GL, Olsen R, Peters J, Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacology. 2009;56(1):2–5. doi: 10.1016/j.neuropharm.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Contractor A, Swanson G, Heinemann SF. Kainate receptors are involved in short- and long-term plasticity at mossy fiber synapses in the hippocampus. Neuron. 2001;29(1):209–216. doi: 10.1016/s0896-6273(01)00191-x. [DOI] [PubMed] [Google Scholar]
- 54.Contractor A, Mulle C, Swanson GT. Kainate receptors coming of age: milestones of two decades of research. Trends Neurosci. 2011;34(3):154–163. doi: 10.1016/j.tins.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nicoletti F, Bockaert J, Collingridge GL, Conn PJ, Ferraguti F, Schoepp DD, Wroblewski JT, Pin JP. Metabotropic glutamate receptors: from the workbench to the bedside. Neuro-pharmacology. 2011;60(7-8):1017–1041. doi: 10.1016/j.neuropharm.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat. Rev. Neurosci. 2007;8(6):413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- 57.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Ann. Rev. Cell Develop. Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 58.Anggono V, Huganir RL. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr. Opin. Neurobiol. 2012;22(3):461–469. doi: 10.1016/j.conb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang G, Gilbert J, Man HY. AMPA receptor trafficking in homeostatic synaptic plasticity: functional molecules and signaling cascades. Neural Plast. 2012;2012:825364. doi: 10.1155/2012/825364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu W, Roche KW. Posttranslational regulation of AMPA receptor trafficking and function. Curr. Opin. Neurobiol. 2012;22(3):470–479. doi: 10.1016/j.conb.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanz-Clemente A, Nicoll RA, Roche KW. Diversity in NMDA receptor composition: many regulators, many consequences. Neuroscientist. 2013;19(1):62–75. doi: 10.1177/1073858411435129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fourie C, Li D, Montgomery JM. The anchoring protein SAP97 influences the trafficking and localisation of multiple membrane channels. Bioch. Biophys. Acta. 2013 doi: 10.1016/j.bbamem.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 63.Shen L, Liang F, Walensky LD, Huganir RL. Regulation of AMPA receptor GluR1 subunit surface expression by a 4.1N-linked actin cytoskeletal association. J. Neurosci. 2000;20(21):7932–7940. doi: 10.1523/JNEUROSCI.20-21-07932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xia J, Zhang X, Staudinger J, Huganir RL. Clustering of AMPA receptors by the synaptic PDZ domain-containing protein. PICK1 Neuron. 1999;22(1):179–187. doi: 10.1016/s0896-6273(00)80689-3. [DOI] [PubMed] [Google Scholar]
- 65.Dong H, O'Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997;386(6622):279–284. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- 66.Nishimune A, Isaac JT, Molnar E, Noel J, Nash SR, Tagaya M, Collingridge GL, Nakanishi S, HeNLGNey JM. NSF binding to GluR2 regulates synaptic transmission. Neuron. 1998;21(1):87–97. doi: 10.1016/s0896-6273(00)80517-6. [DOI] [PubMed] [Google Scholar]
- 67.Li D, Specht CG, Waites CL, Butler-Munro C, Leal-Ortiz S, Foote JW, Genoux D, Garner CC, Montgomery JM. SAP97 directs NMDA receptor spine targeting and synaptic plasticity. J. Physiol. 2011;589 (Pt 18):4491–510. doi: 10.1113/jphysiol.2011.215566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Howard MA, Elias GM, Elias LA, Swat W, Nicoll RA. The role of SAP97 in synaptic glutamate receptor dynamics. Proc. Natl. Acad Sci U S A . 2010;107(8):3805–3810. doi: 10.1073/pnas.0914422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu W. PSD-95-like membrane associated guanylate kinases (PSD-MAGUKs) and synaptic plasticity. Curr. Opin. Neurobiol. 2011;21(2):306–312. doi: 10.1016/j.conb.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bhattacharyya S, Biou V, Xu W, Schluter O, Malenka RC. A critical role for PSD-95/AKAP interactions in endocytosis of synaptic AMPA receptors. Nat. Neurosci. 2009;12(2):172–181. doi: 10.1038/nn.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bats C, Groc L, Choquet D. The interaction between Stargazin and PSD-95 regulates. AMPA receptor surface trafficking Neuron. 2007;53(5):719–734. doi: 10.1016/j.neuron.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 72.Beique JC, Lin DT, Kang MG, Aizawa H, Takamiya K, Huganir RL. Synapse-specific regulation of AMPA receptor function by PSD-95. Proc. Natl. Acad. Sci. USA. 2006;103(51):19535–19540. doi: 10.1073/pnas.0608492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Elias GM, Elias LA, Apostolides PF, Kriegstein AR, Nicoll RA. Differential trafficking of AMPA and NMDA receptors by SAP102 and PSD-95 underlies synapse development. Proc. Natl. Acad. Sci. U.S.A. 2008;105(52):20953–20958. doi: 10.1073/pnas.0811025106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin Y, Jover-Mengual T, Wong J, Bennett MV, Zukin RS. PSD-95 and PKC converge in regulating NMDA receptor trafficking and gating. Proc. Natl. Acad. Sci. U.S.A. 2006;103(52):19902–19907. doi: 10.1073/pnas.0609924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Straub C, Tomita S. The regulation of glutamate receptor trafficking and function by TARPs and other transmembrane auxiliary subunits. Curr. Opin. Neurobiol. 2012;22(3):488–495. doi: 10.1016/j.conb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Groc L, Choquet D. AMPA and NMDA glutamate receptor trafficking: multiple roads for reaching and leaving the synapse. Cell Tissue Res. 2006;326(2):423–438. doi: 10.1007/s00441-006-0254-9. [DOI] [PubMed] [Google Scholar]
- 77.Perez-Otano I, Ehlers MD. Homeostatic plasticity and NMDA receptor trafficking. Trends Neurosci. 2005;28(5):229–238. doi: 10.1016/j.tins.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 78.Carroll RC, Zukin RS. NMDA-receptor trafficking and targeting: implications for synaptic transmission and plasticity. Trends Neurosci. 2002;25(11):571–577. doi: 10.1016/s0166-2236(02)02272-5. [DOI] [PubMed] [Google Scholar]
- 79.Malenka RC. Synaptic plasticity and AMPA receptor trafficking. Ann. N. Y. Acad. Sci. 2003;1003:1–11. doi: 10.1196/annals.1300.001. [DOI] [PubMed] [Google Scholar]
- 80.Carroll RC, Beattie EC, von Zastrow M, Malenka RC. Role of AMPA receptor endocytosis in synaptic plasticity. Nat. Rev. Neurosci. 2001;2(5):315–324. doi: 10.1038/35072500. [DOI] [PubMed] [Google Scholar]
- 81.Choquet D. Fast AMPAR trafficking for a high-frequency synaptic transmission. Eur. J. Neurosci. 2010;32(2):250–260. doi: 10.1111/j.1460-9568.2010.07350.x. [DOI] [PubMed] [Google Scholar]
- 82.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 83.Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc. Natl. Acad. Sci. USA. 2002;99(11):7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Krugers HJ, Hoogenraad CC, Groc L. Stress hormones and AMPA receptor trafficking in synaptic plasticity and memory. Nat. Rev. Neurosci. 2010;11(10):675–681. doi: 10.1038/nrn2913. [DOI] [PubMed] [Google Scholar]
- 85.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440(7087):1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 86.Soden ME, Chen L. Fragile X protein FMRP is required for homeostatic plasticity and regulation of synaptic strength by retinoic acid. J. Neurosci. 2010;30(50):16910–16921. doi: 10.1523/JNEUROSCI.3660-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anggono V, Clem RL, Huganir RL. PICK1 loss of function occludes homeostatic synaptic scaling. J. Neurosci. 2011;31(6):2188–2196. doi: 10.1523/JNEUROSCI.5633-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3. mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52(3):475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hou Q, Zhang D, Jarzylo L, Huganir RL, Man HY. Homeostatic regulation of AMPA receptor expression at single hippocampal synapses. Proc. Natl. Acad. Sci. U.S.A. 2008;105(2):775–780. doi: 10.1073/pnas.0706447105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Isaac JT, Nicoll RA, Malenka RC. Silent glutamatergic synapses in the mammalian brain. Can. J. Physiol. Pharmacol. 1999;77(9):735–737. [PubMed] [Google Scholar]
