Abstract
T cell co-stimulation is a key component of adaptive immunity to viral infection but has also been associated with pathology due to excessive or altered T cell activity. We recently demonstrated that the TNFR family co-stimulatory molecule OX40 (CD134) is critically required to sustain antiviral T cell and antibody responses that enable control of viral replication in the context of chronic LCMV infection. Here, we investigated whether reinforcing OX40 stimulation through an agonist antibody had the potential to prevent LCMV persistence. We observed that anti-OX40 injection early after LCMV clone 13 infection increased CD8 T cell mediated immunopathology. More strikingly, OX40 stimulation of virus-specific CD4 T cells promoted expression of the transcriptional repressor Blimp-1 and diverted the majority of cells away from follicular T helper (Tfh) cell differentiation. This occurred in both acute and chronic infections and resulted in dramatic reductions in germinal center and antibody responses to the viral infection. The effect of the OX40 agonist was dependent on IL-2 signaling and the timing of OX40 stimulation. Collectively, our data demonstrate that excessive OX40 signaling can result in deleterious consequences in the setting of LCMV infection.
Introduction
T cell responses to persistent viruses are often functionally impaired. While this protects the host from overwhelming immunopathology, it is thought to be a contributing factor to the establishment of persistent infection (1, 2). It has been demonstrated that the enhancement of anti-viral T cell responses through blockade or genetic deletion of inhibitory pathways can facilitate rapid clearance of an otherwise protracted viral infection in the murine LCMV cl13 system (1, 3-5). More recently, the importance of immune-stimulatory pathways has been appreciated. IL-6, IL-21, and the co-stimulatory molecule OX40 have each been shown to be required in order to sustain immune system pressure on viral replication and pathogen control (6-10).
OX40 (CD134) is an inducible co-stimulatory receptor that belongs to the TNF receptor superfamily (TNFRSF). It is primarily expressed on activated T cells and OX40-OX40L interactions promote survival but also division and cytokine production of T cells in various settings (11). Therapeutic stimulation of the OX40 receptor through an agonistic monoclonal antibody has been shown to enhance antigen-specific T cell responses in animal models as well as in humans (12, 13). The immune-stimulating capacities of therapeutic OX40 interventions have been employed to strengthen vaccine-induced T cell responses, and also to promote anti-tumor immunity (14-16). Moreover, OX40 signaling has been suggested to be involved in the development of follicular T helper cell (Tfh) responses through association with induction of CXCR5 (17-20) and the importance of humoral immune responses in controlling persistent viruses is increasingly appreciated (9, 10, 21-23). Thus, reagents that trigger OX40 signaling might constitute an interesting approach to boost cellular and humoral immunity that could combat persistent or chronic viral infection. In order to study the effects of exogenous OX40 stimulation in this scenario, we used the LCMV clone 13 model where high viral titers are maintained for several weeks after infection of mice. Previous studies of acute or latent viruses such as vaccinia virus and cytomegalovirus have shown that targeting OX40 can promote beneficial effects in both cytotoxic and helper arms of the adaptive immune response leading to curtailed viral replication (12, 24, 25). Here, we describe the unexpected observation that augmenting OX40 signaling with an agonist antibody during the early stages of LCMV infection profoundly diverted the CD4 T cell response away from Tfh differentiation, and also exacerbated CD8 T cell immunopathology. We demonstrate that agonistic OX40 signaling at an early time drives Blimp-1 expression in LCMV-specific CD4 T cells and Th1 biased CD4 T cell differentiation. As Blimp-1 antagonizes development of follicular helper T cells (Tfh), enforcing OX40 signaling above endogenous levels then becomes deleterious, severely hampering the induction of humoral immunity against LCMV.
Methods
Mice and viruses
All animals were housed at the La Jolla Institute for Allergy and Immunology (LIAI) vivarium under specific pathogen free conditions. C57BL/6 mice were purchased from The Jackson Laboratory. WT and OX40−/− P14 CD8 TCR transgenic mice (LCMV-GP33-41-specific) and wild type, CD25−/− and Blimp-1-YFP reporter Smarta CD4 TCR transgenic mice (LCMV-GP61-80-specific) were bred in house on a C57BL/6 background (26, 27). LCMV infection of 5-8 week old mice was performed either intravenously with 2 × 106 PFU of LCMV cl13 or intraperitoneally with 2 × 105 PFU of LCMV Armstrong or 2 × 103 PFU of LCMV cl13 as indicated. 10 × 105 PFU, and 5 × 105 PFU were used for day 2, and 3 experiments, respectively. All experiments involving mice were reviewed and approved by the La Jolla Institute’s Animal Care Committee (AP152-MvH6).
Cell transfer
Splenocytes from TCR transgenic Smarta and P14 mice (LCMV-GP61-80-specific and LCMV-GP33-41-specific, respectively) on a WT background were harvested and negative CD4 (Smarta) and CD8 (P14) isolation was performed using purified rat antibodies against CD8/CD4, B220, CD11b, CD11c, I-A/I-E, and CD16/32 followed by incubation with sheep anti-rat Dynal Beads (Invitrogen). Isolation of CD25−/− Smarta cells was performed by cell sorting via FACS Aria and CD44-CD62L+CD4+CD19− cells were isolated to exclude activated cells as CD25−/− mice are prone to autoimmunity. Equal numbers of transgenic cells (5,000) were then transferred into naïve WT mice prior to LCMV-infection for analysis on day 7 or later in both Armstrong and cl13 infection. For analysis of transferred cells on days 2 and 3 post infection, 1× 106 and 0.5× 106 Smarta cells were transferred as previously described (10, 26).
In vivo antibody treatment
Mice received a single i.p. injection of 100 μg agonistic OX40 antibody (clone OX86, Biolegend and M. Croft) one day post infection or at later time points post infection as indicated. CD8 depletion experiments were performed by i.p. administration of 500 μg anti-CD8 antibody (clone: 2.43, BioXCell) on days 1 and 3 p.i. Fas ligand was blocked by i.p. administration of 200 μg anti-FasL (clone: MFL4, eBioscience) on days 1 and 3 post infection.
Flow Cytometry
Splenic single cell suspensions were prepared by mashing spleen through a cell strainer into a petir-dish filled with ACK-lysis buffer. Following 90 seconds of red blood cell lysis, cells were washed three times and subsequently counted (countess cell counter, Invitrogen). Surface staining was performed using either fluorescently-labeled or biotinylated antibodies against CD8, CD4, CD19, B220, CXCR5 (26), PD-1, CD150 (SLAM), KLRG-1, CD127, FAS, IgD, CD45.1, CD45.2, and PNA. PE- or APC-labeled Streptavidin was used to stain biotinylated antibodies. PE-labeled GP66-tetramers (NIH tetramer core facility) or GP33-pentamers (Proimmune) were used according to manufacturers instruction. Bcl-6 (clone K112-91) and T-bet (clone 4B10) were stained intracellularly after permeabilization with FoxP3 staining buffer set (eBioscience). To exclude dead cells, LIVE/DEAD (Invitrogen) viability dye was used.
Intracellular cytokine staining was performed following five hours of in vitro stimulation with CD8- (GP33, GP276) and CD4-restricted (GP61) LCMV-epitopes (10 μg/ml) at 37°C in supplemented RPMI-medium (Invitrogen). Permeabilization was performed using Cytofix/Cytoperm (BD) and followed by incubation with fluorescently-labeled antibodies against IL-2, IFN-γ, and TNF. Acquisition was done on a LSRII (BD) using DIVA software (BD) and post-acquisition analysis was performed using FlowJo software (Tree Star).
Plaque Assay
Vero cells (ATCC #CRL-1587) were cultured in supplemented DMEM (Invitrogen) at 37°C and plated in 6 well plates. On the next day, vero cell monolayers were exposed to serum or tissue homogenate of selected mice in 1/10 dilutions and overlaid with agarose and 2× media. 5 (organs) or 6 (serum) days post infection, cells were fixed, stained with crystal violet and plaque forming units (PFU) were counted.
