Abstract
The NH2-terminal amino acid sequences of both structural glycoproteins of each of eight alphaviruses have been obtained. These sequences demonstrate that the alphaviruses are all closely related and have in all probability descended from a common ancestor. Cysteines are conserved as well as several other residues important for secondary structure, suggesting that the three-dimensional conformations of the alphavirus glycoproteins are conserved while considerable variation in the primary sequence has evolved. Secondary structure predictions based upon the amino acid sequences are consistent with this hypothesis. An evolutionary tree for these eight alphaviruses has been constructed from the amino acid sequence data and, at many positions in the sequence, the amino acids present in the ancestral glycoproteins have been deduced.
Full text
PDF

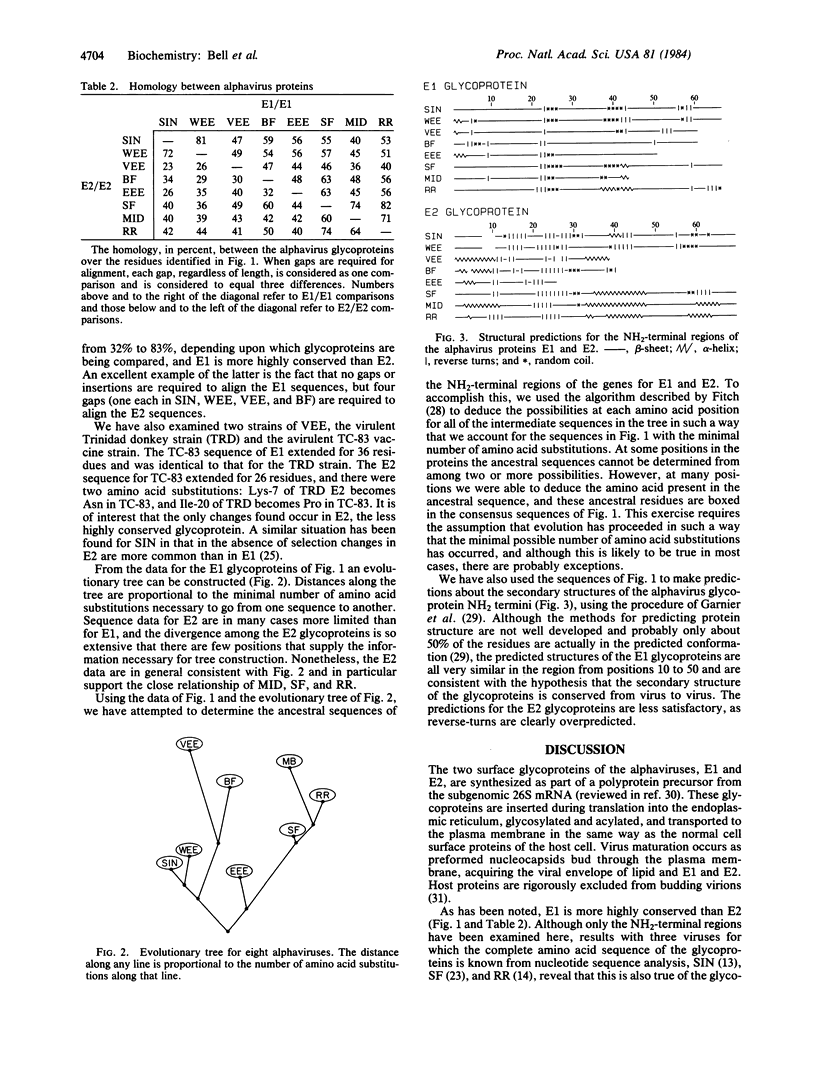

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Air G. M. Sequence relationships among the hemagglutinin genes of 12 subtypes of influenza A virus. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7639–7643. doi: 10.1073/pnas.78.12.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C., Bell J. R., Lenches E. M., Strauss E. G., Strauss J. H. Sequence analysis of two mutants of Sindbis virus defective in the intracellular transport of their glycoproteins. J Mol Biol. 1983 Jul 25;168(1):87–102. doi: 10.1016/s0022-2836(83)80324-6. [DOI] [PubMed] [Google Scholar]
- Bell J. R., Bond M. W., Hunkapiller M. W., Strauss E. G., Strauss J. H., Yamamoto K., Simizu B. Structural proteins of Western equine encephalitis virus: amino acid compositions and N-terminal sequences. J Virol. 1983 Feb;45(2):708–714. doi: 10.1128/jvi.45.2.708-714.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. R., Hunkapiller M. W., Hood L. E., Strauss J. H. Amino-terminal sequence analysis of the structural proteins of Sindbis virus. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2722–2726. doi: 10.1073/pnas.75.6.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. R., Strauss E. G., Strauss J. H. Purification and amino acid compositions of the structural proteins of sindbis virus. Virology. 1979 Sep;97(2):287–294. doi: 10.1016/0042-6822(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Calisher C. H., Shope R. E., Brandt W., Casals J., Karabatsos N., Murphy F. A., Tesh R. B., Wiebe M. E. Proposed antigenic classification of registered arboviruses I. Togaviridae, Alphavirus. Intervirology. 1980;14(5-6):229–232. doi: 10.1159/000149190. [DOI] [PubMed] [Google Scholar]
- Chanas A. C., Gould E. A., Clegg J. C., Varma M. G. Monoclonal antibodies to Sindbis virus glycoprotein E1 can neutralize, enhance infectivity, and independently inhibit haemagglutination or haemolysis. J Gen Virol. 1982 Jan;58(Pt 1):37–46. doi: 10.1099/0022-1317-58-1-37. [DOI] [PubMed] [Google Scholar]
- Dalgarno L., Rice C. M., Strauss J. H. Ross River virus 26 s RNA: complete nucleotide sequence and deduced sequence of the encoded structural proteins. Virology. 1983 Aug;129(1):170–187. doi: 10.1016/0042-6822(83)90404-x. [DOI] [PubMed] [Google Scholar]