- 91.Isaac JT, Crair MC, Nicoll RA, Malenka RC. Silent synapses during development of thalamocortical inputs. Neuron. 1997;8(2):269–280. doi: 10.1016/s0896-6273(00)80267-6. [DOI] [PubMed] [Google Scholar]
- 92.Hall BJ, Ripley B, Ghosh A. NR2B signaling regulates the development of synaptic AMPA receptor current. J. Neurosci. . 2007;27(49):13446–56. doi: 10.1523/JNEUROSCI.3793-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liao D, Zhang X, O'Brien R, Ehlers MD, Huganir RL. Regulation of morphological postsynaptic silent synapses in developing hippocampal neurons. Nat. Neurosci. 1999;2(1):37–43. doi: 10.1038/4540. [DOI] [PubMed] [Google Scholar]
- 94.Adesnik H, Li G, During MJ, Pleasure SJ, Nicoll RA. NMDA receptors inhibit synapse unsilencing during brain development. Proc. Natl. Acad. Sci. USA. 2008;105(14):5597–5602. doi: 10.1073/pnas.0800946105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alvarez VA, Ridenour DA, Sabatini BL. Distinct structural and ionotropic roles of NMDA receptors in controlling spine and synapse stability. J. Neurosci. 2007;27(28):7365–7376. doi: 10.1523/JNEUROSCI.0956-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hall BJ, Ghosh A. Regulation of AMPA receptor recruitment at developing synapses. Trends Neurosci. 2008;31(2):82–89. doi: 10.1016/j.tins.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 97.Luscher C, Malenka RC. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb. Perspect. Biol. 2012;4(6) doi: 10.1101/cshperspect.a005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gainey MA, Hurvitz-Wolff JR, Lambo ME, Turrigiano GG. Synaptic scaling requires the GluR2 subunit of the AMPA receptor. J. Neurosci. 2009;29(20):6479–6489. doi: 10.1523/JNEUROSCI.3753-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Malinow R. AMPA receptor trafficking and long-term potentiation. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2003;358(1432):707–714. doi: 10.1098/rstb.2002.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Citri A, Bhattacharyya S, Ma C, Morishita W, Fang S, Rizo J, Malenka RC. Calcium binding to PICK1 is essential for the intracellular retention of AMPA receptors underlying long-term depression. J. Neurosci. 2010;30(49):16437–16452. doi: 10.1523/JNEUROSCI.4478-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat. Neurosci. 2001;4(11):1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- 102.Xiao MY, Zhou Q, Nicoll RA. Metabotropic glutamate receptor activation causes a rapid redistribution of AMPA receptors. Neuropharmacology. 2001;41(6):664–671. doi: 10.1016/s0028-3908(01)00134-4. [DOI] [PubMed] [Google Scholar]
- 103.Davidkova G, Carroll RC. Characterization of the role of microtubule-associated protein 1B in metabotropic glutamate receptor-mediated endocytosis of AMPA receptors in hippocampus. J. Neurosci. 2007;27(48):13273–13278. doi: 10.1523/JNEUROSCI.3334-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee HK, Takamiya K, He K, Song L, Huganir RL. Specific roles of AMPA receptor subunit GluR1 (GluA1) phosphorylation sites in regulating synaptic plasticity in the CA1 region of hippocampus. J. Neurophysiol. 2010;103(1):479–489. doi: 10.1152/jn.00835.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Makino Y, Johnson RC, Yu Y, Takamiya K, Huganir RL. Enhanced synaptic plasticity in mice with phosphomimetic mutation of the GluA1 AMPA receptor. Proc. Natl. Acad. Sci. U.S.A. 2011;108(20):8450–8455. doi: 10.1073/pnas.1105261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee HK, Kameyama K, Huganir RL, Bear MF. NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron. 1998;21(5):1151–1162. doi: 10.1016/s0896-6273(00)80632-7. [DOI] [PubMed] [Google Scholar]
- 107.Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, Wnthold RJ, Gallagher M, Huganir RL. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112(5):631–643. doi: 10.1016/s0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- 108.Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51(2):213–225. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 109.Bliss TV, Collingridge GL. Expression of NMDA receptor-dependent LTP in the hippocampus: bridging the divide. Mol. Brain. 2013;6 5 doi: 10.1186/1756-6606-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nosyreva ED, Huber KM. Developmental switch in synaptic mechanisms of hippocampal metabotropic glutamate receptor-dependent long-term depression. J. Neurosci. 2005;25(11):2992–3001. doi: 10.1523/JNEUROSCI.3652-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dickinson BA, Jo J, Seok H, Son GH, Whitcomb DJ, Davies CH, Sheng M, Collingridge GL, Cho K. A novel mechanism of hippocampal LTD involving muscarinic receptor-triggered interactions between AMPARs, GRIP and liprin-alpha. Mol. Brain. 2009;2 18 doi: 10.1186/1756-6606-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Volk LJ, Pfeiffer BE, Gibson JR, Huber KM. Multiple Gq-coupled receptors converge on a common protein synthesis-dependent long-term depression that is affected in fragile X syndrome mental retardation. J. Neurosci. 2007;27(43) doi: 10.1523/JNEUROSCI.2266-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bramham CR, Worley PF, Moore MJ, Guzowski JF. The immediate early gene arc/arg3.: regultion mechanisms and function. J. Neurosci. 2008; 28(46):11760–11767. doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Carlsson ML. Hypothesis: is infantile autism a hypoglutamatergic disorder?.Relevance of glutamate - serotonin interactions for pharmacotherapy. J. Neural Transm. 1998;105(4-5):525–535. doi: 10.1007/s007020050076. [DOI] [PubMed] [Google Scholar]
- 115.Bejjani A, O'Neill J, Kim JA, Frew AJ, Yee VW, Ly R, Kitchen C, Salamon N, McCracken JT, Toga AW, Alger JR, Levitt JG. Elevated glutamatergic compounds in pregenual anterior cingulate in pediatric autism spectrum disorder demonstrated by 1H MRS and 1H MRSI. PloS One. 2012;7(7) doi: 10.1371/journal.pone.0038786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Akhondzadeh S, Tajdar H, Mohammadi MR, Mohammadi M, Nouroozinejad GH, Shabstari OL, Ghelichnia HA. A double-blind placebo controlled trial of piracetam added to risperidone in patients with autistic disorder. Child Psychiatry Hum. Dev. 2008;39(3):237–245. doi: 10.1007/s10578-007-0084-3. [DOI] [PubMed] [Google Scholar]
- 117.Chen HS, Pellegrini JW, Aggarwal SK, Lei SZ, Warach S, Jensen FE, Lipton SA. Open-channel block of N-methyl-D-aspartate (NMDA) responses by memantine: therapeutic advantage against NMDA receptor-mediated neurotoxicity. J. Neurosci. 1992;12(11):4427–4436. doi: 10.1523/JNEUROSCI.12-11-04427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ghaleiha A, Asadabadi M, Mohammadi MR, Shahei M, Tabrizi M, Hajiaghaee R, Akhonzhadeh S. Memantine as adjunctive treatment to risperidone in children with autistic disorder: a randomized double-blind placebo-controlled trial. Int. J. Neuropsychopharmacol. 2013;16(4):783–789. doi: 10.1017/S1461145712000880. [DOI] [PubMed] [Google Scholar]
- 119.Chow M, Parmparo T, Winn M, Barnes C, Li H, Weiss L, Fan J. Murray, S. April, C. Belinson, H. Fu, X. Wynshaw-Boris A, Schork N, Courchesne E. Age-dependent brain gene expression and copy number anomalies in autism suggest distinc pathological processes at young versus mature ages. PloS Genet. 2012;8(3):e1002592. doi: 10.1371/journal.pgen.1002592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.O'Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, Levy R, Ko A, Lee C. Smith, J.D. Turner, E.H. Stanaway, I.B. Vernot, B. Malig M, Baker C, Reilly B, Akey JM, Borenstein E, Rieder MJ, Nickerson DA, Bernier R, Shendure J, Eichler EE. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485(7397):246–250. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yoo HJ, Cho IH, Park M, Yang SY, Kim SA. Family based association of GRIN2A and GRIN2B with Korean autism spectrum disorders. Neurosci. Lett. 2012;512(2):89–93. doi: 10.1016/j.neulet.2012.01.061. [DOI] [PubMed] [Google Scholar]