Immunofluorescence
Spleen sections were embedded in optimal cutting temperature (TissueTek) and stored at −80°C. Samples were cut on a cryostat and 6 μm thick samples were stained with fixed with acetone, blocked and stained with CD45.1-PE and B220-FITC. After incubation, samples were washed and stained with a purified rabbit anti-PE antibody. The last staining step was performed with a goat, anti-rabbit IgG-AF594 and a donkey, anti-rat IgG AF488. Sections were washed and mounted with Prolong Gold antifade mounting medium with DAPI (Invitrogen).
LCMV-antibody ELISA
Coating of Elisa plates (Nunc) was performed overnight with LCMV infected cell lysate. Wells were blocked and LCMV-specific IgG was detected in pre-diluted serum samples using HRP-labeled goat anti-mouse IgG (Invitrogen). SureBlue Reserve TMB Kit (KPL) was used as substrate and absorbance was analyzed with a Spectra Max M2e (Molecular Devices).
Statistics
Statistical analyses were performed using GraphPad Prism 5 software (GraphPad). P-values were calculated using the unpaired, two tailed Student’s t-test. All error bars throughout the manuscript are SD. Values of p < 0.05 were considered significant. *p< 0.05, **p < 0.01 and ***p <0.001.
Results
Impaired antiviral immune responses and loss of viral control following early OX40 stimulation
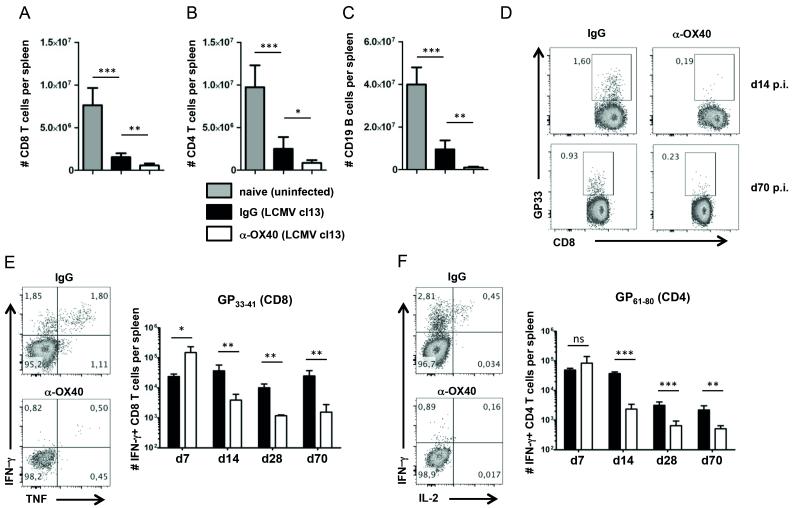
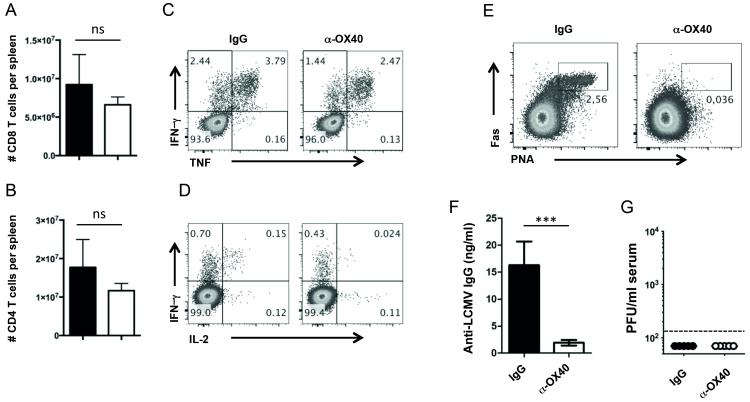
In order to analyze whether exogenous OX40 stimulation has the potential to prevent the development of T cell exhaustion and accelerate virus clearance in LCMV cl13 infection, we infected C57BL/6 mice intravenously with 2×106 PFU of LCMV cl13. One day post infection, we intraperitoneally administered a single injection of 100 μg agonistic OX40 antibody (clone OX86). As LCMV cl13 infected mice develop severe lymphopenia caused by immunopathology, we analyzed T and B cell populations 14 days post LCMV cl13 infection in anti-OX40 treated and control animals. Endogenous OX40 signaling promotes the clonal expansion and survival of both CD4 and CD8 T cells in many immune responses, including during LCMV cl13 infection, but unexpectedly, we found that agonistic anti-OX40 treatment dramatically aggravated LCMV cl13 induced lymphopenia as shown by a profound reduction of T and B cell numbers (Fig. 1, A – C). Similar to our observations on bulk T cell populations, we detected significantly lower numbers of virus-specific CD8 and CD4 T cells in anti-OX40 treated mice (Fig. 1, D – F). Strikingly, while OX40 agonist treatment resulted in a transient increase of IFN-γ producing CD4 and CD8 T cells around day 7 post infection, it caused a sustained reduction of functional effector T cells at all later times (Fig. 1, E and F).
Figure 1. Impaired anti-viral immune responses following OX40 stimulation.
(A – F) C57BL/6 mice were challenged intravenously with 2×106 PFU/ml of LCMV cl13. Mice received a single injection of 100 μg agonistic OX40 antibody (clone OX86) or rat IgG1 (placebo group) one day post infection. (A – C) Bar graphs show number of CD8 T cells, CD4 T cells and B cells per spleen in naïve and untreated (grey bars), infected and IgG treated (black bars) and infected and α-OX40 treated (white bars) mice 14 days p.i. (n = 4-6 per group). (D) GP33 pentamer staining of splenocytes from IgG and α-OX40 treated mice 14 days and 70 days p.i. FACS-plots are gated on CD8+CD19− cells. (E and F) Intracellular cytokine staining (ICCS) was performed on splenocytes of IgG (black bars) and α-OX40 treated (white bars) mice on day 7, 14, 28 and 70 p.i. (n = 4-6 per group and time point) following in vitro stimulation of splenocytes with the GP33 peptide (E) and with the GP61 peptide (F). ICCS FACS-plots from day 14 p.i. analyses are gated on CD8+CD19− cells (E) and CD4+CD19− cells (F). Data are derived from 2 independent experiments per timepoint (1 experiment for day 70). *p< 0.05, **p < 0.01 and ***p <0.001.
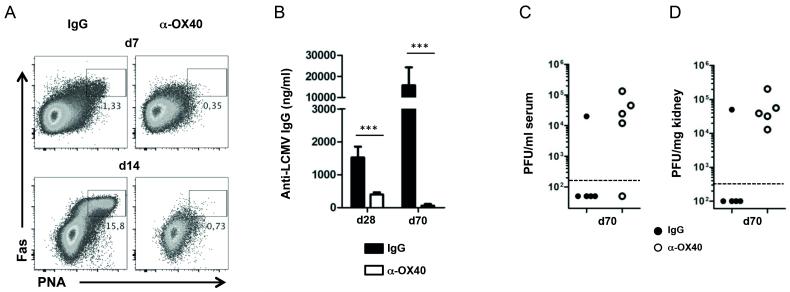
Next, we sought to analyze how the defective T cell response would affect the establishment of LCMV-specific antibody responses, as CD4 T cells are critically required for the development of humoral immunity (28). We found that OX40 stimulation heavily impaired the generation of germinal centers as characterized by the absence of Fas+ PNA+ IgD− germinal center B cells (Fig. 2A), and dramatically reduced anti-LCMV serum IgG titers by day 70 after infection (Fig. 2 B). Thus, while endogenous OX40 signals are critical for antibody responses to LCMV cl13 infection (10), enhancing OX40 engagement surprisingly had powerful negative effects on humoral immunity.
Figure 2. Impaired generation of LCMV-specific antibody responses and loss of viral control after OX40 stimulation.
(A – D) C57BL/6 mice were infected with LCMV cl13 and treated with 100 μg rat IgG1 or α-OX40 one day p.i. (A) Germinal center B cells (Fas+PNA+IgD−) were analyzed 7 and 14 days p.i. (n = 4-5 per group) in IgG and α-OX40 treated mice. FACS plots are gated on CD19+ cells. (B) LCMV-specific IgG antibody titers were analyzed by Elisa on days 28 and 70 post infection (n = 4-5 per group). (C) Serum and (D) kidney samples of treated and untreated mice were harvested on day 70 post infection and viral loads were determined by plaque assay. Data are derived from a total of 2-3 independent experiments (1 experiment for day 70). ***p <0.001.