- Dalgarno L., Short N. J., Hardy C. M., Bell J. R., Strauss J. H., Marshall I. D. Characterization of Barmah forest virus: an alphavirus with some unusual properties. Virology. 1984 Mar;133(2):416–426. doi: 10.1016/0042-6822(84)90407-0. [DOI] [PubMed] [Google Scholar]
- Dalrymple J. M., Schlesinger S., Russell P. K. Antigenic characterization of two sindbis envelope glycoproteins separated by isoelectric focusing. Virology. 1976 Jan;69(1):93–103. doi: 10.1016/0042-6822(76)90197-5. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Similar amino acid sequences: chance or common ancestry? Science. 1981 Oct 9;214(4517):149–159. doi: 10.1126/science.7280687. [DOI] [PubMed] [Google Scholar]
- Foulds L. R., Hendy M. D., Penny D. A graph theoretic approach to the development of minimal phylogenetic trees. J Mol Evol. 1979 Jul 18;13(2):127–149. doi: 10.1007/BF01732868. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Garoff H., Frischauf A. M., Simons K., Lehrach H., Delius H. Nucleotide sequence of cdna coding for Semliki Forest virus membrane glycoproteins. Nature. 1980 Nov 20;288(5788):236–241. doi: 10.1038/288236a0. [DOI] [PubMed] [Google Scholar]
- Helenius A., Fries E., Garoff H., Simons K. Solubilization of the Semliki Forest virus membrane with sodium deoxycholate. Biochim Biophys Acta. 1976 Jun 17;436(2):319–334. doi: 10.1016/0005-2736(76)90197-8. [DOI] [PubMed] [Google Scholar]
- Helenius A., Kartenbeck J., Simons K., Fries E. On the entry of Semliki forest virus into BHK-21 cells. J Cell Biol. 1980 Feb;84(2):404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Analysis of phenylthiohydantoins by ultrasensitive gradient high-performance liquid chromatography. Methods Enzymol. 1983;91:486–493. doi: 10.1016/s0076-6879(83)91045-5. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. New protein sequenator with increased sensitivity. Science. 1980 Feb 1;207(4430):523–525. doi: 10.1126/science.7352258. [DOI] [PubMed] [Google Scholar]
- Krystal M., Elliott R. M., Benz E. W., Jr, Young J. F., Palese P. Evolution of influenza A and B viruses: conservation of structural features in the hemagglutinin genes. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4800–4804. doi: 10.1073/pnas.79.15.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann E., Edwards J., Brown D. T. Polycaryocyte formation mediated by Sindbis virus glycoproteins. J Virol. 1983 Mar;45(3):1083–1089. doi: 10.1128/jvi.45.3.1083-1089.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall I. D., Woodroofe G. M., Hirsch S. Viruses recovered from mosquitoes and wildlife serum collected in the Murray Valley of South-eastern Australia, February 1974, during an epidemic of encephalitis. Aust J Exp Biol Med Sci. 1982 Oct;60(Pt 5):457–470. doi: 10.1038/icb.1982.51. [DOI] [PubMed] [Google Scholar]
- Ou J. H., Rice C. M., Dalgarno L., Strauss E. G., Strauss J. H. Sequence studies of several alphavirus genomic RNAs in the region containing the start of the subgenomic RNA. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5235–5239. doi: 10.1073/pnas.79.17.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J. H., Strauss E. G., Strauss J. H. The 5'-terminal sequences of the genomic RNAs of several alphaviruses. J Mol Biol. 1983 Jul 25;168(1):1–15. doi: 10.1016/s0022-2836(83)80319-2. [DOI] [PubMed] [Google Scholar]