- 122.Barnby G, Abbott A, Sykes N, Morris A, Weeks DE, Mott R, Lamb J, Bailey AJ, Monaco AP. International Molecular Genetics Study of Autism Consortium.Candidate-gene screening and association analysis at the autism-susceptibility locus on chromosome 16p: evidence of association at GRIN2A and ABAT. Am. J. Hum. Genet. 2005;76(6):950–966. doi: 10.1086/430454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tarabeux J, Kebir O, Gauthier J, Hamdan FF, Xiong L, Piton A, Spiegelman D, Henrion É, Millet B, Fathalli F, Joober R, Rapoport JL, DeLisi L.E. Fombonne, É. Mottron, L. Forget-Dubois, N. Boivin, M. Michaud, J.L. Drapeau, P. Lafrenière, R , Rouleau GA, Krebs MO. Rare mutations in N-methyl-D-aspartate glutamate receptors in autism spectrum disorders and schizophrenia. Transl. Psychiatry. 2011;1:e55. doi: 10.1038/tp.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Endele S, Rosenberger G, Geider K, Popp B, Tamer C, Stefanova I, Milh M, Kortüm F, Fritsch A, Pientka FK, Hellenbroich Y, Kalscheuer VM, Kohlhase J, Moog U, Rappold G, Rauch A, Ropers HH, von Spiczak S, Tönnies H, Villeneuve N, Villard L, Zabel B, Zenker M, Laube B, Reis A, Wieczorek D, Van Maldergem L, Kutsche K. Mutations in GRIN2A and GRIN2B encoding regulatory subunits of NMDA receptors cause variable neurodevelopmental phenotypes. Nat. Genet. 2010;42(11):1021–1026. doi: 10.1038/ng.677. [DOI] [PubMed] [Google Scholar]
- 125.Ramanathan S, Woodroffe A, Flodman PL, Mays LZ, Hanouni M, Modahl CB, Steinberg-Epstein R, Bocian ME, Spence MA, Smith M. A case of autism with an interstitial deletion on 4q leading to hemizygosity for genes encoding for glutamine and glycine neurotransmitter receptor sub-units (AMPA 2 GLRA3 GLRB) and neuropeptide receptors. NPY1R NPY5R. BMC Med. Genet. 2004;5:10. doi: 10.1186/1471-2350-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jamain S, Betancur C, Quach H, Philippe A, Fellous M, Giros B, Gillberg C, Leboyer M, Bourgeron T. Linkage and association of the glutamate receptor 6 gene with autism. Mol. Psychiatry. 2002;7(3):302–310. doi: 10.1038/sj.mp.4000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shuang M, Liu J, Jia MX, Yang JZ, Wu SP, Gong XH, Ling YS, Ruan Y, Yang XL, Zhang D. Family-based association study between autism and glutamate receptor 6 gene in Chinese Han trios. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2004;131B(1):48–50. doi: 10.1002/ajmg.b.30025. [DOI] [PubMed] [Google Scholar]
- 128.Motazacker MM, Rost BR, Hucho T, Garshasbi M, Kahrizi K, Ullmann R, Abedini SS, Nieh SE, Amini SH, Goswami C, Tzschach A, Jensen LR, Schmitz D, Ropers HH, Najmabadi H, Kuss AW. A defect in the ionotropic glutamate receptor 6 gene (GRIK2) is associated with autosomal recessive mental retardation. Am. J. Hum. Genet. 2007;81(4):792–798. doi: 10.1086/521275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ramoz N, Reichert JG, Smith CJ, Silverman JM, Bespalova IN, Davis KL, Buxbaum JD. Linkage and association of the mitochondrial aspartate/glutamate carrier SLC25A12 gene with autism. Am. J. Psychiat. 2004;161(4):662–669. doi: 10.1176/appi.ajp.161.4.662. [DOI] [PubMed] [Google Scholar]
- 130.Szatmari P, Paterson AD, Zwaigenbaum L, Roberts W, Brian J. Autism Genome Project Consortium; Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat. Genet. 2007;39(3):319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mejias R, Adamczyk A, Anggono V, Niranjan T, Thomas GM, Sharma K, Skinner C, Schwartz CE, Stevenson RE, Fallin MD, Kaufman W, Pletnikov M, Valle D, Huganir RL, Wang T. Gain-of-function glutamate receptor interacting protein 1 variants alter GluA2 recycling and surface distribution in patients with autism. Proc. Natl. Acad. Sci. U.S.A. 2011;108(12):4920–4925. doi: 10.1073/pnas.1102233108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gauthier J, Spiegelman D, Piton A, Lafreniere RG, Laurent S, St-Onge J. Novel de novo SHANK3 mutation in autistic patients. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2009;150B(3):421–424. doi: 10.1002/ajmg.b.30822. [DOI] [PubMed] [Google Scholar]
- 133.Moessner R, Marshall CR, Sutcliffe JS, Skaug J, Pinto D, Vincent J, Zwaigenbaum L, Fernandez B, Roberts W, Szatmari P, Scherer SW. Contribution of SHANK3 mutations to autism spectrum disorder. Am. J. Hum. Genet. 2007;81(6):1289–1297. doi: 10.1086/522590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chilian B, Abdollahpour H, Bierhals T, Haltrich I, Fekete G, Nagel I, Rosenberger G, Kutsche K. Dysfunction of SHANK2 and CHRNA7 in a patient with intellectual disability and language impairment supports genetic epistasis of the two loci. Clin. Genet. 2013 doi: 10.1111/cge.12105. [DOI] [PubMed] [Google Scholar]
- 135.Bucan M, Abrahams BS, Wang K, Glessner JT, Herman EI, Sonnenblick LI, Alvarez R, A.I. Imielinski, M. Hadley, D. Bradfield, J.P. Kim, C. Gidaya, N.B. Lindquist, I. Hutman, T. Sigman, M. Kustanovich, V. Lajonchere, C.M. Singleton, A. Kim, J. Wassink, T.H. McMahon, W.M. Owley, T. Sweeney, J.A. Coon H, Nurnberger JI, Li M, Cantor RM, Minshew NJ, Sutcliffe JS, Cook EH, Dawson G, Buxbaum JD, Grant SF, Schellenberg GD, Geschwind DH, Hakonarson H. Genome-wide analyses of exonic copy number variants in a family-based study point to novel autism susceptibility genes. PLoS Genet. 2009;5(6):e1000536. doi: 10.1371/journal.pgen.1000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nurmi EL, Bradford Y, Chen Y, Hall J, Arnone B, Gardiner MB, Hutcheson HB, Gilbert JR, Pericak-Vance MA, Copeland-Yates SA, Michaelis RC, Wassink TH, Santangelo SL, Sheffield VC, Piven J, Folstein S.E. Haines JL, Sutcliffe JS. Linkage disequilibrium at the Angelman syndrome gene UBE3A in autism families. Genomics. 2001;77(1-2):105–113. doi: 10.1006/geno.2001.6617. [DOI] [PubMed] [Google Scholar]
- 137.Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T. Paris International Autism Sibpair Study.Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat. Genet. 2003;34(1):27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Laumonnier F, Bonnet-Brilhault F, Gomot M, Blanc R, David A, Moizard MP, Raynaud M. Ronche, N. Calvas, P. Laudier, B. Chelly, J. Fryns, J.P; Ropers HH, Hamel BC, Andres C, Barthelemy C, Moraine C, Briault S. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene a member of the neuroligin family. Am. J. Hum. Genet. 2004;74(3):552–557. doi: 10.1086/382137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yan J, Oliveira G, Coutinho A, Yang C, Feng J, Katz C, Sram J, Bockholt A, Jones IR, Craddock N, Cook EH, Vicente A, Sommer SS. Analysis of the neuroligin 3 and 4 genes in autism and other neuropsychiatric patients. Mol. Psychiatry. 2005;10(4):329–332. doi: 10.1038/sj.mp.4001629. [DOI] [PubMed] [Google Scholar]
- 140.Talebizadeh Z, Lam DY, Theodoro MF, Bittel DC, Lushington GH, Butler MG. Novel splice isoforms for NLGN3 and NLGN4 with possible implications in autism. J. Med. Genet. 2006;43(5):e21. doi: 10.1136/jmg.2005.036897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lawson-Yuen A, Saldivar JS, Sommer S, Picker J. Familial deletion within NLGNGN4 associated with autism and Tourette syndrome. Eur. J. Hum. Genet. 2008;16(5):614–618. doi: 10.1038/sj.ejhg.5202006. [DOI] [PubMed] [Google Scholar]
- 142.Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455(7215):903–11. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pampanos A, Volaki K, Kanavakis E, Papandreou O, Youroukos S, Thomaidis L, Karkelis S, Tzetis M, Kitsiou-Tzeli S. A substitution involving the NLGN4 gene associated with autistic behavior in the Greek population. Genet. Test. Mol. Biomarkers. 2009;13(5):611–615. doi: 10.1089/gtmb.2009.0005. [DOI] [PubMed] [Google Scholar]