As LCMV-specific T cells and antibody responses are required for the long-term control of LCMV cl13 infection, we assessed LCMV titers by plaque assay on day 70 post infection. While control animals eventually control viral replication, LCMV titers remained high in the anti-OX40 treated groups (Fig. 2, C and D), consistent with the defective T cell and antibody responses observed.
Delayed OX40 stimulation does not affect LCMV-specific immunity and virus control
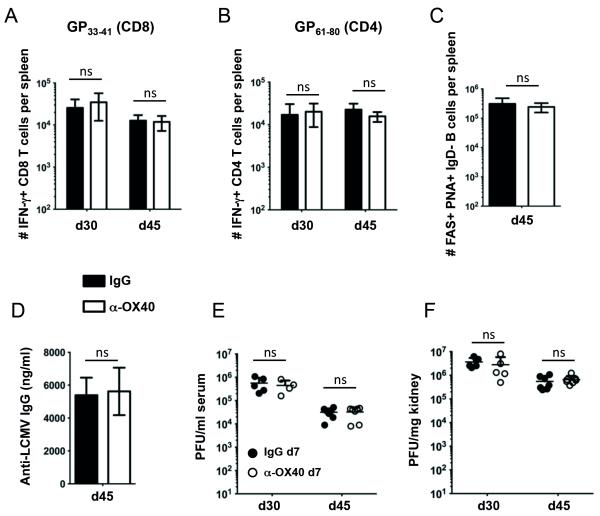
Next, we assessed whether the effect of anti-OX40 was time-dependent. We previously found that OX40 was strongly expressed on LCMV-specific CD4 T cells for at least 20 days throughout the course of LCMV cl13 infection. In contrast, OX40 was only transiently expressed on LCMV-specific CD8 T cells during the initial 5 days of infection (10). Thus, instead of treatment at day 1, anti-OX40 was given 7 days post infection with LCMV cl13. Interestingly, our experiments revealed that delayed OX40 stimulation did not affect LCMV-specific immunity. Indeed, OX40 agonist treatment on day 7 post LCMV cl13 infection did not significantly impact the numbers of LCMV-specific IFN-γ producing CD8 and CD4 T cells (Fig. 3, A and B), Fas+ PNA+ IgD− germinal center B cells (Fig. 3C), or anti-LCMV serum IgG titers (Fig. 3D). Consequently, anti-OX40 treatment on day 7 did not impact viral titers in treated mice (Fig. 3E).
Figure 3. Delayed OX40 stimulation does not alter LCMV-specific immunity and viral control.
(A – F) C57BL/6 mice were challenged intravenously with 2×106 PFU/ml of LCMV cl13. Mice received a single injection of 100 μg agonistic OX40 antibody (clone OX86) or rat IgG1 (placebo group) seven days post infection. (A and B) Intracellular cytokine staining (ICCS) was performed on splenocytes of IgG (black bars) and α-OX40 treated (white bars) mice on day 30, and 45 p.i. (n = 4-7 per group and time point) following in vitro stimulation of splenocytes with the GP33 peptide (A) and with the GP61 peptide (B). (C) Germinal center B cells (Fas+PNA+IgD−) were analyzed 45 days p.i. (n = 5-7 per group) in IgG and α-OX40 treated mice. (D) LCMV-specific IgG antibody titers were analyzed by Elisa on day 45 post infection (n = 5-7 per group). (E) Serum and (F) kidney samples of treated and untreated mice were harvested on days 30 and 45 post infection and viral loads were determined by plaque assay. Data are derived from 2 independent experiments.
OX40 triggers T cell mediated immunopathology
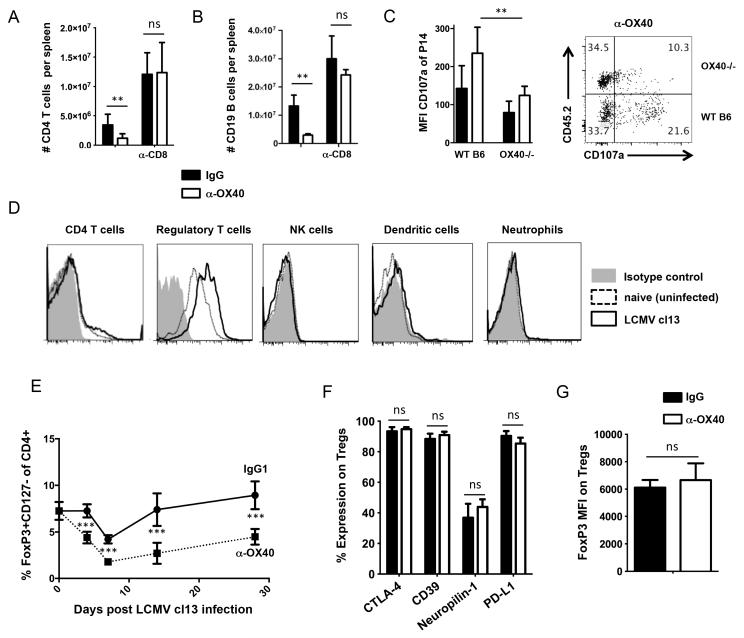
LCMV cl13 induced lymphopenia is predominantly mediated by CD8 T cell immunopathology (29, 30). Thus, we hypothesized that exacerbated lymphopenia in anti-OX40 treated mice was attributed to overstimulation of the CD8 T cell compartment. Notably, we have previously shown that OX40 is strongly expressed on LCMV specific CD8 T cells early after infection, suggesting that they might be direct targets of the agonist antibody (10). In line with this, the more severe lymphopenia in both CD4 T cell and CD19+ B cell compartments triggered by anti-OX40 (Fig. 1) could be prevented by CD8 depletion (Fig. 4, A and B). In order to address whether direct OX40 signaling causes over-activation of CD8 T cells, we co-transferred OX40+/+ and OX40−/− P14 TCR-transgenic CD8 T cells into WT hosts and analyzed CD107a expression seven days post LCMV-infection in IgG and anti-OX40 treated mice directly ex vivo. OX40+/+ P14 cells displayed higher CD107a expression than OX40−/− P14 cells, suggesting that OX40 engagement from the endogenous ligand and from exogenous anti-OX40 treatment directly stimulated LCMV-specific CD8 T cells to degranulate and this might have contributed to immunopathological events (Fig. 4C).
Figure 4. OX40 triggered lymphopenia is mediated by CD8 T cells.
(A, B) C57BL/6 mice were infected with LCMV cl13 and treated with 100 μg rat IgG1 or α-OX40 one day p.i. CD8 T cells were depleted by i.p. administration of 500 μg anti-CD8 antibody on days 1 and 3 post infection. CD4 T cell and B cell numbers were assessed by flow cytometry 14 days post infection. (C) 2,000 naive LCMV-GP33-41-specific cells from CD8 TCRtg (P14) OX40+/+(WT B6, CD45.1+/+) and OX40−/− (CD45.1+CD45.2+) mice were transferred into CD45.2+/+ recipients prior to LCMV cl13 infection. Mice were treated with 100 μg rat IgG1 or α-OX40 one day p.i. and CD107a expression was assessed directly ex vivo 7 days p.i. Representative FACS-plot gated on live CD8+CD45.1+ cells. (D) OX40 expression on different cell subsets 2 days post LCMV cl13 infection. (E) Longitudinal analysis of regulatory T cell frequencies (Tregs, CD4+CD127-FoxP3+) during LCMV cl13 infection in mice treated with IgG1 (circles) or 100 μg α-OX40 one day p.i. (squares). (F) Expression levels of CTLA-4 (intra- and extracellular), CD39, Neuropilin-1 and PD-L1 on Tregs seven days p.i. in mice treated with 100 μg rat IgG1 or α-OX40 one day p.i. (G) FoxP3 MFI on Tregs seven days p.i. in mice treated with 100 μg rat IgG1 or α-OX40 one day p.i. Data are derived from at least 2 independent experiments with 4-6 mice per group. *p < 0.05, **p <0.01 and ***p <0.001.
