Summary
Background
Achalasia is a not frequent esophageal disorder characterized by the absence of esophageal peristalsis and incomplete relaxation of the lower esophageal sphincter (LES). Its cause is unknown. The aim of treatment is to improve the symptoms. We report the results of the treatment of this condition achieved in one center.
Patients and methods
We conducted a retrospective study of patients with esophageal achalasia. In the period 2010–2012 we observed 64 patients, of whom 19 were referred for medical treatment. Three of the remaining patients underwent botulinum toxin injection, 17 underwent multiple endoscopic dilation procedures and 25 underwent laparoscopic surgery.
Results
There were no complications in the group undergoing endoscopic therapy, but symptom remission was only temporary. Patients undergoing surgery showed a significant improvement in symptoms and no recurrence throughout the follow-up period, that is still ongoing (3 years). There were no major complications in any case and no morbidity or mortality.
Conclusions
Surgical treatment of esophageal achalasia with laparoscopic Heller myotomy and Dor fundoplication gives the best and longest-lasting results in suitably selected patients. The extension of the myotomy and reduction in LES pressure are the most important parameters to achieve a good result.
Keywords: Laparoscopy, Heller myotomy, Intraoperative manometry
Introduction
Achalasia is a not frequent esophageal motor disorder that affects both the body of the esophagus and the lower esophageal sphincter (LES). It involves loss of normal peristalsis and sphincter hypertonia, and symptoms are debilitating (1). Its etiology is still unknown, for which reason no specific treatment is available. The possible therapeutic options are all symptomatic and involve the use of drugs, endoscopy (injection of botulinum toxin, pneumatic dilatation) and surgery. Treatment is based on the delicate balance between removing the functional obstruction, thus improving symptoms, and the need to avoid uncomfortable major consequences such as gastroesophageal reflux, peptic stricture, Barrett’s esophagus and esophageal adenocarcinoma (2). The aim of this article is to analyze the results achieved by our unit in the treatment of esophageal achalasia over the last three years.
Patients and methods
Achalasia is routinely treated in our unit. We conducted a retrospective analysis for the period 2010–2012 to assess the impact of new technologies, and particularly laparoscopic surgery, in the treatment of this disease (3–5, 33).
In all, 64 patients (36 females and 28 males) with a mean age of 63.1 years (range 17–75 years) were diagnosed with achalasia. These patients were divided according to the treatment received. The first group included 19 patients referred for medical treatment due to general contraindications to or refusal of more invasive procedures (6); 5 patients initially referred for medical treatment subsequently decided to undergo the endoscopic procedures and were thus merged into another group.
The remaining 45 patients underwent treatment as follows:
- injection of botulinum toxin repeated on average every 7 months (range 2 – 11 months) in 3 cases (7);
- endoscopic pneumatic dilatation in 17 cases; most of these (78%) underwent an average of two dilations during the same procedure (8, 9);
- laparoscopic Heller myotomy and Dor fundoplication in 25 patients; intraoperative esophageal perforation was immediately recognized in two cases and was treated with direct suturing, without the need for conversion to open surgery (10).
Techniques
Botulinum toxin (BoTox) injection
Two administrations of 100 IU each are injected into the LES via endoscopy around 30 days apart (8 portions: 4 into the four cardiac quadrants and 4 about 1 cm above the cardia) (11).
Endoscopic pneumatic dilatation
Balloons (such as Rigiflex®) are introduced orally, connected to a probe and positioned endoscopically by guide wire or fluoroscopy (12). Over the first session an insufflation pressure of 5 PSI (pounds per square inch) is reached and maintained for one minute. This is subsequently increased to 7–10 PSI for another minute, using a 30 mm diameter balloon. For the next session, we use a 35 mm balloon with the same pressures as in the first session. In patients requiring further dilatation, we use a 40 mm balloon (13, 14).