- 144.Daoud H, Bonnet-Brilhault F, Vedrine S, Demattei MV, Vourc'h P, Bayou N, Andres CR, Barthelemy C, Laumonnier F, Briault S. Autism and nonsyndromic mental retardation associated with a de novo mutation in the NLGN4X gene promoter causing an increased expression level. Biol. Psychiatry. 2009;66(10):906–910. doi: 10.1016/j.biopsych.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 145.Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L, Anagnostou E, Wasserman S. Oxytocin increases retention of social cognition in autism. Biol. Psychiatry. 2007;61(4):498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 146.Anagnostou E, Soorya L, Chaplin W, Bartz J, Halpern D, Wasserman S, Wang AT, Pepa L, Tanel N, Kushki A, Hollander E. Intranasal oxytocin versus placebo in the treatment of adults with autism spectrum disorders: a randomized controlled trial. Mol. Autism. 2012;3(1):16. doi: 10.1186/2040-2392-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ninan I. Oxytocin suppresses basal glutamatergic transmission but facilitates activity-dependent synaptic potentiation in the medial prefrontal cortex. J. Neurochem. 2011;119(2):324–331. doi: 10.1111/j.1471-4159.2011.07430.x. [DOI] [PubMed] [Google Scholar]
- 148.Hardan AY, Jou RJ, Handen BL. A retrospective assessment of topiramate in children and adolescents with pervasive developmental disorders. J. Child Adolesc. Psychopharmacol. 2004;14(3):426–432. doi: 10.1089/cap.2004.14.426. [DOI] [PubMed] [Google Scholar]
- 149.Rezaei V, Mohammadi MR, Ghanizadeh A, Sahraian A, Tabrizi M, Rezazadeh SA, Akhonzhadeh S. Double-blind placebo-controlled trial of risperidone plus topiramate in children with autistic disorder. Prog. Neuro-psychopharmacol. Biol. Psychiatry. 2010;34(7):1269–1272. doi: 10.1016/j.pnpbp.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 150.Doyle CA, McDougle CJ. Pharmacologic treatments for the behavioral symptoms associated with autism spectrum disorders across the lifespan. Dialog. Clin. Neurosci. 2012;14(3):263–279. doi: 10.31887/DCNS.2012.14.3/cdoyle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Berlin HA, Koran LM, Jenike MA, Shapira NA, Chaplin W, Pallanti S, Hollander E. Double-blind placebo-controlled trial of topiramate augmentation in treatment-resistant obsessive-compulsive disorder. J. Clin. Psychiatry. 2011;72(5):716–721. doi: 10.4088/JCP.09m05266gre. [DOI] [PubMed] [Google Scholar]
- 152.Erickson CA, Posey DJ, Stigler KA, Mullett J, Katschke AR, McDougle CJ. A retrospective study of memantine in children and adolescents with pervasive developmental disorders. Psychopharmacology. 2007;191(1):141–147. doi: 10.1007/s00213-006-0518-9. [DOI] [PubMed] [Google Scholar]
- 153.Owley T, Salt J, Guter S, Grieve A, Walton L, Ayuyao N, Leventhal BL, Cook EH. A prospective, open-label trial of memantine in the treatment of cognitive behavioral and memory dysfunction in pervasive developmental disorders. J. Child Adolesc. Psychopharmacol. 2006;16(5):517–524. doi: 10.1089/cap.2006.16.517. [DOI] [PubMed] [Google Scholar]
- 154.Chez MG, Burton Q, Dowling T, Chang M, Khanna P, Kramer C. Memantine as adjunctive therapy in children diagnosed with autistic spectrum disorders: an observation of initial clinical response and maintenance tolerability. J. Child Neurol. 2007;22(5):574–579. doi: 10.1177/0883073807302611. [DOI] [PubMed] [Google Scholar]
- 155.Reiser G, Binmoller FJ, Koch R. Memantine (1-amino-3b 5-dimethyladamantane) blocks the serotonin-induced depolarization response in a neuronal cell line. Brain Res. 1988;443(1-2):338–344. doi: 10.1016/0006-8993(88)91630-7. [DOI] [PubMed] [Google Scholar]
- 156.Rammes G, Rupprecht R, Ferrari U, Zieglgansberger W, Parsons CG. The N-methyl-D-aspartate receptor channel blockers memantine, MRZ 2/579 and other amino-alkyl-cyclohexanes antagonise 5-HT(3) receptor currents in cultured HEK-293 and N1E-115 cell systems in a non-competitive manner. Neurosci. Lett. 2001;306(1-2):81–84. doi: 10.1016/s0304-3940(01)01872-9. [DOI] [PubMed] [Google Scholar]
- 157.Becker B, Klein EM, Striepens N, Mihov Y, Schlaepfer TE, Reul J, Goosens L, Schruers K, Kendrick KM, Hurlemann R. Nicotinic Acetylcholine Receptors Contribute to Learning-induced Metaplasticity in the Hippocampus. J. Cogn. Neurosci. 2013;25(7):986–997. doi: 10.1162/jocn_a_00383. [DOI] [PubMed] [Google Scholar]
- 158.Seeman P, Caruso C, Lasaga M. Memantine agonist action at dopamine D2High receptors. Synapse. 2008;62(2):149–153. doi: 10.1002/syn.20472. [DOI] [PubMed] [Google Scholar]
- 159.Blanpied TA, Clarke RJ, Johnson JW. Amantadine inhibits NMDA receptors by accelerating channel closure during channel block. J. Neurosci. 2005;25(13):3312–3322. doi: 10.1523/JNEUROSCI.4262-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.King BH, Wright DM, Handen BL, Sikich L, Zimmerman AW, McMahon W, Cantwell E, Davanzo PA, Dourish CT, Dykens EM, Hooper SR, Jaselskis CA, Leventhal BL, Levitt J, Lord C, Lubetsky MJ, Myers SM, Ozonoff S, Shah BG, Snape M, Shernoff EW, Williamson K, Cook EH Jr. Double-blind, placebo-controlled study of amantadine hydrochloride in the treatment of children with autistic disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40(6):658–665. doi: 10.1097/00004583-200106000-00010. [DOI] [PubMed] [Google Scholar]
- 161.Mann K, Kiefer F, Spanagel R, Littleton J. Acamprosate: recent findings and future research directions. Alcohol. Clin. Exp. Res. 2008;32(7):1105–1110. doi: 10.1111/j.1530-0277.2008.00690.x. [DOI] [PubMed] [Google Scholar]
- 162.Mason BJ, Crean R. Acamprosate in the treatment of alcohol dependence: clinical and economic considerations. Expert Rev. Neurother. 2007;7(11):1465–1477. doi: 10.1586/14737175.7.11.1465. [DOI] [PubMed] [Google Scholar]
- 163.Littleton JM. Acamprosate in alcohol dependence: implications of a unique mechanism of action. J. Addict. Med. 2007;1(3):115–125. doi: 10.1097/ADM.0b013e318156c26f. [DOI] [PubMed] [Google Scholar]
- 164.Erickson CA, Early M, Stigler KA, Wink LK, Mullett JE, McDougle CJ. An open-label naturalistic pilot study of acamprosate in youth with autistic disorder. J. Child Adolesc. Psychopharmacol. 2011;21(6):565–569. doi: 10.1089/cap.2011.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Erickson CA, Wink LK, Ray B, Early MC, Stiegelmeyer E, Mathieu-Frasier L, Patrick V, Lahiri DK, McDougle CJ. Impact of acamprosate on behavior and brain-derived neurotrophic factor: an open-label study in youth with fragile X syndrome. Psychopharmacology. 2013;228(1):75–84. doi: 10.1007/s00213-013-3022-z. [DOI] [PubMed] [Google Scholar]
- 166.Goff DC. D-cycloserine: an evolving role in learning and neuroplasticity in schizophrenia. Schizophr. Bull. 2012;38(5):936–941. doi: 10.1093/schbul/sbs012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Posey DJ, Kem DL, Swiezy NB, Sweeten TL, Wiegand RE, McDougle CJ. A pilot study of D-cycloserine in subjects with autistic disorder. Am. J. Psychiatry. 2004;61(11):2115–2117. doi: 10.1176/appi.ajp.161.11.2115. [DOI] [PubMed] [Google Scholar]
- 168.Purcell AE, Jeon OH, Zimmerman AW, Blue ME, Pevsner J. Postmortem brain abnormalities of the glutamate neuro- transmitter system in autism. Neurology. 2001;57(9):1618–28. doi: 10.1212/wnl.57.9.1618. [DOI] [PubMed] [Google Scholar]
- 169.Bernardi S, Anagnostou E, Shen J, Kolevzon A, Buxbaum JD, Hollander E, Hof PR, Fa J. In vivo 1H-magnetic resonance spectroscopy study of the attentional networks in autism. Brain Res. 2011;1380:198–205. doi: 10.1016/j.brainres.2010.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2(5):255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Li J, Pelletier MR, Perez V JL, Carlen PL. Reduced cortical synaptic plasticity and GluR1 expression associated with fragile X mental retardation protein deficiency. Mol. Cell. Neurosci. 2002;19(2):138–251. doi: 10.1006/mcne.2001.1085. [DOI] [PubMed] [Google Scholar]