In order to determine whether other cell populations might be targets of anti-OX40, we additionally analyzed OX40 expression on CD4 T cells, NK cells, dendritic cells and neutrophils 48 hours following cl13 infection (Fig 4D). Interestingly, LCMV cl13 infection resulted in an upregulation of OX40 on regulatory T cells (Fig. 4D). We previously published that OX40 agonist treatment can expand CD4 T cells with a regulatory phenotype that are able to prevent autoimmune diabetes in mice (31). Thus, in order to address the role of Tregs in anti-OX40 treated mice during LCMV-infection, we longitudinally analyzed Treg numbers (Fig. 4E). Interestingly, we observed that Tregs were significantly reduced in mice treated with anti-OX40 early after LCMV cl13 infection. OX40 signals have been reported to cause Treg-exhaustion that is characterized by lower levels of FoxP3 (32). To determine whether the Tregs in anti-OX40 treated mice displayed an exhausted phenotype, we analyzed several molecules that have been implicated in the suppressive activities of Tregs, such as PD-L1, CTLA-4, CD39 and Neuropilin-1 (33-35). These experiments revealed that Tregs from anti-OX40 treated mice were indistinguishable from Tregs derived from IgG-treated mice regarding FoxP3, PD-L1, CTLA-4, CD39 and Neuropilin-1 expression levels (Fig. 4, F and G). Collectively, our findings suggest that CD8 mediated immunopathology following anti-OX40 treatment is likely a combined consequence both of direct activation of LCMV-specific CD8 T cells and reduced numbers of Tregs.
In contrast to LCMV cl13 infection, immunopathology and lymphopenia are typically not observed during LCMV Armstrong infection (4). Thus, we assessed the effects of anti-OX40 treatment following acute LCMV Arm infection. Analysis was performed 20 days post infection, as this is a time point at which global and virus-specific lymphopenia was most severe in LCMV cl13 infection (Fig. 1). Anti-OX40 did not induce lymphopenia with LCMV Arm infection (Fig. 5, A and B). Similarly, there was minimal change in virus-specific CD8 and CD4 T cell numbers (Fig. 5, C and D). Unexpectedly, a pronounced reduction in germinal center B cells and LCMV-specific antibodies occurred in acute LCMV Arm infection (Fig. 5, E and F), indicating that the anti-OX40 triggered loss of humoral immunity was not a consequence of immunopathology. Despite the loss of a functional germinal center response, LCMV Armstrong infected mice treated with anti-OX40 did not display prolonged viremia, since LCMV Arm is primarily controlled by CD8 T cells (Fig. 5 G).
Figure 5. Impaired humoral immunity following OX40 stimulation during LCMV-Armstrong despite absence of enhanced immunopathology.
(A – G) C57BL/6 mice were infected with LCMV Armstrong and treated with 100 μg rat IgG1 or α-OX40 one day p.i. Mice were euthanized 20 days post infection. CD4 and CD8 T cell numbers were determined by flow cytometry (A and B). (C and D) Intracellular cytokine staining (ICCS) was performed following in vitro stimulation of splenocytes with the GP33 peptide (CD8 T cells) or GP61 peptide (CD4 T cells). FACS-plots are gated on CD8+CD19− cells (C) and CD4 T cells (D). (E) GC B cells were analyzed in IgG and α-OX40 treated mice, FACS-plot are gated on CD19 B cells. (F) Anti-LCMV IgG responses were analyzed by Elisa. (G) Serum samples of treated and untreated mice were harvested on day 20 post infection and viral loads were determined by plaque assay. Data are derived from at least 2 independent experiments with 4-6 mice per group. ***p <0.001.
Loss of follicular helper cell responses in anti-OX40 treated mice
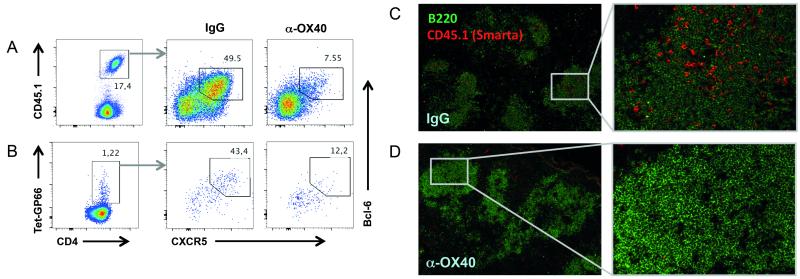
The observation that early anti-OX40 treatment profoundly inhibited LCMV-specific humoral immunity with both acute and persistent infection was particularly surprising as OX40 deficiency results in a loss of germinal centers and anti-LCMV IgG during chronic LCMV infection (10). Given the profound reduction in germinal center B cells and LCMV-specific antibody titers observed here, we hypothesized that enhanced OX40 signaling negatively impacted the follicular T helper cell (Tfh) population. Tfh cells constitute a distinct CD4 T cell lineage that is committed to the establishment of germinal center responses and characterized by the expression of Bcl-6 and CXCR5 (28, 36). Interestingly, recent work has highlighted the importance of Tfh responses in the context of persistent viral infection (9, 10, 22, 37). Thus, we analyzed the Tfh response in anti-OX40 treated mice. We stained for CXCR5 and the Tfh transcription factor Bcl-6 on day 7 post infection on previously transferred TCR transgenic (TCRtg) CD4 (Smarta) T cells and endogenous virus-specific CD4 T cells (Fig. 6, A and B). In line with the poor antibody response, the virus-specific Bcl-6+CXCR5+ Tfh cell compartment was substantially reduced in mice receiving anti-OX40 (6.8% ± 0.7 N=4) compared to the control group (46.1% ± 1.6 N=5) (Fig. 6A). Moreover, immunofluorescent staining of spleen sections revealed a dramatic reduction of transferred Smarta cells in splenic B cell zones, suggesting impaired T cell-B cell interactions in treated mice compared to control animals (Fig. 6, C and D).
Figure 6. OX40 stimulation inhibits Tfh formation.
Naïve congenic CD4 T cells from TCR transgenic (TCRtg) Smarta mice were transferred into C57BL/6 mice prior to LCMV cl13 infection and treatment with 100 μg rat IgG1 or α-OX40 one day p.i. (A and B) T follicular helper cell (Tfh) responses of IgG and α-OX40 treated mice were analyzed on congenic Smarta cells (A) and endogenous GP66-tetramer-positive cells (B) by CXCR5 surface staining and intracellular staining for Bcl-6 on day 7 post LCMV cl13 infection. (C and D) Spleens were harvested on day 7 p.i., and optimal cutting temperature (OCT) compound embedded. Immunofluorescence analysis was performed on cryostat sections using antibodies to B220 (for B cells) and CD45.1 (for transferred Smarta CD4 T cells) as described in the method section. Representative stainings from 2 independent experiments with 3-5 mice per group are displayed.