Surgery
The following refers to the last 3 years only, since incorporating the use of laparoscopy for this condition. Heller extra-mucosal cardiomyotomy and subsequent 180° Dor fundoplication to prevent reflux was performed in all patients (15, 16), who had fasted for at least 24 hours. A nasogastric tube (NGT) is positioned the evening before surgery. Patients are placed on the operating table in the reverse Trendelenburg position with legs apart and slightly bent (the classic French position). Five trocars are positioned after induction of pneumoperitoneum with a Veress needle (Fig. 1a,b). The esophagus gastric junction access is created from left to right with sectioning of the phrenoesophageal membrane, without affecting the anatomy of the crura of the diaphragm. The distal esophagus is prepared on the anterior wall, sparing the vagal branches. We do not prepare the posterior wall and do not use intramuscular epinephrine injections prior to the myotomy (17). The myotomy begins on the esophagus and proceeds towards above for at least 4–6 cm, before passing to the gastric side for another 2 cm. Once the myotomy is complete, we perform an intraoperative control manometry to check that the high pressure zone has been substantially eliminated; if it persists, the myotomy is extended (18, 19). Intraoperative esophageal gastroscopy was performed in just one case, to exclude any iatrogenic perforation (20). A 180° Dor fundoplication is performed for the dual purpose of controlling gastroesophageal reflux and protecting the esophageal submucosa (21, 22). No abdominal drainage was used in any patient.
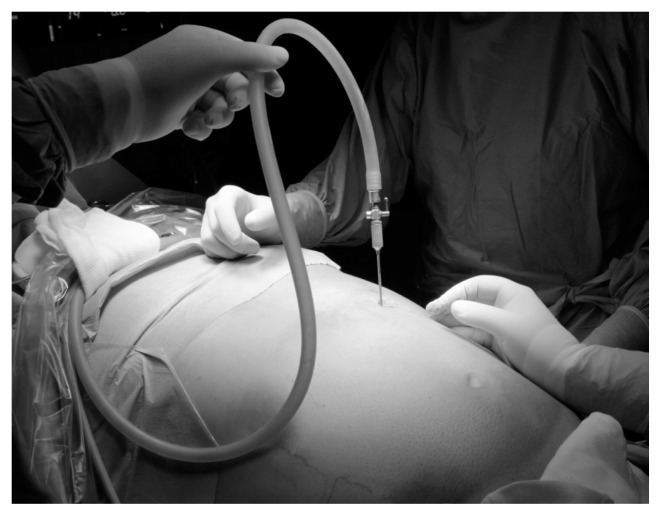
Fig. 1a.
Induction of pneumoperitoneum with Veress needle.
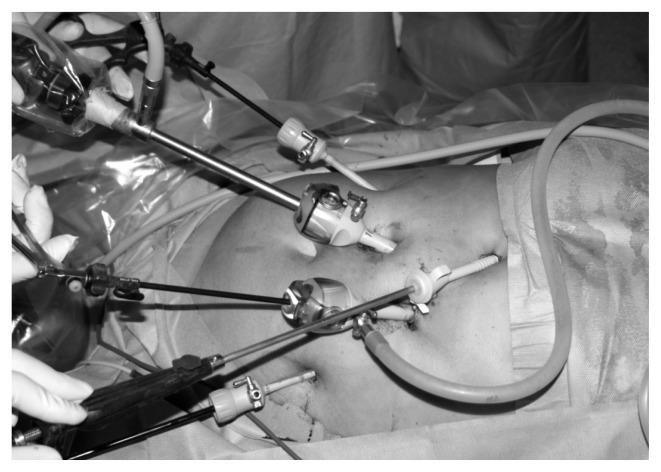
Fig. 1b.
Placement of trocars.
Results
The results refer only to the surgical treatment; the other methods provided only transient improvement. The mean duration of the procedure was 105 minutes (range 85–135 min). The NGT inserted before surgery was removed on postoperative day (POD) 1, and patients underwent barium swallow to assess esophageal transit and/or integrity. They recommenced feeding and were discharged on POD 3. There were no major intra- or postoperative complications requiring transfer to intensive care in any patient (23). As already noted, iatrogenic perforation of the distal esophagus occurred in just two cases (8%), both of which were in the early days of our use of the technique. These were treated intraoperatively with direct suturing (3, 24, 25). In these two cases the NGT was only removed on POD 3 and barium swallow to assess transit was performed on POD 4.
There was no morbidity or mortality. This is probably due in part to the youth and good health of the patients undergoing surgery in comparison with those referred to other treatments (26).
Discussion
There is as yet no consensus about the follow-up of patients treated for achalasia, but it is good practice to follow up the patients every 6–12 months, even if no clinical symptoms are reported. Our clinical experience in these years has led us to apply a strict follow-up program, at least in the first year. About a month after surgery, the patient undergoes another clinical and radiological examination with esophageal videofluorography to assess the anatomy of the residual gastroesophageal junction and the results of the myotomy. Manometry and pH testing are repeated at 6 months to assess any reflux and revise the therapy accordingly. In asymptomatic patients with a good response to the treatment, esophagogastroduodenoscopy (EGDS) is performed at 1 year. Patients are subsequently followed up annually, but this can of course be revised if the patient experiences any symptoms (27, 28).