- 172.Godfraind JM, Reyniers E, De Boulle K, D'Hooge R, De Deyn PP, Bakker CE, Oostra BA, Kooy RF, Willems PJ. Long-term potentiation in the hippocampus of fragile X knockout mice. Am. J. Med. Genet. 1996;64(2):246–251. doi: 10.1002/(SICI)1096-8628(19960809)64:2<246::AID-AJMG2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 173.Gocel J, Larson J. Synaptic NMDA receptor-mediated currents in anterior piriform cortex are reduced in the adult fragile X mouse. Neuroscience. 2012;221:170–181. doi: 10.1016/j.neuroscience.2012.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pilpel Y, Kolleker A, Berberich S, Ginger M, Frick A, Mientjes E, Oostra B, Seeburg PH. Synaptic ionotropic glutamate receptors and plasticity are developmentally altered in the CA1 field of Fmr1 knockout mice. J. Physiol. 2009;587 (Pt 4):787–804. doi: 10.1113/jphysiol.2008.160929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Larson J, Jessen RE, Kim D, Fine AK, du Hoffmann J. Age-dependent and selective impairment of long-term potentiation in the anterior piriform cortex of mice lacking the fragile X mental retardation protein. J. Neurosci. 2005;25(41) doi: 10.1523/JNEUROSCI.2638-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gantois I, Pop AS, de Esch CE, Buijsen RA, Pooters T, Gomez-Mancilla B, Gasparini F, Oostra BA, D'Hooge R, Willemsen R. Chronic administration of AFQ056/Mavoglurant restores social behaviour in Fmr1 knockout mice. Behav. Brain Res. 2013;239:72–79. doi: 10.1016/j.bbr.2012.10.059. [DOI] [PubMed] [Google Scholar]
- 177.Pop AS, Levenga J, de Esch CE, Buijsen RA, Nieuwenhuizen IM, Li T, Isaacs A, Gasparini F, Oostra B, Willemsen R. Rescue of dendritic spine phenotype in Fmr1 KO mice with the mGluR5 antagonist AFQ056/Mavoglurant. Psycho- pharmacology. 2012 doi: 10.1007/s00213-012-2947-y. [DOI] [PubMed] [Google Scholar]
- 178.Michalon A, Sidorov M, Ballard TM, Ozmen L, Spooren W, Wettstein JG, Jaeschke G, Bear MF, Lindemann L. Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice. Neuron. 2012;74(1):49–56. doi: 10.1016/j.neuron.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Levenga J, Hayashi S, de Vrij FM, Koekkoek SK, van der Linde HC, Nieuwenhuizen I, Song C, Buisen RA, Pop AS, Gomez-Mancilla B, Nelson DL, Willemsen R, Gasparini F, Osstra BA. AFQ056 a new mGluR5 antagonist for treatment of fragile X syndrome. Neurobiol. Dis. 2011;42(3):311–317. doi: 10.1016/j.nbd.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 180.de Vrij FM, Levenga J, van der Linde HC, Koekkoek SK, De Zeeuw CI, Nelson DL, Oostra BA, Willemsen R. Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiol. Dis. 2008;31(1):127–132. doi: 10.1016/j.nbd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Silverman JL, olu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsycho- pharmacology. 2010;35(4):976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Suvrathan A, Hoeffer CA, Wong H, Klann E, Chattarji S. Characterization and reversal of synaptic defects in the amygdala in a mouse model of fragile X syndrome. Proc. Natl. Acad. Sci. U S A . 2010;107(25):11591–11596. doi: 10.1073/pnas.1002262107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wei H, Dobkin C, Sheikh AM, Malik M, Brown WT, Li X. The therapeutic effect of memantine through the stimulation of synapse formation and dendritic spine maturation in autism and fragile X syndrome. PloS One. 2012;7(5):e36981. doi: 10.1371/journal.pone.0036981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gambrill AC, Barria A. NMDA receptor subunit composition controls synaptogenesis and synapse stabilization. Proc. Natl. Acad. Sci. U S A . 2011;108(14):5855–5860. doi: 10.1073/pnas.1012676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48(2):289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 186.Wang H, Wu LJ, Kim S.S. Lee, F.J. Gong, B. Toyoda, H. Ren, M. Shang, Y.Z. Xu H, Liu F, Zhao MG, Zhuo M. FMRP acts as a key messenger for dopamine modulation in the forebrain. Neuron. 2008;59(4):634–47. doi: 10.1016/j.neuron.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 187.Xu ZH, Yang Q, Feng B, Liu SB, Zhang N, Xing JH, Li XQ, Wu YM, Gao GD, Zhao MG. Group I mGluR antagonist rescues the deficit of D1-induced LTP in a mouse model of fragile X syndrome. Mol. Neurodegener. 2012;7:24. doi: 10.1186/1750-1326-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Krueger DD, Osterweil EK, Chen SP, Tye LD, Bear MF. Cognitive dysfunction and prefrontal synaptic abnormalities in a mouse model of fragile X syndrome. Proc.Natl. Acad. Sci. USA. 2011;108(6):2587–92. doi: 10.1073/pnas.1013855108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yun SH, Trommer BL. Fragile X mice: reduced long- term potentiation and N-Methyl-D-Aspartate receptor-mediated neurotransmission in dentate gyrus. J. Neurosci. Res. 2011;89(2):176–182. doi: 10.1002/jnr.22546. [DOI] [PubMed] [Google Scholar]
- 190.Mientjes EJ, Nieuwenhuizen I, Kirkpatrick L, Zu T, Hoogeveen-Westerveld M, Severijnen L, Rife M, Willemsen R, Nelson DL, Oostra BA. The generation of a conditional Fmr1 knock out mouse model to study Fmrp function in vivo. Neurobiol. Dis. 2006;21(3):549–555. doi: 10.1016/j.nbd.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 191.Kooy RF, D'Hooge R, Reyniers E, Bakker CE, Nagels G, De Boulle K, Storm K, Clincke G, De Deyn PP, Oostra BA, Willems PJ. Transgenic mouse model for the fragile X syndrome. Am. J. Med. Genet. 1996;64(2):241–245. doi: 10.1002/(SICI)1096-8628(19960809)64:2<241::AID-AJMG1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 192.Edbauer D, Neilson JR, Foster KA, Wang CF, Seeburg DP, Batterton MN, Tada T, Dolan BM, Sharp PA, Sheng M. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron. 2010;65(3):373–384. doi: 10.1016/j.neuron.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhu T, Uzunova G, Cohen IJ, Musnier A, D'Hulst C, Ng M, Drouet V, Dictenberg J. Altered early synaptic glutamate receptor expression through FMRP-dependent translational CAM mRNA regulation. Society for Neuroscience Annual Meeting October 13-17 New Orleans LA. 2012 [Google Scholar]
- 194.Hu H, Qin Y, Bochorishvili G, Zhu Y, van Aelst L, Zhu JJ. Ras signaling mechanisms underlying impaired GluR1-dependent plasticity associated with fragile X syndrome. J. Neurosci. 2008;28(31):7847–7862. doi: 10.1523/JNEUROSCI.1496-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kaufmann WE, Abrams MT, Chen W, Reiss AL. Genotype, molecular phenotype, and cognitive phenotype: correlations in fragile X syndrome. Am. J. Med. Genet. 1999;83(4):286–295. [PubMed] [Google Scholar]
- 196.Hagerman R, Au J, Hagerman P. FMR1 premutation and full mutation molecular mechanisms related to autism. J. Neurodev. Disord. 2011;3(3):211–224. doi: 10.1007/s11689-011-9084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hessl D, Wang JM, Schneider A, Koldewyn K, Le L, Iwahashi C, Cheung K, Tassone F. Hagerman PJ, Rivera SM. Decreased fragile X mental retardation protein expression underlies amygdala dysfunction in carriers of the fragile X premutation. Biol. Psychiatry. 2011;70(9):859–865. doi: 10.1016/j.biopsych.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kron M, Howell CJ, Adams IT, Ransbottom M, Christian D, Ogier M, Katz DM. Brain activity mapping in Mecp2 mutant mice reveals functional deficits in forebrain circuits including key nodes in the default mode network that are reversed with ketamine treatment. J. Neurosci. 2012;32(40):13860–13872. doi: 10.1523/JNEUROSCI.2159-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nguyen MV, Du F, Felice C.A. Shan, X. Nigam, A. Mandel, G. Robinson, J.K. Ballas N. MeCP2 is critical for maintaining mature neuronal networks and global brain anatomy during late stages of postnatal brain development and in the mature adult brain. J. Neurosci. 2012;32(29):10021–10034. doi: 10.1523/JNEUROSCI.1316-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bateup HS, Takasaki KT, Saulnier JL, Denefrio CL, Sabatini BL. Loss of Tsc1 in vivo impairs hippocampal mGluR-LTD and increases excitatory synaptic function. J. Neurosci. 2011;31(24):8862–8869. doi: 10.1523/JNEUROSCI.1617-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pfeiffer BE, Huber KM. The state of synapses in fragile X syndrome. Neuroscientist. 2009;15(5):549–567. doi: 10.1177/1073858409333075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jacome LF, Burket JA, Herndon AL, Deutsch SI. Genetically inbred Balb/c mice differ from outbred Swiss Webster mice on discrete measures of sociability: relevance to a genetic mouse model of autism spectrum disorders. Autism Res. 2011;4(6):393–400. doi: 10.1002/aur.218. [DOI] [PubMed] [Google Scholar]