Early anti-OX40 treatment drives Blimp-1 expression in LCMV-specific CD4 T cells
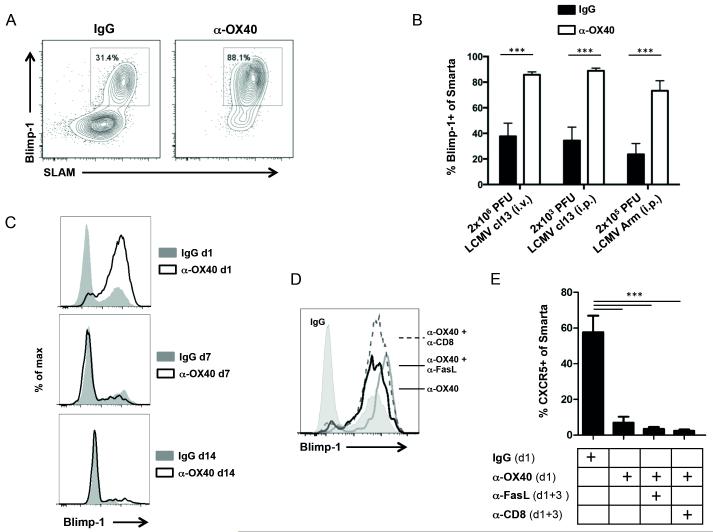
The transcription factors Bcl-6 and Blimp-1 are functional antagonists in CD4 T cell differentiation regulating divergence into Tfh versus effector pathways respectively (36). We hypothesized that exogenous OX40 stimulation during LCMV infection might have driven more T cells to express Blimp-1 and then become effector T helper cells rather than Tfh cells. In order to analyze how OX40 engagement affects Blimp-1 expression by virus-specific CD4 T cells, we transferred 5,000 congenic, naïve Blimp-1-YFP reporter Smarta T cells into B6 mice prior to LCMV cl13 infection. Significantly, Blimp-1 and the Th1 associated surface marker SLAM were strongly expressed by the vast majority of virus-specific CD4 T cells following injection of anti-OX40 (85.8% ± 0.85 N=7), compared to treatment with a control antibody (37.7% ± 3.88 N=7) (Fig. 7A). Interestingly, this was also observed in acute LCMV Armstrong infection (2×105 PFU) given intraperitoneally, as well as low dose LCMV cl13 infection (2×103 PFU, intraperitoneally), suggesting that Blimp-1 induction by OX40 is a broad phenomenon with LCMV infections not determined by the strain, inoculation dose, and route of infection (Fig. 7B). However, when we administered anti-OX40 on days 7 or 14 post LCMV infection, instead of day 1, Blimp-1 expression on virus-specific CD4 T cells was unaltered (Fig. 7C). This indicated that CD4 T cell fate commitment was perhaps already established by day 7 (38).
Figure 7. OX40 stimulation drives Blimp-1 expression.
(A – E) 5,000 naïve congenic CD4 T cells from TCR transgenic (TCRtg) Smarta Blimp-1-YFP reporter mice were transferred into C57BL/6 mice prior to LCMV infection. (A and B) Blimp-1 expression and the Th1 associated surface marker SLAM (CD150) were analyzed on transferred Smarta cells 7 days post infection in IgG and α-OX40 treated mice. (B) The same analysis was performed in mice that have been infected intravenously with high-dose LCMV cl13, intraperitoneally with low-dose LCMV cl13 or LCMV Armstrong. Data are representative of 2-3 independent experiments with 3-5 mice per group. (C) 100 μg IgG or α-OX40 was administered 1, 7 or 14 days post LCMV cl13 infection. Analysis of Blimp-1 expression on adoptively transferred Smarta Blimp-1-YFP cells was performed on days 7, 14 and 28 respectively. (D and E) Following adoptive transfer of naïve Smarta Blimp-1-YFP reporter cells and LCMV cl13 infection, mice were treated with 100 μg rat IgG1 or α-OX40 either alone or in combination with CD8 depletion or Fas ligand blockade as indicated. Transferred Smarta cells were assessed for Blimp-1 expression and Tfh markers (CXCR5, PD-1) 7 days post infection. Data are representative of 2 independent experiments with 2-4 mice per group. ***p <0.001.
Both GC B cells and Tfh cells express high levels of the death receptor Fas (CD95). In addition, anti-OX40 treatment resulted in upregulation of Fas on both CD4 and CD8 T cells (data not shown). In order to evaluate if this contributed to poor Tfh development we administered a blocking antibody to Fas Ligand. Analysis of the virus-specific CD4 T cells 7 days p.i. revealed that this did not rescue the Tfh population, suggesting that Fas-mediated depletion was not the underlying cause for the loss of Tfh cells in anti-OX40 treated mice (Fig. 7, D and E). Similarly, we performed CD8 depletion experiments that essentially yielded the same results (Fig. 7, D and E). These data then suggested that the loss of Tfh cells resulted from an early action of OX40 signaling leading to skewed differentiation into Th1 effectors due to Blimp-1 expression.
Altered CD4 T cell differentiation following OX40 stimulation
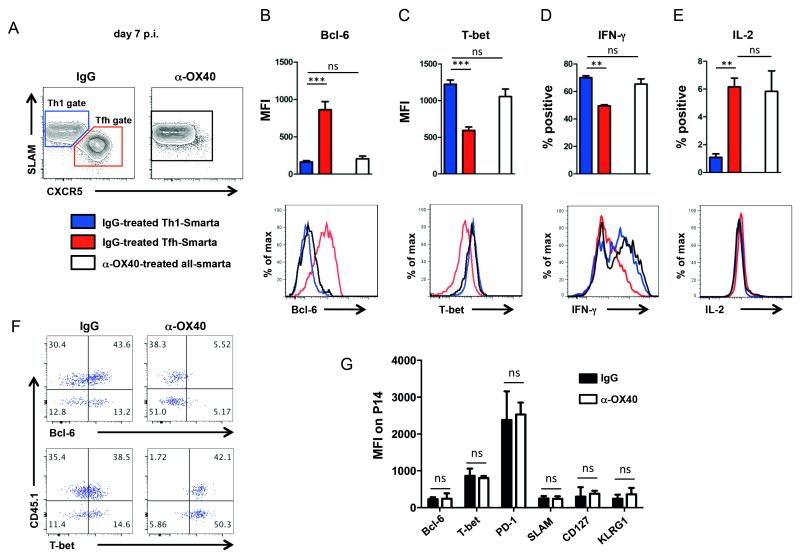
In response to LCMV infection, virus-specific CD4 T cells can be divided into two distinct populations, a Th1 population and a Tfh population (Fig. 8A) (39). Th1 cells are typically characterized by the expression of the transcription factor T-bet (40), the signaling leukocyte activation molecule CD150 (SLAM) (41) and secretion of IFN-gamma (42), whereas Tfh cells primarily express CXCR5 and their transcription factor Bcl-6 (28). In order to more closely define phenotypic and functional properties of the virus-specific CD4 T cells following anti-OX40 treatment, we examined the Th1 and Tfh transcription factors T-bet and Bcl-6, respectively, as well as IFN-γ and IL-2 production. CD4 T cells from anti-OX40 treated mice were phenotypically and functionally almost indistinguishable from Th1 cells in control animals as they expressed low levels of Bcl-6 (Fig. 8B), high levels of T-bet (Fig. 8C), and produced similar amounts of IFN-γ in response to in vitro peptide stimulation (Fig. 8D). Intriguingly, IL-2 expression by the anti-OX40 treated cells was substantially higher than Th1 cells from untreated mice (Fig. 8E). Increases in IL-2 production have been observed following OX40 engagement on CD4 T cells (43). Importantly, the loss of Bcl-6 expression and upregulation of T-bet was observed on both transferred and endogenous LCMV-specific CD4 T cells in response to early anti-OX40 administration (Fig. 8F). Collectively, these results demonstrate that anti-OX40 treatment potently drives Th1 cell differentiation by upregulating Blimp-1 and T-bet expression at the expense of Tfh cell responses.
Figure 8. Altered CD4 T cell differentiation following OX40 stimulation.
(A – E) 5,000 naïve congenic CD4 T cells from TCR transgenic (TCRtg) Smarta Blimp-1-YFP reporter mice were transferred into C57BL/6 mice prior to LCMV infection and analyzed 7 days post infection. T-bet and Bcl-6 expression as well as IFN-γ and IL-2 production (by ICCS) was analyzed on transferred Smarta cells within the Th1 gate (SLAM+CXCR5−, blue) or Tfh gate (SLAM-CXCR5+, red) of IgG treated mice and on transferred Smarta cells of α-OX40 treated mice (white). (F) Plots are gated on CD4+GP66-tetramer+ T cells. CD45.1+ cells are transferred Smarta cells, CD45.1-negative cells are endogenous LCMV-specific CD4 T cells. T-bet and Bcl-6 expression are identically altered in endogenous and transferred LCMV-specific CD4 T cells following OX40 stimulation. (G) 2,000 naive LCMV-GP33–41-specific cells from CD8 TCRtg mice (P14) were transferred into CD45.1/2 mismatched recipients prior to LCMV cl13 infection. Mice were treated with 100 μg rat IgG1 or α-OX40 one day p.i. Bcl-6, T-bet, PD-1, SLAM, CD127 and KLRG-1 expression was analyzed 7 days post LCMV-infection. Data are representative of 2-3 independent experiments with 3-4 mice per group. **p < 0.01 and ***p <0.001.
