Abstract
A ring fragmentation and intramolecular azomethine ylide 1,3-dipolar cycloaddition sequence of reactions was successfully used in the preparation of a known (±)-cycloclavine precursor in good overall yield. Results of efforts to incorporate the tetrasubstituted cyclopropane ring present in cycloclavine are also discussed.
Keywords: Cycloclavine; Ergot Alkaloid; Natural Product Synthesis; Ring Fragmentation; 1,3-Dipolar Cycloaddition
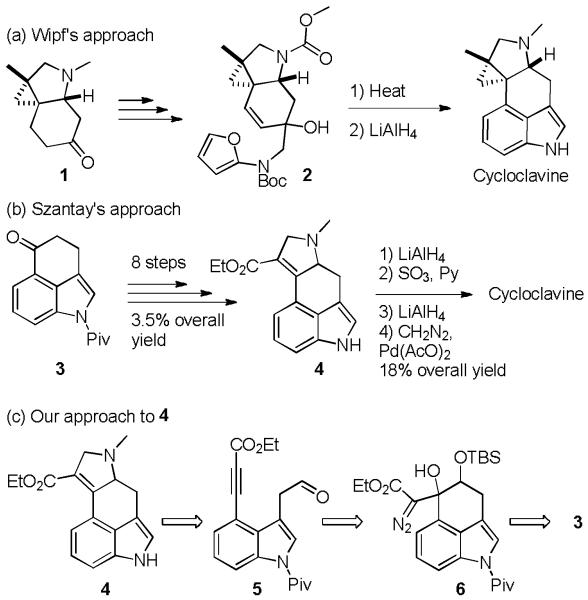
Cycloclavine, an indole alkaloid first isolated in 1969 from seeds of ipomoea hildebrandtii by Hofmann and coworkers,1 is a member of the ergot alkaloid family. The unique tetracyclic core of cycloclavine contains a tetrasubstituted cyclopropane moiety and has garnered interest from the synthetic community; to date two total syntheses of cycloclavine have been reported.2 Most recently, Petronijevic and Wipf2b prepared (±)-cycloclavine in 14 linear steps with 1.2% overall yield by an elegant and unconventional approach in which the aromatic indole portion of the molecule was formed at a late stage in the synthesis by their intramolecular Diels-Alder cycloaddition of furan (IMDAF reaction) approach.2b Prior to this synthesis, Szántay and coworkers2a reported a more traditional approach to (±)-cycloclavine starting from pivalate protected Uhle’s ketone (3)3 (Scheme 1), which was advanced to tetracycle 4 in 8 steps. Reduction of the ethyl ester of 4 to the corresponding methyl group and Pd catalyzed cyclopropanation with diazomethane provided the target molecule. While this is a nice straightforward approach to (±)-cycloclavine, the route to tetracycle 4 was hampered by several low yielding steps and provided 4 in only 3.5% overall yield.
Scheme 1.
Synthetic approaches to cycloclavine
We recently reported an efficient synthetic approach to polycyclic 2,5-dihydropyrroles4 that we have applied in the synthesis of the steroidal alkaloid demissidine.5 This strategy is based on our report that cyclic γ-siloxy-β-hydroxy-α-diazocarbonyl compounds fragment when treated with SnCl4 to provide tethered aldehyde ynoates,6 which are excellent substrates for intramolecular azomethine ylide 1,3-dipolar cycloadditions, which in turn provide the desired 2,5-dihydropyrrole products. The fragmentation cycloaddition sequence appears to be fairly general, and we recognized that this reaction sequence might provide an efficient route to Szántay’s tetracyclic 2,5-dihydropyrrole intermediate 4. In this letter we report our results on the formation of 4 and provide details about our efforts to advance 4 to (±)-cycloclavine.