- 203.Deutsch SI, Pepe GJ, Burket JA, Winebarger EE, Herndon AL, Benson AD. D-cycloserine improves sociability and spontaneous stereotypic behaviors in 4-week old mice. Brain Res. 2012;1439:96–107. doi: 10.1016/j.brainres.2011.12.040. [DOI] [PubMed] [Google Scholar]
- 204.Burket JA, Herndon AL, Winebarger EE, Jacome LF, Deutsch SI. Complex effects of mGluR5 antagonism on sociability and stereotypic behaviors in mice: possible implications for the pharmacotherapy of autism spectrum disorders. Brain Res. Bull. 2011;86(3-4):152–158. doi: 10.1016/j.brainresbull.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 205.Saunders JA, Gandal MJ, Roberts TP, Siegel SJ. NMDA antagonist MK801 recreates auditory electrophysiology disruption present in autism and other neurodevelopmental disorders. Behav. Brain Res. 2012;234(2):233–237. doi: 10.1016/j.bbr.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gandal MJ, Sisti J, Klook K, Ortinski PI, Leitman V, Liang Y, Thieu T, Anderson R, Pierce RC, Jonak G, Gur RE, Carlson G, Siegel SJ. GABAB-mediated rescue of altered excitatory-inhibitory balance, gamma synchrony and behavioral deficits following constitutive NMDAR-hypofunction. Transl. Psychiatry. 2012;2:e142. doi: 10.1038/tp.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Gandal MJ, Anderson RL, Billingslea EN, Carlson GC, Roberts TP, Siegel SJ. Mice with reduced NMDA receptor expression: more consistent with autism than schizophrenia?. Genes Brain Behav. 2012;11(6):740–750. doi: 10.1111/j.1601-183X.2012.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Saunders JA, Tatard-Leitman VM, Suh J, Billingslea EN, Roberts TP, Siegel SJ. Knockout of NMDA Receptors in Parvalbumin Interneurons Recreates Autism-Like Phenotypes. Autism Res. 2013 doi: 10.1002/aur.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Henderson C, Wijetunge L, Kinoshita MN, Shumway M, Hammond RS, Postma FR, Brynzska C, Rush R, Thomas A, Paylor A, Warren ST, Vanderklish PW, Kind PC, Carpenter RL, Bear MF, Healy AM. Reversal of disease-related pathologies in the fragile X mouse model by selective activation of GABA(B) receptors with arbaclofen. Science Transl. Med. 2012;4(152):152ra28. doi: 10.1126/scitranslmed.3004218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hagerman R, Lauterborn J, Au J, Berry-Kravis E. Fragile X syndrome and targeted treatment trials. Res. Probl. Cell Differ. 2012;54:297–335. doi: 10.1007/978-3-642-21649-7_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Corbett BA, Schupp CW, Lanni KE. Comparing biobehavioral profiles across two social stress paradigms in children with and without autism spectrum disorders. Mol. Autism. 2012;3(1):3. doi: 10.1186/2040-2392-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Anderson CJ, Colombo J, Unruh KE. Pupil and salivary indicators of autonomic dysfunction in autism spectrum disorder. Dev. Psychobiol. 2013;55(5):465–482. doi: 10.1002/dev.21051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wisbeck JM, Huffman LC, Freund L, Gunnar MR, Davis EP, Reiss AL. Cortisol and social stressors in children with fragile X: a pilot study. J. Dev. Behav. Pediatr. 2000;21(4):278–282. doi: 10.1097/00004703-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 214.Hessl D, Glaser B, Dyer-Friedman J, Blasey C, Hastie T, Gunnar M, Reiss AL. Cortisol and behavior in fragile X syndrome. Psychoneuroendocrinology. 2002;27(7):855–872. doi: 10.1016/s0306-4530(01)00087-7. [DOI] [PubMed] [Google Scholar]
- 215.Markham JA, Beckel-Mitchener AC, Estrada CM, Greenough WT. Corticosterone response to acute stress in a mouse model of Fragile X syndrome. Psychoneuroendocrinology. 2006;31(6):781–785. doi: 10.1016/j.psyneuen.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 216.Qin M, Entezam A, Usdin K, Huang T, Liu ZH, Hoffman GE, Smith CB. A mouse model of the fragile X premutation: effects on behavior dendrite morphology and regional rates of cerebral protein synthesis. Neurobiol. Dis. 2011;42(1):85–98. doi: 10.1016/j.nbd.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Caudal D, Godsil BP, Mailliet F, Bergerot D, Jay TM. Acute stress induces contrasting changes in AMPA receptor subunit phosphorylation within the prefrontal cortex, amygdala and hippocampus. PloS One. 2010;5(12):e15282. doi: 10.1371/journal.pone.0015282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Schmidt MV, Trumbach D, Weber P, Wagner K, Scharf SH, Liebl C, Datson N, Namendorf C, Gerlach T, Kuhne C, Uhr M, Deussing JM, Wurst W, Binder EB, Holsboer F, Muller MB. Individual stress vulnerability is predicted by short-term memory and AMPA receptor subunit ratio in the hippocampus. J. Neurosci. 2010;30(50):16949–16958. doi: 10.1523/JNEUROSCI.4668-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Fumagalli F, Caffino L, Vogt MA, Frasca A, Racagni G, Sprengel R, Gass P, Riva M. AMPA GluR-A receptor subunit mediates hippocampal responsiveness in mice exposed to stress. Hippocampus. 2011;21(9):1028–1035. doi: 10.1002/hipo.20817. [DOI] [PubMed] [Google Scholar]
- 220.Conboy L, Sandi C. Stress at learning facilitates memory formation by regulating AMPA receptor trafficking through a glucocorticoid action. Neuropsychopharmacology. 2010;35(3):674–685. doi: 10.1038/npp.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Martin , Henley JM, Holman D, Zhou M, Wiegert O, van Spronsen M, Joels M, Hoogenraad CC, Krugers HJ. Corticosterone alters AMPAR mobility and facilitates bidirectional synaptic plasticity. PloS One. 2009;4(3):5. doi: 10.1371/journal.pone.0004714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hazlett HC, Poe MD, Lightbody AA, Styner M, MacFall JR, Reiss AL, Piven J. Trajectories of early brain volume development in fragile X syndrome and autism. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51(9):921–33. doi: 10.1016/j.jaac.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Steinlin M. The cerebellum in cognitive processes: supporting studies in children. Cerebellum. 2007;6(3):237–241. doi: 10.1080/14734220701344507. [DOI] [PubMed] [Google Scholar]
- 224.Huber KM. The fragile X-cerebellum connection. Trends Neurosci. 2006;29(4):183–185. doi: 10.1016/j.tins.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 225.Giza J, Urbanski MJ, Prestori F, Bandyopadhyay B, Yam A, Friedrich V, Kelley K, D'Angelo E, Goldfarb M. Behavioral and cerebellar transmission deficits in mice lacking the autism-linked gene islet brain-2. J. Neurosci. 2010;30(44):14805–14816. doi: 10.1523/JNEUROSCI.1161-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Safo PK, Regehr WG. Endocannabinoids control the induction of cerebellar LTD. Neuron. 2005;48(4):647–659. doi: 10.1016/j.neuron.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 227.Carey MR, Myoga MH, McDaniels KR, Marsicano G, Lutz B, Mackie K, Regher WG. Presynaptic CB1 receptors regulate synaptic plasticity at cerebellar parallel fiber synapses. J. Neurophysiology. 2011;105(2):958–963. doi: 10.1152/jn.00980.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27(7):370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 229.Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, Huganir RL, Linden DJ, Worley PF. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3. essential for mGluR-LTD. Neuron. 2008;59(1):70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Garber KB, Visootsak J, Warren ST. Fragile X syndrome. Eur. J. Hum. Genet. 2008;16(6):666–672. doi: 10.1038/ejhg.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Waung MW, Huber KM. Protein translation in synaptic plasticity: mGluR-LTD. Fragile X Curr. Opin. Neurobiol. 2009;19(3):319–326. doi: 10.1016/j.conb.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Luscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 2010;65(4):445–459. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Nakamoto M, Nalavadi V, Epstein MP, Narayanan U, Bassell GJ, Warren ST. Fragile X mental retardation protein deficiency leads to excessive mGluR5-dependent internalization of AMPA receptors. Proc. Natl. Acad. Sci. U S A. 2007;104(39):15537–15542. doi: 10.1073/pnas.0707484104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Nosyreva ED, Huber KM. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome. J. Neurophysiol. 2006;95(5):3291–3295. doi: 10.1152/jn.01316.2005. [DOI] [PubMed] [Google Scholar]