Next, we analyzed whether early OX40 stimulation would also affect the differentiation fate of LCMV-specific CD8 T cells. In contrast to the pronounced effect observed in the CD4 T cell population, LCMV-specific CD8 T cells remained unaffected following OX40 stimulation as shown by unaltered expression of Bcl-6, T-bet, PD-1, SLAM, CD127 and KLRG1 on GP33-specific CD8 T cells 7 days post infection (Fig. 8G).
IL-2 signaling is required for OX40 mediated effector differentiation
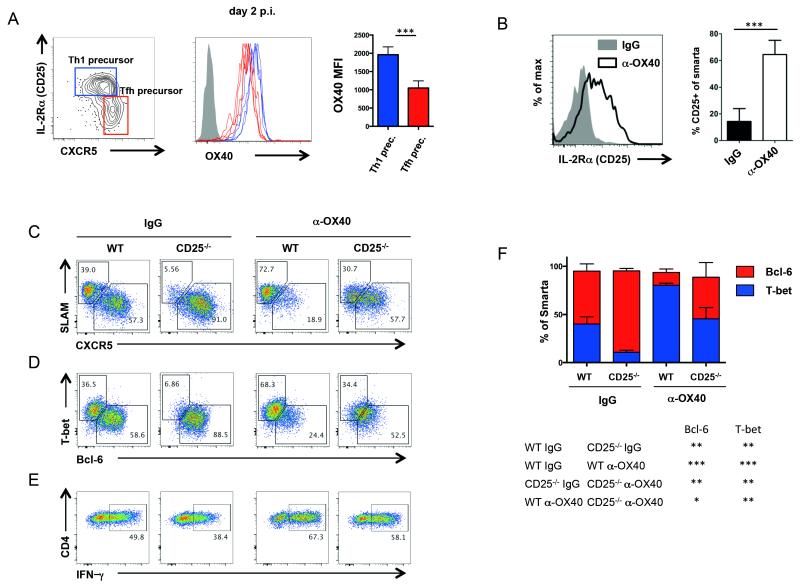
Several factors are known to co-determine the differentiation fate of a naive CD4 T cell in response to LCMV infection. IL-2, in particular, is known to inhibit Tfh differentiation and promote effector T helper cell differentiation through induction of STAT5 activation and Blimp-1 expression (26, 27). Indeed, IL-2 was dose limiting for Tfh versus Th1 differentiation (27). Given that increased IL-2 expression was observed by LCMV-specific CD4 T cells in anti-OX40 treated mice, we explored this pathway in greater detail. Tfh cell precursors can be distinguished from effector T helper precursors through differential expression of Bcl-6, CXCR5, and the alpha unit of the IL-2 receptor (CD25) as early as two days post infection (26). Thus, we analyzed the OX40 expression levels on both populations 48 hours after LCMV infection. Although both populations expressed OX40, we observed significantly higher levels on the effector T helper precursors (Fig. 9A), suggesting that during CD4 T cell differentiation, OX40 may be of particular importance in the generation of non-Tfh effector responses.
Figure 9. Involvement of IL-2 in OX40 mediated Th1 effector differentiation.
(A) 500,000 naïve congenic CD4 T cells from TCR transgenic (TCRtg) Smarta Blimp-1-YFP reporter mice were transferred into C57BL/6 mice prior to LCMV infection and analyzed 2 days post infection. OX40 expression was analyzed on effector Th1 precursors expressing the IL-2 receptor α-chain (CD25) and Blimp-1 and on Tfh precursors expressing CXCR5. (B) Naïve congenic CD4 T cells from TCR transgenic (TCRtg) Smarta mice were transferred into C57BL/6 mice prior to LCMV cl13 infection and treatment with 100 μg rat IgG1 or α-OX40 one day p.i. Expression of CD25 was analyzed on CD4 Smarta cells in both groups on day 7 p.i. (C – F) Naïve WT or CD25−/− CD4 T cells from TCR transgenic (TCRtg) Smarta mice were transferred into congenically mismatched C57BL/6 mice prior to LCMV cl13 infection and treatment with 100 μg rat IgG1 or α-OX40 one day p.i. Representative FACS-plot gated on CD4 Smarta T cells. On day 7 post infection, Th1 and Tfh surface markers (C), transcription factors (D and F) as well as IFN-γ (E) were analyzed in WT and CD25−/− CD4 Smarta T cells. Data are representative for 2 independent experiments (n = 3-5 per group). *p< 0.05, **p < 0.01 and ***p <0.001.
Since OX40 signaling has the ability to enhance IL-2 production, we sought to analyze whether IL-2R signaling was involved in the loss of Tfh responses following OX40 stimulation. Notably, we found that anti-OX40 treatment resulted in upregulation of the alpha unit of the IL-2 receptor on virus-specific CD4 T cells (Fig. 9B). Therefore, we isolated naïve CD4 T cells from WT and IL-2Rα (CD25)-deficient Smarta transgenic mice and co-transferred those cells into WT hosts in order to determine the biological impact of IL-2 signaling on the altered T cell differentiation following anti-OX40 treatment. In mice receiving WT Smarta T cells, anti-OX40 treatment resulted in the loss of Tfh cells expressing Bcl-6 and CXCR5 (Fig. 9, C - E). We found that the vast majority of CD25−/− Smarta T cells in control mice acquired a Tfh phenotype (CXCR5+ Bcl-6+), consistent with previous findings (27, 44). Following anti-OX40 treatment, we observed an increase of Th1 associated markers T-bet and SLAM, a loss of Tfh markers CXCR5 and Bcl-6, and an increase in IFN-γ production by CD25−/− Smarta T cells. However, the effects of anti-OX40 treatment on CD25−/− cells was significantly less than for WT T cells (Fig. 9, C - F), suggesting that IL-2 contributed to the altered T cell differentiation following anti-OX40 treatment. Collectively these data suggest that exogenous OX40 stimulation enhances IL-2 expression and employs the IL-2-STAT5-Blimp1 axis to hamper Tfh differentiation and enhance Th1 development in the context of LCMV infection.
Discussion
In summary, we demonstrate that exogenously targeting the costimulatory receptor OX40 during infection with LCMV results in skewed T cell differentiation, CD8 mediated immunopathology, and loss of LCMV-specific antibody responses. Interestingly, while the impairment of humoral immunity was observed during both acute and persistent infection, the severe immunopathology was largely restricted to persistent LCMV infection. Thus, although endogenous OX40 signaling is required to sustain LCMV-specific immune responses that eventually facilitate control of LCMV cl13 infection (10), providing further stimulation via this receptor results in deleterious consequences.
Although endogenous OX40-OX40L interactions or exogenous treatment with agonists to OX40 can promote clonal expansion and survival of CD8 T cells in a number of settings, including responses to LCMV and several other viruses and to tumors, our findings demonstrate that the lymphopenia during chronic LCMV infection after anti-OX40 treatment was a consequence of CD8 mediated immunopathology. Interestingly, OX40-OX40L interactions have previously been demonstrated to mediate immunopathology in response to respiratory influenza infection (45) suggesting that if excessive OX40 stimulation occurs in certain contexts, this can result in over-exuberant CD8 T cell activity that does not favor the host. Besides direct stimulation of CD8 T cell activity, early OX40 stimulation in the context of LCMV cl13 infection also resulted in a significant reduction in the percentage of Foxp3+ regulatory T cells. Thus, our findings suggest that CD8 mediated immunopathology in OX40-treated mice is a consequence of direct OX40 signaling on CD8 T cells as well as a reduction of Treg frequency resulting in a failure to control the overwhelming T cell response. Recent studies have shown OX40 can influence the activity of certain inflammatory cell types other than T cells. Indeed, under certain conditions, OX40 can regulate neutrophil activity and impact the NK/NKT-pDC axis (11, 46, 47). Although we have not seen relevant levels of OX40 expressed on any of these cells early after LCMV cl13 infection, we cannot exclude the possibility that innate cells contributed to the immunopathology in LCMV cl13 infection following OX40 stimulation.