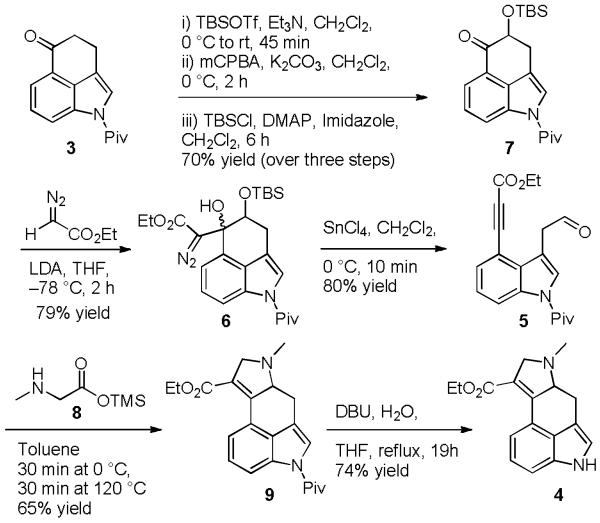
Our synthetic efforts, shown in Scheme 2, also began with pivalate protected Uhle’s ketone (3),3 which was converted into the corresponding enoxy silane by treatment with TBSOTf and Et3N. This enoxy silane proved to be unstable to silica gel chromatography and after extractive workup it was subjected directly to Rubottom oxidation7 under biphasic conditions containing aqueous base.8 The oxidation proceeded efficiently but provided the α-hydroxy ketone as a mixture of the free and silyl protected alcohols. Treating the crude reaction mixture with TBSCl provided the desired α-silyloxy ketone (7) in 70% yield over the three steps. Aldol type addition of ethyl lithiodiazoacetate to ketone 7 furnished the corresponding γ-silyloxy-β-hydroxy-α-diazo ester derivative 6 as an inseparable mixture of diastereomers in 79% yield. We were pleased to observe that treating the diastereomeric mixture of 6 with SnCl4 resulted in efficient rupture of the Cβ-Cγ bond to provide tethered aldehyde ynoate 5 in 80% isolated yield. Reacting aldehyde ynoate 5 with the trimethylsilyl ester of sarcosine (8) resulted in the expected intramolecular azomethine ylide 1,3-dipolar cycloaddition to give the tetracyclic core of cycloclavine as 2,5-dihydropyrrole 9 in 65% yield. We attribute the moderate yield of this reaction to the instability of the sarcosine silyl ester which tended to dimerize under the reaction conditions. While optimizing this fragmentation cycloaddition sequence we observed that purifying the fragmentation product by chromatography prior to cycloaddition was not necessary. In fact, when the crude fragmentation product was used the overall yield for the two step sequence increased to 62%. We attribute this 10% increase in yield to losses of aldehyde ynoate 5 during chromatography. DBU mediated pivaloyl cleavage9 from 9 provided tetracycle 4, which intersects with Szántay’s prior synthesis and thus completes a formal synthesis of cycloclavine. Overall, this ring fragmentation/cycloaddition approach provided Szántay’s intermediate in seven steps and 25% overall yield.
Scheme 2.
Formal synthesis of cycloclavine
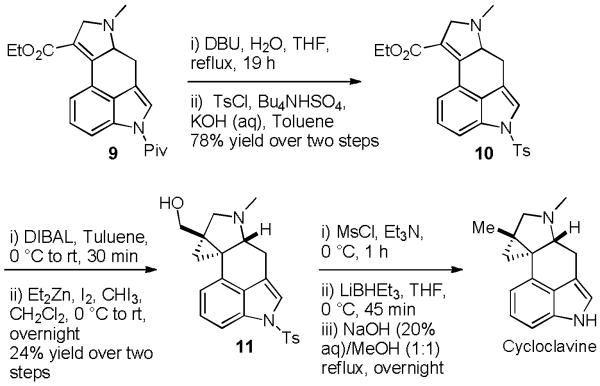
With efficient access to protected indole intermediate 9 in place, we assessed various alternative strategies to incorporate the tetrasubstituted cyclopropane ring present in cycloclavine. Pyrazolines are well known cyclopropane precursors10 and we envisioned converting enoate 9 into the corresponding pyrazoline via 1,3-dipolar cycloaddition with diazomethane. Unfortunately, enoate 9 failed to react with diazomethane even after prolonged reaction times. We next attempted to incorporate the cyclopropane moiety by a Corey-Chaykovsky cyclopropanation reaction.11 However, the pivaloyl protecting group proved to be too labile for these nucleophilic conditions and only N-deprotected indole 4 was returned. To circumvent this problem, we exchanged the pivaloyl group with a more stable tosyl group via a two-step sequence (Scheme 3).9, 12 Unfortunately, Corey-Chaykovsky cyclopropanation of tosyl derivative 10 also failed to furnish any cyclopropane product; only starting material and decomposition products were returned. These results were surprising to us because Corey-Chaykovsky cyclopropanation of similar systems has been reported.13
Scheme 3.