- 235.Davidkova G, Ng M, Wu H, Drouet V, D'Hulst C, Dictenberg G. Altered activity-induced neuroligin translation and synapse homeostasis in Fragile X syndrome Society for Neuroscience Annual Meeting. November 12-16 Washington D C . 2011 [Google Scholar]
- 236.Isaac JT, Ashby MC, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54(6):859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 237.Zhang Y, Venkitaramani DV, Gladding CM, Zhang Y, Kurup P, Molnar E, Collingridge GL, Lombroso PJ. The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. J. Neurosci. 2008;28(42):10561–10566. doi: 10.1523/JNEUROSCI.2666-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron. 2006;51(4):441–454. doi: 10.1016/j.neuron.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 239.Lu R, Wang H, Liang Z, Ku L, O'Donnell WT, Li W, Warren ST, Feng Y. The fragile X protein controls microtubule-associated protein 1B translation and microtubule stability in brain neuron development. Proc. Natl. Acad. Sci. U S A . 2004;101(42):15201–15206. doi: 10.1073/pnas.0404995101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Johnson MA, Lombroso PJ. A common STEP in the synaptic pathology of diverse neuropsychiatric disorders. Yale J. Biol.Med. 2012;85(4):481–490. [PMC free article] [PubMed] [Google Scholar]
- 241.Goebel-Goody SM, Baum M, Paspalas CD, Fernandez SM, Carty NC, Kurup P, Lombroso PJ. Therapeutic implications for striatal-enriched protein tyrosine phosphatase (STEP) in neuropsychiatric disorders. Pharmacol. Rev. 2012;64(1):65–87. doi: 10.1124/pr.110.003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Goebel-Goody SM, Wilson-Wallis ED, Royston S, Tagliatela SM, Naegele JR, Lombroso PJ. Genetic manipulation of STEP reverses behavioral abnormalities in a fragile X syndrome mouse model. Genes Brain Behav. 2012;11(5):586–600. doi: 10.1111/j.1601-183X.2012.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Muddashetty RS, Kelic S, Gross C, Xu M, Bassell GJ. Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X syndrome. J. Neurosci. 2007;27(20):5338–5348. doi: 10.1523/JNEUROSCI.0937-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Nalavadi VC, Muddashetty RS, Gross C, Bassell GJ. Dephosphorylation-induced ubiquitination and degradation of FMRP in dendrites: a role in immediate early mGluR-stimulated translation. J. Neurosci. 2012;32(8):2582–2587. doi: 10.1523/JNEUROSCI.5057-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Greer PL, Hanayama R, Bloodgood BL, Mardinly AR, Lipton DM, Flavell SW, Kim TK, Griffith TC, .Waldon Z, Maehr R, Pleogh HL, Chowdhury S, Worley PF, Steen J, Greenberg ME. The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell. 2010;140(5):704–716. doi: 10.1016/j.cell.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Bateup HS, Johnson CA, Denefrio CL, Saulnier JL, Kornacker K, Sabatini BL. Excitatory/inhibitory synaptic imbalance leads to hippocampal hyperexcitability in mouse models of tuberous sclerosis. Neuron. 2013;78(3):510–522. doi: 10.1016/j.neuron.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007;30(3):126–134. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 248.Grooms SY, Opitz T, Bennett MV, Zukin RS. Status epilepticus decreases glutamate receptor 2 mRNA and protein expression in hippocampal pyramidal cells before neuronal death. Proc. Natl. Acad. Sci. U S A . 2000;97(7):3631–3636. doi: 10.1073/pnas.050586497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Hu Y, Jiang L, Chen H, Zhang X. Expression of AMPA receptor subunits in hippocampus after status convulsion. Child's Nerv. Syst. 2012;28(6):911–918. doi: 10.1007/s00381-012-1747-3. [DOI] [PubMed] [Google Scholar]
- 250.Rogawski MA. AMPA receptors as a molecular target in epilepsy therapy. Acta Neurol. Scand. Suppl. 2013;(197):9–18. doi: 10.1111/ane.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lanore F, Labrousse VF, Szabo Z, Normand E, Blanchet C, Mulle C. Deficits in morphofunctional maturation of hippocampal mossy fiber synapses in a mouse model of intellectual disability. J. Neurosci. 2012;32(49):17882–17893. doi: 10.1523/JNEUROSCI.2049-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Strutz-Seebohm N, Korniychuk G, Schwarz R, Baltaev R, Ureche ON, Mack AF, Ma ZL, Hollman M, Lang F, Seebohm G. Functional significance of the kainate receptor GluR6(M836I) mutation that is linked to autism. Cell. Physiol. Biochem. 2006;18(4-5):287–294. doi: 10.1159/000097675. [DOI] [PubMed] [Google Scholar]
- 253.Gauthier J, Siddiqui TJ, Huashan P, Yokomaku D, Hamdan FF, Champagne N, Lapointe M, Spiegelman D, Noreau A, Lafreniere RG, Fathalli F, Joober R, Krebs MO, DeLisi LE, Mottron L, Fombonne E, Michaud JL, Drapeau P, Carbonetto S, Craig AM, Rouleau GA. Truncating mutations in NRXN2 and NRXN1 in autism spectrum disorders and schizophrenia. Hum. Genet. 2011;130(4):563–73. doi: 10.1007/s00439-011-0975-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Sato D, Lionel AC, Leblond CS, Prasad A, Pinto D, Walker S, O'Connor I, Russel C, Drmic IE, Hamdan FF, Michaud JL, Endris V, Roeth R, Delorme , Huguet G, Leboyer M, Rastam M, Gillberg C, Lathrop M, Stavropolous DJ, Anagnostou E, Weksberg R, Fombonne E, Zwaigenbaum L, Fernandez BA, Roberts W, Rappold GA, Marshall CR, Bourgeron T, Szatmari P, Scherer SW. SHANK1 Deletions in Males with Autism Spectrum Disorder. Am. J. Hum. Genet. 2012;90(5):879–887. doi: 10.1016/j.ajhg.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Jiang YH, Ehlers MD. Modeling autism by SHANK gene mutations in mice. Neuron. 2013;78(1):8–27. doi: 10.1016/j.neuron.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Etherton MR, Tabuchi K, Sharma M, Ko J, Sudhof TC. An autism-associated point mutation in the neuroligin cytoplasmic tail selectively impairs.AMPA receptor-mediated synaptic transmission in hippocampus. EMBO J. 2011;30(14):2908–2919. doi: 10.1038/emboj.2011.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Etherton M, Foldy C, Sharma M, Tabuchi K, Liu X, Shamloo M, Malenka RC, Sudhof TC. Autism-linked neuroligin-3 R451C mutation differentially alters hippocampal and cortical synaptic function. Proc. Natl. Acad. Sci. U S A . 2011;108(33):13764–13769. doi: 10.1073/pnas.1111093108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307(5713):1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- 259.Soler-Llavina GJ, Fuccillo MV, Ko J, Sudhof TC, Malenka RC. The neurexin ligands, neuroligins and leucine-rich repeat transmembrane proteins, perform convergent and divergent synaptic functions in vivo. Proc. Natl. Acad. Sci. U S A. 2011;108(40):16502–16509. doi: 10.1073/pnas.1114028108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Siddiqui TJ, Pancaroglu R, Kang Y, Rooyakkers A, Craig AM. LRRTMs and neuroligins bind neurexins with a differential code to cooperate in glutamate synapse development. J. Neurosci. 2010;30(22):7495–7506. doi: 10.1523/JNEUROSCI.0470-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Ko J, Fuccillo MV, Malenka RC, Sudhof TC. LRRTM2 functions as a neurexin ligand in promoting excitatory synapse formation. Neuron. 2009;64(6):791–8. doi: 10.1016/j.neuron.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Fu Z, Vicini S. Neuroligin-2 accelerates GABAergic synapse maturation in cerebellar granule cells. Mol. Cell. Neurosci. 2009;42(1):45–55. doi: 10.1016/j.mcn.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Budreck EC, Scheiffele P. Neuroligin-3 is a neuronal adhesion protein at GABAergic and glutamatergic synapses. Eur. J. Neurosci. 2007;26(7):1738–1748. doi: 10.1111/j.1460-9568.2007.05842.x. [DOI] [PubMed] [Google Scholar]