- Rentier-Delrue F., Young N. A. Genomic divergence among Sindbis virus strains. Virology. 1980 Oct 15;106(1):59–70. doi: 10.1016/0042-6822(80)90221-4. [DOI] [PubMed] [Google Scholar]
- Rice C. M., Bell J. R., Hunkapiller M. W., Strauss E. G., Strauss J. H. Isolation and characterization of the hydrophobic COOH-terminal domains of the sindbis virion glycoproteins. J Mol Biol. 1982 Jan 15;154(2):355–378. doi: 10.1016/0022-2836(82)90069-9. [DOI] [PubMed] [Google Scholar]
- Rice C. M., Strauss J. H. Association of sindbis virion glycoproteins and their precursors. J Mol Biol. 1982 Jan 15;154(2):325–348. doi: 10.1016/0022-2836(82)90067-5. [DOI] [PubMed] [Google Scholar]
- Rice C. M., Strauss J. H. Nucleotide sequence of the 26S mRNA of Sindbis virus and deduced sequence of the encoded virus structural proteins. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2062–2066. doi: 10.1073/pnas.78.4.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrig J. T., Day J. W., Kinney R. M. Antigenic analysis of the surface glycoproteins of a Venezuelan equine encephalomyelitis virus (TC-83) using monoclonal antibodies. Virology. 1982 Apr 30;118(2):269–278. doi: 10.1016/0042-6822(82)90346-4. [DOI] [PubMed] [Google Scholar]
- Strauss E. G. Mutants of Sindbis virus. III. Host polypeptides present in purified HR and ts103 virus particles. J Virol. 1978 Nov;28(2):466–474. doi: 10.1128/jvi.28.2.466-474.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E. G., Rice C. M., Strauss J. H. Sequence coding for the alphavirus nonstructural proteins is interrupted by an opal termination codon. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5271–5275. doi: 10.1073/pnas.80.17.5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E. G., Strauss J. H. Replication strategies of the single stranded RNA viruses of eukaryotes. Curr Top Microbiol Immunol. 1983;105:1–98. doi: 10.1007/978-3-642-69159-1_1. [DOI] [PubMed] [Google Scholar]
- Trent D. W., Clewley J. P., France J. K., Bishop D. H. Immunochemical and oligonucleotide fingerprint analyses of Venezuelan equine encephalomyelitis complex viruses. J Gen Virol. 1979 May;43(2):365–381. doi: 10.1099/0022-1317-43-2-365. [DOI] [PubMed] [Google Scholar]
- Trent D. W., Grant J. A. A comparison of New World alphaviruses in the western equine encephalomyelitis complex by immunochemical and oligonucleotide fingerprint techniques. J Gen Virol. 1980 Apr;47(2):261–282. doi: 10.1099/0022-1317-47-2-261. [DOI] [PubMed] [Google Scholar]
- Wengler G., Wengler G., Filipe A. R. A study of neucleotide sequence homology between the nucleic acids of different alphaviruses. Virology. 1977 May 1;78(1):124–134. doi: 10.1016/0042-6822(77)90084-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Simizu B. Purification of the envelope glycoproteins of western equine encephalitis virus by glass wool column chromatography. Appl Environ Microbiol. 1980 Aug;40(2):240–243. doi: 10.1128/aem.40.2.240-243.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemiecki A., Garofff H. Subunit composition of the membrane glycoprotein complex of Semliki Forest virus. J Mol Biol. 1978 Jul 5;122(3):259–269. doi: 10.1016/0022-2836(78)90189-4. [DOI] [PubMed] [Google Scholar]