Synthesis of cycloclavine
Allylic hydroxyl groups are well known to act as directing groups in metal mediated cyclopropanation reactions.14 With this in mind we subjected 10 to DIBALH reduction to generate the corresponding allylic alcohol and subjected this material to various metal carbenoid cyclopropanation reactions. Unfortunately, cyclopropanation reactions mediated by Zn,15 Sm,16 Pd,17 Mg18 and Cr19 all failed to provide any of the desired cyclopropane product. Ultimately, we observed that the gemdizinc carbenoid conditions developed by Charette and coworkers20 provided the desired cyclopropane (11), albeit in low and variable yields. For reasons we do not understand, this reaction was only successful when the allylic alcohol used was crude material isolated from the preceding DIBALH reduction after aqueous workup, in which case cyclopropane 11 was formed in up to 24% yield over the two steps. When the allylic alcohol was purified by chromatography, the cyclopropanation consistently failed to provide any desired cyclopropane product.
With small quantities of cyclopropane intermediate 11 in hand we were able to complete the total synthesis of (±)-cycloclavine. This was achieved by mesylation of the primary alcohol followed by nucleophilic displacement with hydride to give the requisite methyl, and subsequent hydrolysis of the sulfonamide. Unfortunately, due to limited and inconsistent access to cyclopropyl intermediate 11 these final steps were not optimized.
In summary, a ring fragmentation/intramolecular azomethine ylide 1,3-dipolar cycloaddition route to 2,5-dihydropyrroles was successfully used in the preparation of (±)-cycloclavine. Attempts to incorporate the cyclopropane moiety of cycloclavine through a directed cyclopropanation reaction were moderately successful, but did not represent an improvement over Szántay’s prior work. However, this route to known cycloclavine precursor 4 was significantly higher yielding (25% overall yield) than the published route to 4, and increases the overall yield of the prior synthesis of (±)-cycloclavine to 4%. These results further highlight the synthetic utility of the fragmentation/cycloaddition sequence for the construction of polycyclic 2,5-dihydropyrroles in natural product synthesis.
Supplementary Material
Acknowledgments
We thank Bruce O’Rourke (University of Vermont) for obtaining mass spectral data. This work was financially supported by the NIH (National Institute of General Medical Sciences Award Number R01GM092870), and was made possible by use of a facility supported by the Vermont Genetics Network through Grant Number 8P20GM103449 from the INBRE Program of the National Institute of General Medical Sciences (NIGMS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIGMS or NIH. The National Science Foundation supported this work through instrumentation grants CHE-1126265 and CHE-0821501.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1).Stauffacher D, Niklaus P, Tscherter H, Weber HP, Hofmann A. Tetrahedron. 1969;25(24):5879–5887. doi: 10.1016/s0040-4020(01)83095-7. [DOI] [PubMed] [Google Scholar]
- 2).a) Incze M, Doernyei G, Moldvai I, Temesvari-Major E, Egyed O, Szántay C. Tetrahedron. 2008;64(13):2924–2929. [Google Scholar]; b) Petronijevic FR, Wipf P. J. Am. Chem. Soc. 2011;133(20):7704–7707. doi: 10.1021/ja2026882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).a) Teranishi K, Hayashi S, Nakatsuka S.-i., Goto T. Tetrahedron Lett. 1994;35(44):8173–8176. [Google Scholar]; b) Teranishi K, Hayashi S, Nakatsuka S, Goto T. Synthesis. 1995;(5):506–508. [Google Scholar]