- 264.Hoon M, Soykan T, Falkenburger B, Hammer M, Patrizi A, Schmidt KF, Sassoe-Pognetto M, Lowel S, Moser T, Taschenberger H, Brose N, Varoqueaux F. Neuroligin-4 is localized to glycinergic postsynapses and regulates inhibition in the retina. Proc. Natl. Acad. Sci. U S A. 2011;108(7):3053–3058. doi: 10.1073/pnas.1006946108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Baudouin SJ, Gaudias J, Gerharz S, Hatstatt L, Zhou K, Punnakkal P, Tanaka K.F. Spooren W, Hen R, De Zeewuv CI, Vogt K, Scheiffele P. Shared synaptic pathophysiology in syndromic and nonsyndromic rodent models of autism. Science. 2012;338(6103):128–132. doi: 10.1126/science.1224159. [DOI] [PubMed] [Google Scholar]
- 266.Nam CI, Chen L. Postsynaptic assembly induced by neurexin-neuroligin interaction and neurotransmitter. Proc. Natl. Acad. Sci. U.S.A. 2005;102(17):6137–6142. doi: 10.1073/pnas.0502038102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Heine M, Thoumine O, Mondin M, Tessier B, Giannone G, Choquet D. Activity-independent and subunit-specific recruitment of functional AMPA receptors at neurexin/neuroligin contacts. Proc. Natl. Acad. Sci. USA . 2008;105(52):20947–20952. doi: 10.1073/pnas.0804007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Mondin M, Labrousse V, Hosy E, Heine M, Tessier B, Levet F, Poujol C, Blanchet C, Choquet D, Thoumine O. Neurexin-neuroligin adhesions capture surface-diffusing AMPA receptors through PSD-95 scaffolds. J. Neurosci. 2011;31(38):13500–13515. doi: 10.1523/JNEUROSCI.6439-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Zeidan A, Ziv NE. Neuroligin-1 loss is associated with reduced tenacity of excitatory synapses. PloS One. 2012;7(7):e42314. doi: 10.1371/journal.pone.0042314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Ripley B, Otto S, Tiglio K, Williams ME, Ghosh A. Regulation of synaptic stability by AMPA receptor reverse signaling. Proc. Natl. Acad. Sci. USA. 2011;108(1):367–372. doi: 10.1073/pnas.1015163108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Shipman SL, Nicoll RA. A subtype-specific function for the extracellular domain of neuroligin 1 in hippocampal LTP. Neuron. 2012;76(2):309–16. doi: 10.1016/j.neuron.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Budreck EC, Kwon OB, Jung JH, Baudouin S, Thommen A, Kim HS, Fukazawa Y, Harada H, Tabuchi K, Shigemoto R, Scheiffele P, Kim JH. Neuroligin-1 controls synaptic abundance of NMDA-type glutamate receptors through extracellular coupling. Proc. Natl. Acad. Sci. USA. 2013;110(2):725–730. doi: 10.1073/pnas.1214718110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Dahlhaus R, Hines RM, Eadie BD, Kannangara TS, Hines DJ, Brown CE, Christie BR, El-Husseini A. Overexpression of the cell adhesion protein neuroligin-1 induces learning deficits and impairs synaptic plasticity by altering the ratio of excitation to inhibition in the hippocampus. Hippocampus. 2010;20(2):305–322. doi: 10.1002/hipo.20630. [DOI] [PubMed] [Google Scholar]
- 274.Dahlhaus R, El-Husseini A. Altered neuroligin expression is involved in social deficits in a mouse model of the fragile X syndrome. Behav. Brain Res. 2010;208(1):96–105. doi: 10.1016/j.bbr.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 275.Blundell J, Blaiss CA, Etherton MR, Espinosa F, Tabuchi K, Walz C, Bolliger MF, Sudhof TC, Powell CM. Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. J. Neurosci. 2010;30(6):2115–2129. doi: 10.1523/JNEUROSCI.4517-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Peixoto RT, Kunz PA, Kwon H, Mabb AM, Sabatini BL, Philpot BD, Ehlers MD. Transsynaptic signaling by activity-dependent cleavage of neuroligin-1. Neuron. 2012;76(2):396–409. doi: 10.1016/j.neuron.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Boeckers TM, Bockmann J, Kreutz MR, Gundelfinger ED. ProSAP/Shank proteins - a family of higher order organizing molecules of the postsynaptic density with an emerging role in human neurological disease. J. Neurochem. 2002;81(5):903–910. doi: 10.1046/j.1471-4159.2002.00931.x. [DOI] [PubMed] [Google Scholar]
- 278.Arons MH, Thynne CJ, Grabrucker AM, Li D, Schoen M, Cheyne JE, Boeckers TM, Montgomery JM, Garner CC. Autism-associated mutations in ProSAP2/Shank3 impair synaptic transmission and neurexin-neuroligin-mediated transsynaptic signaling. J. Neurosci. 2012;32(43):14966–14978. doi: 10.1523/JNEUROSCI.2215-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Schmeisser MJ, Ey E, Wegener S, Bockmann J, Stempel AV, Kuebler A. Janssen, A.L. Udvardi PT, Shiban E, Spiker C, Balschun D, Skryabin BV, Dieck St, Smalla KH, Montag D, Leblond CS, Faure , Tourquet N, Le Sourd AM, Toro R, Grabrucker AM, Shoichet SA, Schmitz D, Kreutz MR, Bourgeron T, Gundelfinger ED, Boeckers TM. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature. 2012;486(7402):256–260. doi: 10.1038/nature11015. [DOI] [PubMed] [Google Scholar]
- 280.Won H, Lee HR, Gee HY, Mah W, Kim JI, Lee J, Ha S, Chung C, Jung ES, Cho YS, Park SG, Lee JS, Lee K, Kim D, Bae YC, Kaang BK, Lee MG, Kim E. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature. 2012;486(7402):261–265. doi: 10.1038/nature11208. [DOI] [PubMed] [Google Scholar]
- 281.Gong X, Jiang YW, Zhang X, An Y, Zhang J, Wu Y, Wang J, Sun Y, Liu Y, Gao X, Shen Y, Wu X, Qiu Z, Jin L, Wu BL, Wang H. High proportion of 22q13 deletions and SHANK3 mutations in Chinese patients with intellectual disability. PloS One. 2012;7(4):e34739. doi: 10.1371/journal.pone.0034739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Phelan K, McDermid HE. The 22q13. Deletion Syndrome (Phelan-McDermid Syndrome). Mol. Syndromol. 2012;2(3-5):186–201. doi: 10.1159/000334260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Bozdagi O, Sakurai T, Papapetrou D, Wang X, Dickstein DL, Takahashi N, Kajiwara Y, Wang M, Katz AM, Scattoni ML, Harris MJ, Saxena R, Silverman JL, Crawley JN, Zhou Q, Hof PR, Buxbaum JD. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function social interaction and social communication. Mol. Autism. 2010;1(1):15. doi: 10.1186/2040-2392-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Wang X, McCoy PA, Rodriguiz RM, Pan Y, Je HS, Roberts A.C. Kim CJ, Berrios J, Colvin JS, Bousquet-Moore D, Lorenzo I, Wu G, Weinberg RJ, Ehlers MD, Philpot BD, Beaudet AL, Wetsel WC, Jiang YH. Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Hum. Mol. Genet. 2011;20(15):3093–3108. doi: 10.1093/hmg/ddr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Xia J, Chung HJ, Wihler C, Huganir RL, Linden DJ. Cerebellar long-term depression requires PKC-regulated inter- actions between GluR2/3 and PDZ domain-containing proteins. Neuron. 2000;28(2):499–510. doi: 10.1016/s0896-6273(00)00128-8. [DOI] [PubMed] [Google Scholar]
- 286.Marsden KC, Beattie JB, Friedenthal J, Carroll RC. NMDA receptor activation potentiates inhibitory transmission through GABA receptor-associated protein-dependent exocytosis of GABA(A) receptors. J. Neurosci. 2007;27(52):14326–14337. doi: 10.1523/JNEUROSCI.4433-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Ma DQ, Cuccaro ML, Jaworski JM, Haynes CS, Stephan DA, Parod J, Abramson RK, Wright HH, Gilbert JR, Haines JL, Pericak-vance MA. Dissecting the locus heterogeneity of l. Psychiatry. 2007;12(4):376–84. doi: 10.1038/sj.mp.4001927. [DOI] [PubMed] [Google Scholar]