The recent identification of various preoperative manometric patterns in different patients also provides information on their probable subsequent clinical outcome (29–31). The patients discussed herein have so far been followed up for just 3 years, but the results so far are satisfactory. There have been no recurrences to date. One patient experienced transient postoperative dysphagia, which resolved with medical treatment in two weeks. Follow-up videofluorography showed complete esophageal emptying once the LES obstruction had been removed. Manometry revealed that the fundoplication was of normal length, position and caliber and that the LES pressure was insignificant or significantly reduced with respect to preoperative values in the same patient.
Intraoperative manometry was performed in all patients (Fig. 2). Intraoperative EGDS was only necessary in one case. Despite the conflicting literature evidence, our experience and the lack of recurrence even in the longest follow-up period (patient group treated by traditional laparotomy before we introduced the laparoscopic technique) have convinced us of the importance of manometry, as it provides information which is fundamental for the complete success of the myotomy. Specifically, it enables confident identification of the position and extension of the highest pressure area, real-time verification of the drop in LES tone following the myotomy and recognition (and consequent surgical correction) of any high pressure areas (32).
Fig. 2.
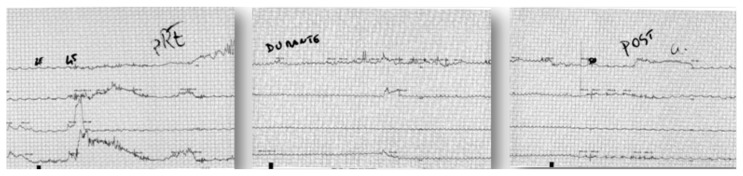
Intraoperative manometry: LES pression before, during and after myotomy.
Conclusions
In line with literature reports, our caseload of patients with esophageal achalasia were often young, female and with a long and varied history of pharmacological and even psychiatric treatment, with negative consequences for both the patients and the evolution of the disease. Evaluation of such patients thus requires a multidisciplinary diagnostic and therapeutic approach and care in dedicated centers.
Laparoscopic Heller myotomy can be carried out safely by expert surgeons, leading to a substantial, long-lasting improvement in symptoms in comparison with other treatments. The laparoscopic approach is also beneficial with respect to postoperative pain, mobilization and duration of hospitalization. The use of intraoperative manometry enables the efficacy of the myotomy to be assessed in real time, with significant benefits for patients’ symptoms in subsequent follow-up.
References
- 1.Triadafilopoulos G, Boeckxstaens GE, Gullo R, Patti MG, Pandolfino JE, Kahrilas PJ, Duranceau A, Jamieson G, Zaninotto G. The Kagoshima consensus on esophageal achalasia. Dis Esophagus. 2012;25(4):337–48. doi: 10.1111/j.1442-2050.2011.01207.x. [DOI] [PubMed] [Google Scholar]
- 2.Bresadola V, Feo CV. Minimally invasive myotomy for the treatment of esophageal achalasia: evolution of the surgical procedure and the therapeutic algorithm. Surg Laparosc Endosc Percutan tech. 2012;22(2):83–7. doi: 10.1097/SLE.0b013e318243368f. [DOI] [PubMed] [Google Scholar]
- 3.Stefanidis D, Richardson W, Farrell TM, Kohn GP, Augenstein V, Fanelli RD. SAGES Guidelines for the surgical treatment of esophageal achalasia. Surg Endosc. 2012;26:296–311. doi: 10.1007/s00464-011-2017-2. [DOI] [PubMed] [Google Scholar]
- 4.Agrusa A, Romano G, Di Buono G, Dafnomili A, Gulotta G. Laparoscopic approach in abdominal emergencies: a 5-year experience at a single center. G Chir. 2012;33(11–12):400–3. [PubMed] [Google Scholar]