In contrast to the immunopathology we observed in anti-OX40 treated mice, the loss of humoral immunity could not be attributed to the action of CD8 T cells. A profound reduction of Tfh cells was observed in acute LCMV Armstrong and persistent cl13 infection, even in the absence of CD8 T cells. Our findings clearly demonstrate that anti-OX40 profoundly altered the differentiation fate of LCMV-specific CD4 T cells. There are several known pathways by which OX40 stimulation promotes the development of effector CD4 T cells (43, 48-50). One is the induction of anti-apoptotic Bcl-2 family members and cell cycle proteins that contribute to clonal expansion and survival (51, 52). Very recently it has been shown that anti-OX40 treatment can induce the up-regulation of eomesodermin on CD4 T cells which results in terminal differentiation and enhanced cell lytic capabilities of these cells in a tumor environment (53). OX40 can also promote IL-2 production and IL-2R expression (43, 49, 50). Our data now imply that Blimp-1 induction by autocrine IL-2 is another mechanism by which early and strong OX40 signaling favors effector generation. We demonstrate that OX40 stimulation resulted in a higher frequency of IL-2 secreting CD4 T cells in LCMV infected mice, consistent with enhanced Th1 polarizing conditions, as IL-2+ Th1 cells are regarded as “multifunctional” or highly polarized Th1 cells. In addition, IL-2Ra expression on LCMV-specific CD4 T cells was dramatically increased in anti-OX40 treated animals. Given recent findings that IL-2R signaling potently interferes with Tfh development (27), we tested the importance of IL2 signaling pathways downstream of OX40 engagement. We indeed observed that Tfh differentiation was partially restored in anti-OX40 treated mice when the virus-specific CD4 T cells were CD25−/−.
Our findings here are surprising in the context of the previous observation that endogenous OX40 signaling is critically required for sustaining adaptive immune responses in the context of LCMV infection (10). Antiviral T cell and antibody responses were profoundly reduced in OX40-deficient mice, and while those mice displayed reduced signs of immunopathology (due to weaker CD8 responses), they were incapable of controlling LCMV cl13 replication. Although the molecular machinery downstream of OX40 is likely similar in both cases, the dramatic effects caused by agonist targeting of OX40 in this viral infection demonstrate how tightly OX40 usage is controlled in vivo as both the absence of this molecule and enforced triggering of this molecule resulted in negative consequences for the host. Interestingly, the deleterious activity of anti-OX40 we report here has not been seen in other settings (14, 16, 24, 54). In the context of MCMV infection, which causes persistent viral infection with lower viremia than LCMV, early exogenous OX40 stimulation profoundly enhanced anti-viral CD8 T cell responses but immunopathology was not observed (12). It is likely that skewing toward effector Th1 differentiation and inhibition of Tfh differentiation seen here with LCMV requires co-factors present in the inflammatory environment when OX40 is engaged. Exactly what these may be is unknown but of obvious importance for future agonist therapeutics that might target OX40. Furthermore, the striking outcomes observed here help illuminate the multiple downstream signaling pathways that can be regulated by OX40. Moreover, a virulent virus-induced inflammatory environment also likely varies compared to the environment induced by non-virulent viruses, or adjuvants used for vaccination, or for example in the context of tumor growth. These are all scenarios where reagents to OX40 might be clinically useful. Therapeutic ligation of OX40 using a monoclonal antibody with agonistic properties has already been tested in a phase I cancer trial (ClinicalTrial.gov Identifier: NCT01303705) with no reported adverse activity (AgonOx, Providence Portland Medical Center). This reagent was also safe with no major toxicity when injected into non-human primates following immunization with SIV gp130, and it enhanced both T cell and antibody responses to gp130 (55). However, it will be of great importance to precisely characterize those factors that co-determine the fate of a T cell after OX40 engagement as the possible deleterious effects may constitute an obstacle for successful clinical targeting in humans whether in cancer or vaccination for infectious disease.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH) to MvH (AI089624-02, AI068818-04), MC (CA91837, AI67341, AI49453) and SC (AI072543 and AI063107) and a research fellowship to TB (BO 3361/1-1 and 1-2) from the German Research foundation (DFG).
References
- 1.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 2.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Tinoco R, Alcalde V, Yang Y, Sauer K, Zuniga EI. Cell-intrinsic transforming growth factor-beta signaling mediates virus-specific CD8+ T cell deletion and viral persistence in vivo. Immunity. 2009;31:145–157. doi: 10.1016/j.immuni.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, von Herrath MG. Resolution of a chronic viral infection after interleukin-10 receptor blockade. The Journal of experimental medicine. 2006;203:2461–2472. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nature medicine. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frohlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, Marsland BJ, Oxenius A, Kopf M. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 8.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harker JA, Lewis GM, Mack L, Zuniga EI. Late Interleukin-6 Escalates T Follicular Helper Cell Responses and Controls a Chronic Viral Infection. Science. 2011;334:825–829. doi: 10.1126/science.1208421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boettler T, Moeckel F, Cheng Y, Heeg M, Salek-Ardakani S, Crotty S, Croft M, von Herrath MG. OX40 Facilitates Control of a Persistent Virus Infection. PLoS pathogens. 2012;8:e1002913. doi: 10.1371/journal.ppat.1002913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134) Annual review of immunology. 2010;28:57–78. doi: 10.1146/annurev-immunol-030409-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humphreys IR, Loewendorf A, de Trez C, Schneider K, Benedict CA, Munks MW, Ware CF, Croft M. OX40 costimulation promotes persistence of cytomegalovirus-specific CD8 T Cells: A CD4-dependent mechanism. J Immunol. 2007;179:2195–2202. doi: 10.4049/jimmunol.179.4.2195. [DOI] [PubMed] [Google Scholar]
- 13.Sugamura K, Ishii N, Weinberg AD. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nat Rev Immunol. 2004;4:420–431. doi: 10.1038/nri1371. [DOI] [PubMed] [Google Scholar]
- 14.Goulding J, Tahiliani V, Salek-Ardakani S. OX40:OX40L axis: emerging targets for improving poxvirus-based CD8(+) T-cell vaccines against respiratory viruses. Immunological reviews. 2011;244:149–168. doi: 10.1111/j.1600-065X.2011.01062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirschhorn-Cymerman D, Rizzuto GA, Merghoub T, Cohen AD, Avogadri F, Lesokhin AM, Weinberg AD, Wolchok JD, Houghton AN. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. The Journal of experimental medicine. 2009;206:1103–1116. doi: 10.1084/jem.20082205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salek-Ardakani S, Moutaftsi M, Sette A, Croft M. Targeting OX40 promotes lung-resident memory CD8 T cell populations that protect against respiratory poxvirus infection. Journal of virology. 2011;85:9051–9059. doi: 10.1128/JVI.00619-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brocker T, Gulbranson-Judge A, Flynn S, Riedinger M, Raykundalia C, Lane P. CD4 T cell traffic control: in vivo evidence that ligation of OX40 on CD4 T cells by OX40-ligand expressed on dendritic cells leads to the accumulation of CD4 T cells in B follicles. Eur J Immunol. 1999;29:1610–1616. doi: 10.1002/(SICI)1521-4141(199905)29:05<1610::AID-IMMU1610>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 18.Walker LS, Gulbranson-Judge A, Flynn S, Brocker T, Raykundalia C, Goodall M, Forster R, Lipp M, Lane P. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5-positive CD4 cells and germinal centers. The Journal of experimental medicine. 1999;190:1115–1122. doi: 10.1084/jem.190.8.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu D, Vinuesa CG. The elusive identity of T follicular helper cells. Trends Immunol. 2010;31:377–383. doi: 10.1016/j.it.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Akiba H, Oshima H, Takeda K, Atsuta M, Nakano H, Nakajima A, Nohara C, Yagita H, Okumura K. CD28-independent costimulation of T cells by OX40 ligand and CD70 on activated B cells. J Immunol. 1999;162:7058–7066. [PubMed] [Google Scholar]
- 21.Bergthaler A, Flatz L, Verschoor A, Hegazy AN, Holdener M, Fink K, Eschli B, Merkler D, Sommerstein R, Horvath E, Fernandez M, Fitsche A, Senn BM, Verbeek JS, Odermatt B, Siegrist CA, Pinschewer DD. Impaired antibody response causes persistence of prototypic T cell-contained virus. PLoS Biol. 2009;7:e1000080. doi: 10.1371/journal.pbio.1000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fahey LM, Wilson EB, Elsaesser H, Fistonich CD, McGavern DB, Brooks DG. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. The Journal of experimental medicine. 2011;208:987–999. doi: 10.1084/jem.20101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Streeck H, D’Souza MP, Littman DR, Crotty S. Harnessing CD4(+) T cell responses in HIV vaccine development. Nature medicine. 2013;19:143–149. doi: 10.1038/nm.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Humphreys IR, de Trez C, Kinkade A, Benedict CA, Croft M, Ware CF. Cytomegalovirus exploits IL-10-mediated immune regulation in the salivary glands. The Journal of experimental medicine. 2007;204:1217–1225. doi: 10.1084/jem.20062424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salek-Ardakani S, Flynn R, Arens R, Yagita H, Smith GL, Borst J, Schoenberger SP, Croft M. The TNFR family members OX40 and CD27 link viral virulence to protective T cell vaccines in mice. The Journal of clinical investigation. 2011;121:296–307. doi: 10.1172/JCI42056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, Lao C, Crotty S. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. The Journal of experimental medicine. 2012;209:243–250. doi: 10.1084/jem.20111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crotty S. Follicular helper CD4 T cells (TFH) Annual review of immunology. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 29.Wu-Hsieh BA, Whitmire JK, de Fries R, Lin JS, Matloubian M, Ahmed R. Distinct CD8 T cell functions mediate susceptibility to histoplasmosis during chronic viral infection. J Immunol. 2001;167:4566–4573. doi: 10.4049/jimmunol.167.8.4566. [DOI] [PubMed] [Google Scholar]
- 30.Crotty S, McCausland MM, Aubert RD, Wherry EJ, Ahmed R. Hypogammaglobulinemia and exacerbated CD8 T-cell-mediated immunopathology in SAP-deficient mice with chronic LCMV infection mimics human XLP disease. Blood. 2006;108:3085–3093. doi: 10.1182/blood-2006-04-018929. [DOI] [PubMed] [Google Scholar]
- 31.Bresson D, Fousteri G, Manenkova Y, Croft M, von Herrath M. Antigen-specific prevention of type 1 diabetes in NOD mice is ameliorated by OX40 agonist treatment. Journal of autoimmunity. 2011;37:342–351. doi: 10.1016/j.jaut.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao X, Gong W, Demirci G, Liu W, Spoerl S, Chu X, Bishop DK, Turka LA, Li XC. New insights on OX40 in the control of T cell immunity and immune tolerance in vivo. J Immunol. 2012;188:892–901. doi: 10.4049/jimmunol.1101373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Hopner S, Centonze D, Bernardi G, Dell’Acqua ML, Rossini PM, Battistini L, Rotzschke O, Falk K. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 34.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 35.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. The Journal of experimental medicine. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petrovas C, Yamamoto T, Gerner MY, Boswell KL, Wloka K, Smith EC, Ambrozak DR, Sandler NG, Timmer KJ, Sun X, Pan L, Poholek A, Rao SS, Brenchley JM, Alam SM, Tomaras GD, Roederer M, Douek DC, Seder RA, Germain RN, Haddad EK, Koup RA. CD4 T follicular helper cell dynamics during SIV infection. The Journal of clinical investigation. 2012;122:3281–3294. doi: 10.1172/JCI63039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi YS, Yang JA, Yusuf I, Johnston RJ, Greenbaum J, Peters B, Crotty S. Bcl6 expressing follicular helper CD4 T cells are fate committed early and have the capacity to form memory. J Immunol. 2013;190:4014–4026. doi: 10.4049/jimmunol.1202963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hale JS, Youngblood B, Latner DR, Mohammed AU, Ye L, Akondy RS, Wu T, Iyer SS, Ahmed R. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity. 2013;38:805–817. doi: 10.1016/j.immuni.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 41.Howie D, Okamoto S, Rietdijk S, Clarke K, Wang N, Gullo C, Bruggeman JP, Manning S, Coyle AJ, Greenfield E, Kuchroo V, Terhorst C. The role of SAP in murine CD150 (SLAM)-mediated T-cell proliferation and interferon gamma production. Blood. 2002;100:2899–2907. doi: 10.1182/blood-2002-02-0445. [DOI] [PubMed] [Google Scholar]
- 42.Lederer JA, Perez VL, DesRoches L, Kim SM, Abbas AK, Lichtman AH. Cytokine transcriptional events during helper T cell subset differentiation. The Journal of experimental medicine. 1996;184:397–406. doi: 10.1084/jem.184.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gramaglia I, Weinberg AD, Lemon M, Croft M. Ox-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J Immunol. 1998;161:6510–6517. [PubMed] [Google Scholar]
- 44.Ballesteros-Tato A, Leon B, Graf BA, Moquin A, Adams PS, Lund FE, Randall TD. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity. 2012;36:847–856. doi: 10.1016/j.immuni.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Humphreys IR, Walzl G, Edwards L, Rae A, Hill S, Hussell T. A critical role for OX40 in T cell-mediated immunopathology during lung viral infection. The Journal of experimental medicine. 2003;198:1237–1242. doi: 10.1084/jem.20030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baumann R, Yousefi S, Simon D, Russmann S, Mueller C, Simon HU. Functional expression of CD134 by neutrophils. Eur J Immunol. 2004;34:2268–2275. doi: 10.1002/eji.200424863. [DOI] [PubMed] [Google Scholar]
- 47.Diana J, Griseri T, Lagaye S, Beaudoin L, Autrusseau E, Gautron AS, Tomkiewicz C, Herbelin A, Barouki R, von Herrath M, Dalod M, Lehuen A. NKT cell-plasmacytoid dendritic cell cooperation via OX40 controls viral infection in a tissue-specific manner. Immunity. 2009;30:289–299. doi: 10.1016/j.immuni.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 48.Gramaglia I, Jember A, Pippig SD, Weinberg AD, Killeen N, Croft M. The OX40 Costimulatory Receptor Determines the Development of CD4 Memory by Regulating Primary Clonal Expansion. The Journal of Immunology. 2000;165:3043–3050. doi: 10.4049/jimmunol.165.6.3043. [DOI] [PubMed] [Google Scholar]
- 49.Huddleston CA, Weinberg AD, Parker DC. OX40 (CD134) engagement drives differentiation of CD4+ T cells to effector cells. Eur J Immunol. 2006;36:1093–1103. doi: 10.1002/eji.200535637. [DOI] [PubMed] [Google Scholar]
- 50.Williams CA, Murray SE, Weinberg AD, Parker DC. OX40-mediated differentiation to effector function requires IL-2 receptor signaling but not CD28, CD40, IL-12Rbeta2, or T-bet. J Immunol. 2007;178:7694–7702. doi: 10.4049/jimmunol.178.12.7694. [DOI] [PubMed] [Google Scholar]
- 51.Rogers PR, Song J, Gramaglia I, Killeen N, Croft M. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity. 2001;15:445–455. doi: 10.1016/s1074-7613(01)00191-1. [DOI] [PubMed] [Google Scholar]
- 52.Song J, So T, Cheng M, Tang X, Croft M. Sustained Survivin Expression from OX40 Costimulatory Signals Drives T Cell Clonal Expansion. Immunity. 2005;22:621–631. doi: 10.1016/j.immuni.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 53.Hirschhorn-Cymerman D, Budhu S, Kitano S, Liu C, Zhao F, Zhong H, Lesokhin AM, Avogadri-Connors F, Yuan J, Li Y, Houghton AN, Merghoub T, Wolchok JD. Induction of tumoricidal function in CD4+ T cells is associated with concomitant memory and terminally differentiated phenotype. The Journal of experimental medicine. 2012 doi: 10.1084/jem.20120532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pan PY, Zang Y, Weber K, Meseck ML, Chen SH. OX40 ligation enhances primary and memory cytotoxic T lymphocyte responses in an immunotherapy for hepatic colon metastases. Molecular therapy: the journal of the American Society of Gene Therapy. 2002;6:528–536. doi: 10.1006/mthe.2002.0699. [DOI] [PubMed] [Google Scholar]
- 55.Weinberg AD, Thalhofer C, Morris N, Walker JM, Seiss D, Wong S, