Abstract
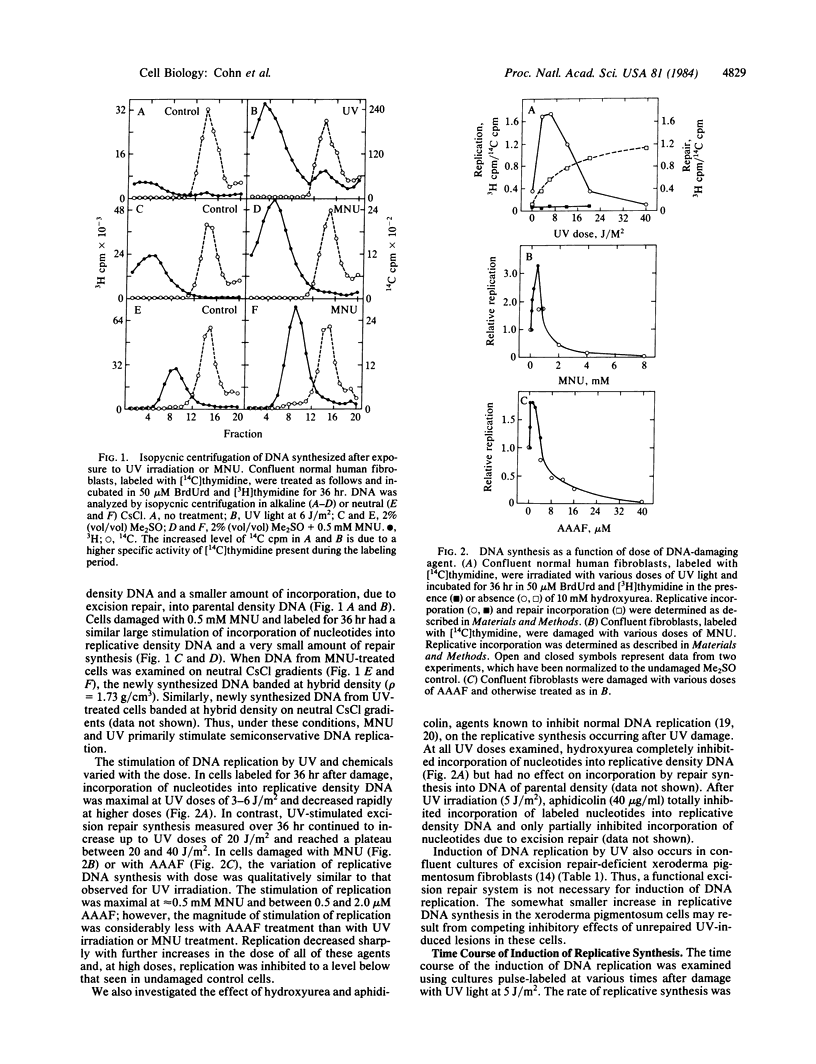
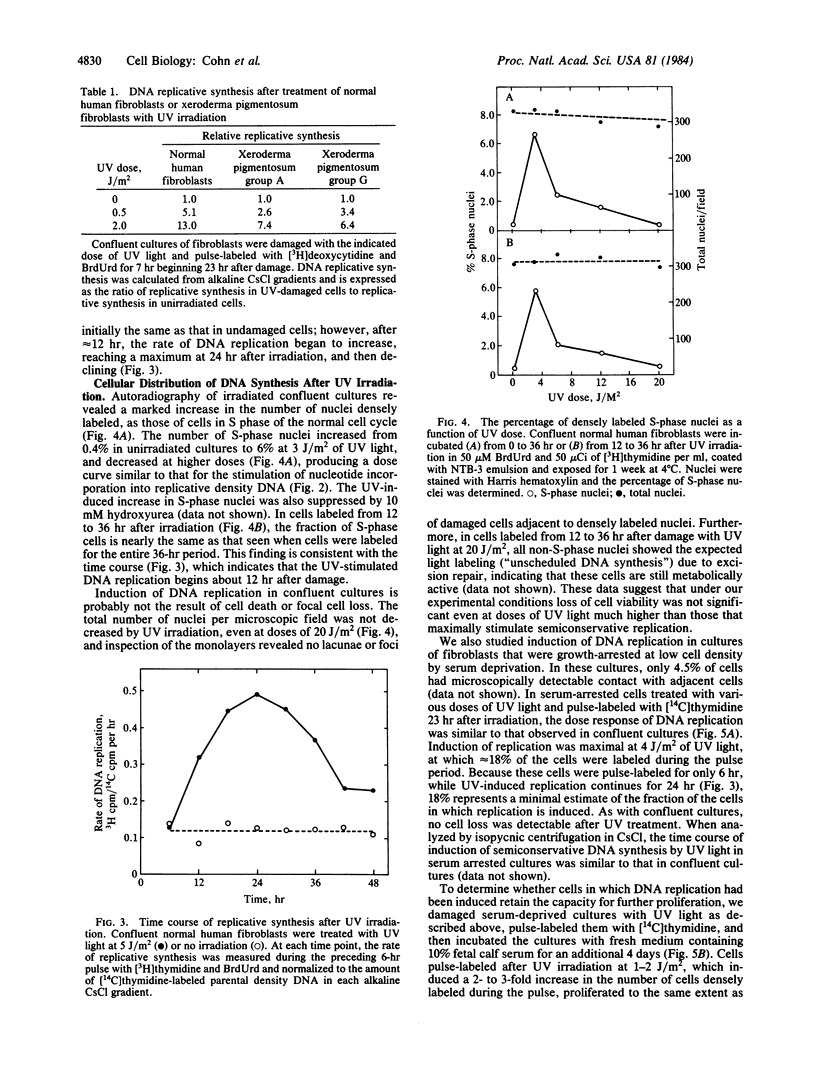
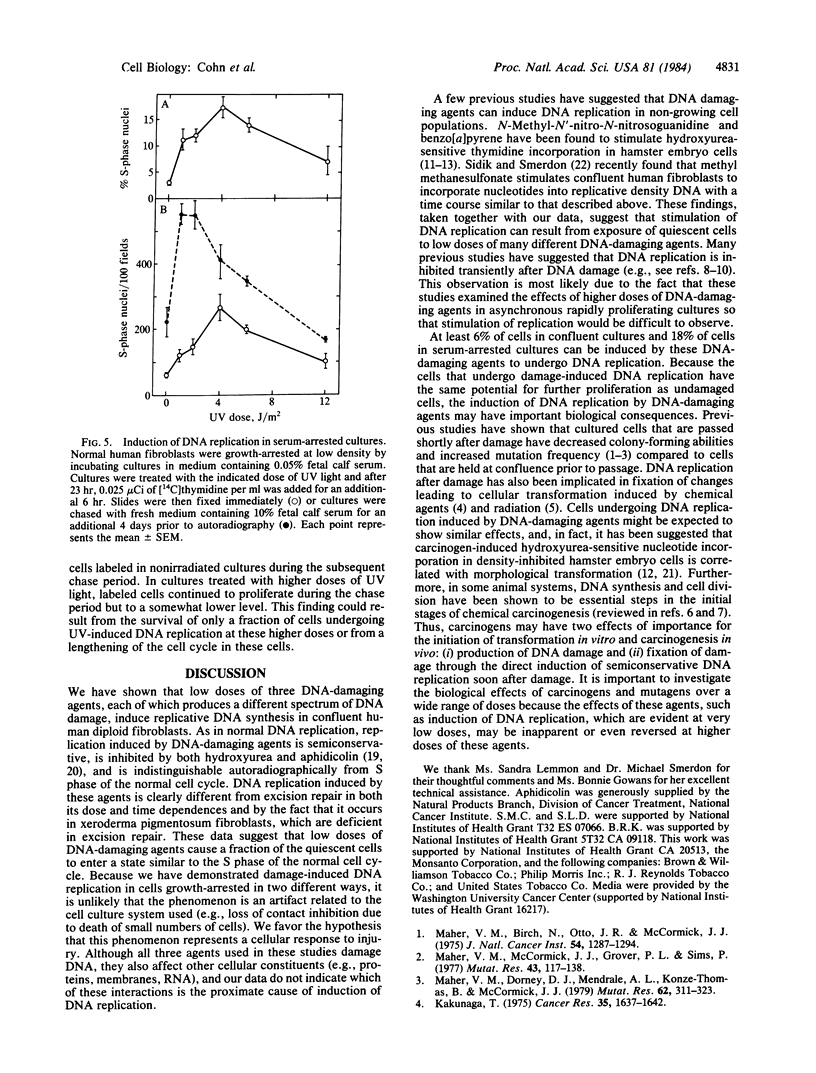
A marked induction of DNA replication was observed in confluent human diploid fibroblast cultures treated with low relatively nontoxic doses of UV radiation, N-methyl-N-nitrosourea (MNU), and N-acetoxy-2-acetylaminofluorene (AAAF). Isopycnic CsCl density gradient analysis of newly synthesized DNA labeled with BrdUrd indicated that most of the synthesis was semiconservative. The rate of semiconservative DNA synthesis was maximal 24 hr after damage. This induction of DNA replication was greatest at approximately equal to 3 J/m2 UV, 0.5 mM MNU, or 1.0 microM AAAF; was inhibited by hydroxyurea and aphidicolin; and also occurred in repair-deficient xeroderma pigmentosum fibroblasts. Autoradiographic examination of both confluent cultures and serum-arrested cultures showed a large increase in the fraction of densely labeled (S phase) cells after UV treatment. These densely labeled cells retain the capacity for cell division and subsequent proliferation. We conclude that low doses of at least three different DNA damaging agents are capable of recruiting quiescent cells into a state of DNA replication similar to that observed in the normal cell cycle.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borek C., Sachs L. In vitro cell transformation by x-irradiation. Nature. 1966 Apr 16;210(5033):276–278. doi: 10.1038/210276a0. [DOI] [PubMed] [Google Scholar]
- Brandt W. N., Flamm W. G., Bernheim N. J. The value of hydroxyurea in assessing repair synthesis of DNA in HeLa cells. Chem Biol Interact. 1972 Oct;5(5):327–339. doi: 10.1016/0009-2797(72)90072-5. [DOI] [PubMed] [Google Scholar]
- Davis R. W., Thomas M., Cameron J., St John T. P., Scherer S., Padgett R. A. Rapid DNA isolations for enzymatic and hybridization analysis. Methods Enzymol. 1980;65(1):404–411. doi: 10.1016/s0076-6879(80)65051-4. [DOI] [PubMed] [Google Scholar]
- Dresler S. L., Lieberman M. W. Identification of DNA polymerases involved in DNA excision repair in diploid human fibroblasts. J Biol Chem. 1983 Aug 25;258(16):9990–9994. [PubMed] [Google Scholar]
- Dresler S. L., Roberts J. D., Lieberman M. W. Characterization of deoxyribonucleic acid repair synthesis in permeable human fibroblasts. Biochemistry. 1982 May 11;21(10):2557–2564. doi: 10.1021/bi00539a040. [DOI] [PubMed] [Google Scholar]
- Farber E., Cameron R. The sequential analysis of cancer development. Adv Cancer Res. 1980;31:125–226. doi: 10.1016/s0065-230x(08)60658-2. [DOI] [PubMed] [Google Scholar]
- Hirsch K. S., Wilson J. G., Scott W. J., O'Flaherty E. J. Acetazolamide teratology and its association with carbonic anhydrase inhibition in the mouse. Teratog Carcinog Mutagen. 1983;3(2):133–144. doi: 10.1002/1520-6866(1990)3:2<133::aid-tcm1770030205>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Huberman J. A. New views of the biochemistry of eucaryotic DNA replication revealed by aphidicolin, an unusual inhibitor of DNA polymerase alpha. Cell. 1981 Mar;23(3):647–648. doi: 10.1016/0092-8674(81)90426-8. [DOI] [PubMed] [Google Scholar]
- Kakunaga T. The role of cell division in the malignant transformation of mouse cells treated with 3-methylcholanthrene. Cancer Res. 1975 Jul;35(7):1637–1642. [PubMed] [Google Scholar]
- Kaufmann W. K., Cleaver J. E., Painter R. B. Ultraviolet radiation inhibits replicon initiation in S phase human cells. Biochim Biophys Acta. 1980 Jun 27;608(1):191–195. doi: 10.1016/0005-2787(80)90147-1. [DOI] [PubMed] [Google Scholar]
- Maher V. M., Birch N., Otto J. R., MacCormick J. J. Cytotoxicity of carcinogenic aromatic amides in normal and xeroderma pigmentosum fibroblasts with different DNA repair capabilities. J Natl Cancer Inst. 1975 Jun;54(6):1287–1294. doi: 10.1093/jnci/54.6.1287. [DOI] [PubMed] [Google Scholar]
- Maher V. M., Dorney D. J., Mendrala A. L., Konze-Thomas B., McCormick J. J. DNA excision-repair processes in human cells can eliminate the cytotoxic and mutagenic consequences of ultraviolet irradiation. Mutat Res. 1979 Sep;62(2):311–323. doi: 10.1016/0027-5107(79)90087-3. [DOI] [PubMed] [Google Scholar]
- Maher V. M., McCormick J. J., Grover P. L., Sims P. Effect of DNA repair on the cytotoxicity and mutagenicity of polycyclic hydrocarbon derivatives in normal and xeroderma pigmentosum human fibroblasts. Mutat Res. 1977 Apr;43(1):117–138. doi: 10.1016/0027-5107(77)90137-3. [DOI] [PubMed] [Google Scholar]
- Mironescu S. G., Epstein S. M., DiPaolo J. A. Relationship between morphological transformation and [3H]thymidine incorporation stimulated by a chemical carcinogen in postconfluent cultures of hamster embryo cells. Cancer Res. 1980 Jul;40(7):2411–2416. [PubMed] [Google Scholar]
- Mironescu S. G. Inhibition of morphological transformation induced with N-methyl-N'-nitro-N-nitrosoguanidine in cultures of hamster embryo cells by 5'-bromo-2'-deoxyuridine-photolysis. Int J Cancer. 1978 Sep 15;22(3):304–314. doi: 10.1002/ijc.2910220314. [DOI] [PubMed] [Google Scholar]
- Mironescu S., Love R. DNA synthesis and transformation induced in density-inhibited cultures of hamster embryo cells by the carcinogen benzo(alpha)pyrene. Cancer Res. 1974 Oct;34(10):2562–2570. [PubMed] [Google Scholar]
- Painter R. B. Inhibition of DNA replicon initiation by 4-nitroquinoline 1-oxide, adriamycin, and ethyleneimine. Cancer Res. 1978 Dec;38(12):4445–4449. [PubMed] [Google Scholar]
- Povirk L. F., Painter R. B. The effect of 313 nanometer light on initiation of replicons in mammalian cell DNA containing bromodeoxyuridine. Biochim Biophys Acta. 1976 May 19;432(3):267–272. doi: 10.1016/0005-2787(76)90135-0. [DOI] [PubMed] [Google Scholar]
- Sidik K., Smerdon M. J. Nuclease sensitivity of repair-incorporated nucleotides in chromatin and nucleosome rearrangement in human cells damaged by methyl methanesulfonate and methylnitrosourea. Carcinogenesis. 1984 Feb;5(2):245–253. doi: 10.1093/carcin/5.2.245. [DOI] [PubMed] [Google Scholar]
- Smerdon M. J., Kastan M. B., Lieberman M. W. Distribution of repair-incorporated nucleotides and nucleosome rearrangement in the chromatin of normal and xeroderma pigmentosum human fibroblasts. Biochemistry. 1979 Aug 21;18(17):3732–3739. doi: 10.1021/bi00584a014. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Hanawalt P. C. Repair replication in human cells. Simplified determination utilizing hydroxyurea. Biochim Biophys Acta. 1976 May 19;432(3):336–347. doi: 10.1016/0005-2787(76)90143-x. [DOI] [PubMed] [Google Scholar]