- 5.Tuveri M, Calò PG, Medas F, Tuveri A, Nicolosi A. Limits and advantages of fundus-first laparoscopic cholecystectomy: lessons learned. J Laparoendosc Adv Surg Tech A. 2008;18(1):69–75. doi: 10.1089/lap.2006.0194. [DOI] [PubMed] [Google Scholar]
- 6.Annese V, Bassotti G. Non-surgical treatment of esophageal achalasia. World J Gastroenterol. 2006;12(36):5763–6. doi: 10.3748/wjg.v12.i36.5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinek J, Siroky M, Plottova Z, Bures J, Hep A, Spicak J. treatment of patients with achalasia with botulinum toxin: a multicenter prospective cohort study. Dis Esophagus. 2003;16:204–209. doi: 10.1046/j.1442-2050.2003.00329.x. [DOI] [PubMed] [Google Scholar]
- 8.Katsinelos P, Kountouras J, Parautoglou G, Beltsis A, Zavos C, Papaziogas B, Mimidis K. Long-term results of pneumatic dilation for achalasia: a 15 years’ experience. World J Gastroenterol. 2005;11:5701–5705. doi: 10.3748/wjg.v11.i36.5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zerbib F, Thetiot V, Richy F, Benajah DA, Message L, Lamouliatte H. Repeated pneumatic dilation as long-term maintenance therapy for esophageal achalasia. Am J Gastroenterol. 2006;101:692–697. doi: 10.1111/j.1572-0241.2006.00385.x. [DOI] [PubMed] [Google Scholar]
- 10.El Hak MG, Hamdy E, Abdalla T, Kandel T, El Raof AA, El Hemaly M, Salah T, El Hanafy E. Laparoscopic Heller myotomy for achalasia: analysis of successes and failure. Hepatogastroenterology. 2012;59(117):1450–4. doi: 10.5754/hge10060. [DOI] [PubMed] [Google Scholar]
- 11.Kroupa R, Hep A, Dolina J, Valek V, Matyasova Z, Prokesova J, Mrazova J, Sedmik J, Novotny Combined treatment of achalasia - botulinum toxin injection followed by pneumatic dilatation: long-term results. Dis Esophagus. 2010;23(2):100–5. doi: 10.1111/j.1442-2050.2009.01005.x. [DOI] [PubMed] [Google Scholar]
- 12.Chuah SK, Hu TH, Wu KL, Kuo CM, Fong TV, Lee CM, Chang-chien CS. Endoscope-guided pneumatic dilatation of esophageal achalasia without fluoroscopy is another safe and effective treatment option: a report of Taiwan. Surg Laparosc Endosc Percutan Tech. 2008;18(1):8–12. doi: 10.1097/SLE.0b013e31815c1ba2. [DOI] [PubMed] [Google Scholar]
- 13.Kadakia SC, Wong RK. Pneumatic balloon dilation for esophageal achalasia. Gastrointest Endosc Clin N Am. 2001;11:325–346. [PubMed] [Google Scholar]
- 14.Boeckxstaens GE, Annese V, Des Varannes SB, Chaussade S, Costantini M, Cuttitta A, Elizalde JI, Fumagalli U, Gaudric M, Rohof WO, Smout AJ, Tack J, Zwinderman AH, Zaninotto G, Busch OR. European Achalasia Trial Investigators: Pneumatic dilation versus laparoscopic Heller’s myotomy for idiopathic achalasia. N Engl J Med. 2011;364(19):1807–16. doi: 10.1056/NEJMoa1010502. [DOI] [PubMed] [Google Scholar]
- 15.Rebecchi F, Giaccone C, Farinella E, Campaci R, Morino M. Randomized controlled tria of laparoscopic Heller myotomy plus Dor fundoplicatio versus Nissen fundoplicatio for achalasia: long-term results. Ann Surg. 2008;248:1023–1030. doi: 10.1097/SLA.0b013e318190a776. [DOI] [PubMed] [Google Scholar]
- 16.Torquati A, Richards WO, holzman MD, Sharp KW. Laparoscopic myotomy for achalasia: predictors of successful outcome after 200 cases. Ann Surg. 2006;243:587–591. doi: 10.1097/01.sla.0000216782.10502.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuster GG. Local epinephrine facilitates laparoscopic Heller myotomy. Surg Endosc. 1998;12:79–81. doi: 10.1007/s004649900600. [DOI] [PubMed] [Google Scholar]
- 18.Endo S, Nakajima K, Nishikawa K, Takahashi T, Souma Y, Taniguchi E, Ito T, Nishida T. Laparoscopic Heller-Dor surgery for esophageal achalasia: impact of intraoperative real time manometric feedback on post-operative outcome. Dig Surg. 2009;26:342–348. doi: 10.1159/000244512. [DOI] [PubMed] [Google Scholar]