- 4).Draghici C, Huang Q, Brewer M. J. Org. Chem. 2009;74(21):8410–8413. doi: 10.1021/jo901978y. [DOI] [PubMed] [Google Scholar]
- 5).Zhang Z, Giampa GM, Draghici C, Huang QF, Brewer M. Org. Lett. 2013;15(9):2100–2103. doi: 10.1021/ol4004993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).a) Bayir A, Draghici C, Brewer M. J. Org. Chem. 2010;75(2):296–302. doi: 10.1021/jo902405f. [DOI] [PubMed] [Google Scholar]; b) Draghici C, Brewer M. J. Am. Chem. Soc. 2008;130(12):3766–3767. doi: 10.1021/ja801004d. [DOI] [PubMed] [Google Scholar]
- 7).Rubottom GM, Vazquez MA, Pelegrina DR. Tetrahedron Lett. 1974;15(49-50):4319–4322. [Google Scholar]
- 8).Novikov YY, Sampson P. Org Lett. 2003;5(13):2263–2266. doi: 10.1021/ol034594x. [DOI] [PubMed] [Google Scholar]
- 9).Ruiz M, Sanchez JD, Lopez-Alvarado P, Menendez JC. Tetrahedron. 2012;68(2):705–710. [Google Scholar]
- 10).a) Taber DF, Guo P. J. Org. Chem. 2008;73(23):9479–9481. doi: 10.1021/jo8017704. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wang Z. Comprehensive Organic Name Reactions and Reagents. John Wiley & Sons, Inc.; 2010. Kishner Decomposition. [Google Scholar]; c) Chowdhury MA, Senboku H, Tokuda M. Tetrahedron Lett. 2003;44(16):3329–3332. [Google Scholar]
- 11).Corey EJ, Chaykovsky M. J. Am. Chem. Soc. 1965;87(6):1353–1364. [Google Scholar]
- 12).Wang K, Liu Z. Synth. Commun. 2010;40(1):144–150. [Google Scholar]
- 13).a) Inoue T, Watanabe S, Yamagishi T, Arano Y, Morita M, Shimada K. WO2010137351 Aryl substituted carboxamide derivatives as calcium or sodium channel blockers. 2010; b) Yohannes D, Procko K, Lebel LA, Fox CB, O’Neill BT. Bioorg. Med. Chem. Lett. 2008;18(7):2316–2319. doi: 10.1016/j.bmcl.2008.02.078. [DOI] [PubMed] [Google Scholar]
- 14).Lebel H, Marcoux J-F, Molinaro C, Charette AB. Chem. Rev. 2003;103(4):977–1050. doi: 10.1021/cr010007e. [DOI] [PubMed] [Google Scholar]
- 15).a) Fujii K, Shiine K, Misaki T, Sugimura T. Appl. Organomet. Chem. 2013;27(2):69–72. [Google Scholar]; b) Charette AB, Lacasse M-C. Org. Lett. 2002;4(20):3351–3353. doi: 10.1021/ol0264051. [DOI] [PubMed] [Google Scholar]; c) Charette AB, Lebel H. J. Org. Chem. 1995;60(10):2966–2967. [Google Scholar]; d) Voituriez A, Zimmer LE, Charette AB. J. Org. Chem. 2010;75(4):1244–1250. doi: 10.1021/jo902618e. [DOI] [PubMed] [Google Scholar]; e) Lorenz JC, Long J, Yang Z, Xue S, Xie Y, Shi Y. J. Org. Chem. 2004;69(2):327–334. doi: 10.1021/jo030312v. [DOI] [PubMed] [Google Scholar]; f) Charette AB, Juteau H, Lebel H, Molinaro C. J. Am. Chem. Soc. 1998;120(46):11943–11952. [Google Scholar]
- 16).Molander GA, Harring LS. J. Org. Chem. 1989;54(15):3525–3532. [Google Scholar]
- 17).Simoneau B, Thavonekham B, Landry S, O’meara J, Yoakim C, Faucher A-M. WO2004050643. Non-nucleoside reverse transcriptase inhibitors. 2004 doi: 10.1016/j.bmcl.2007.03.097. [DOI] [PubMed]
- 18).Brunner G, Eberhard L, Oetiker J, Schroder F. J. Org. Chem. 2008;73(19):7543–7554. doi: 10.1021/jo8007397. [DOI] [PubMed] [Google Scholar]
- 19).Concellón JM, Rodríguez-Solla H, Méjica C, Blanco EG. Org. Lett. 2007;9(16):2981–2984. doi: 10.1021/ol070896d. [DOI] [PubMed] [Google Scholar]
- 20).Fournier J-F, Mathieu S, Charette AB. J. Am. Chem. Soc. 2005;127(38):13140–13141. doi: 10.1021/ja054328+. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.