- 19.Mattioli S, Ruffato A, Lugaresi M, Pilotti V, Aramini B, D’ovidio F. Long-term results of the Heller-Dor myotomy operation with intraoperative manometry for the treatment of esophageal achalasia. J Thorac Cardiovasc Surg. 2010;140(5):962–9. doi: 10.1016/j.jtcvs.2010.07.053. [DOI] [PubMed] [Google Scholar]
- 20.Adikibi BT, MacKinlay GA, Munro FD, Khan LR, Gillett PM. Intraoperative upper GI endoscopy ensures an adeguate laparoscopic Heller’s myotomy. J Laparoendosc Adv Surg Tech A. 2009;19(5):687–9. doi: 10.1089/lap.2008.0156. [DOI] [PubMed] [Google Scholar]
- 21.Patti MG, Herbella FA. Fundoplicatio after laparoscopic Heller myotomy for esophagea achalasia: what type? J Gastrointest Surg. 2010;10:1453–8. doi: 10.1007/s11605-010-1188-9. [DOI] [PubMed] [Google Scholar]
- 22.Engstrom C, Lonroth H, Mardani J, Lundell L. An anterior or posterior approach to partial fundoplicatio? Long-term results of a randomized trial. World J Surg. 2007;31:1221–1225. doi: 10.1007/s00268-007-9004-8. [DOI] [PubMed] [Google Scholar]
- 23.Del Genio G, Tolone S, Del Genio F, D’Alessandro A, Brusciano L, Aggarwal R, Conzo G, Orditura M, Docimo L, Del Genio A. Impact of total fundoplication on esophageal transit: analysis by combined multichannel intraluminal impedance and manometry. J Clin Gastroenterol. 2012;46(1):1–5. doi: 10.1097/MCG.0b013e31822f3735. [DOI] [PubMed] [Google Scholar]
- 24.Campos GM, Vittinghoff E, Rabl C, Takata M, Gadenstatter M, Lin F, Ciovica R. Endoscopic and surgical treatments for achalasia: a systematic review and meta-analysis. Ann Surg. 2009;249:45–57. doi: 10.1097/SLA.0b013e31818e43ab. [DOI] [PubMed] [Google Scholar]
- 25.Bloomston M, Serafini F, Boyce HW, et al. The “learning curve” in videscopic Heller myotomy. JSLS. 2002;11:351–359. [PMC free article] [PubMed] [Google Scholar]
- 26.Rakita S, Bloomston M, Villadolid D, Thometz D, Boe B, Rosemurgy A. Age affects presenting symptoms of achalasia and outcome after myotomy. Am Surg. 2005;71:424–429. [PubMed] [Google Scholar]
- 27.Ferulano GP, Dilillo S, D’Ambra M, Lionetti R, Saviano C, Fico D. Oesophageal achalasia in elderly people: results of the laparoscopic Heller-Dor Myotomy. Actabiomed. 2005;76:37–41. [PubMed] [Google Scholar]
- 28.Boeckxstaens G. The European experience of achalasia treatment; advances in GERD. Gastroenterology & Hepatology. 2011;7:609–611. [PMC free article] [PubMed] [Google Scholar]
- 29.Zaninotto G, Costantini M, Rizzetto C, et al. Four hundred laparoscopic myotomies for esophageal achalasia: a single centre experience. Ann surg. 2008;248:986–993. doi: 10.1097/SLA.0b013e3181907bdd. [DOI] [PubMed] [Google Scholar]
- 30.Pandolfino JE, Kwiatek MA, Nealis T, et al. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology. 2008;135:1526–1533. doi: 10.1053/j.gastro.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salvador R, Costantini M, Zaninotto G, et al. the preoperative manometric pattern predicts the outcome of surgical treatment for esophageal achalasia. J Gastrointest Surg. 2010;14:1635–1645. doi: 10.1007/s11605-010-1318-4. [DOI] [PubMed] [Google Scholar]
- 32.Bonventre S, Frazzetta M, Lucania M, Frazzetta F, Sciortino AS, Sammartano A, Vetri G, Di Gesù G. The role of the intraoperative in the esophageal achalasia surgical treatment. G Chir. 2008;29:373–377. [PubMed] [Google Scholar]
- 33.Cucinella G, Rotolo S, Calagna G, Granese R, Agrusa A, Perino A. Laparoscopic management of interstitial pregnancy: the “purse-string” technique. Acta Obstet Gynecol Scand. 2012 Aug;91(8):996–9. doi: 10.1111/j.1600-0412.2012.01437.x.. [DOI] [PubMed] [Google Scholar]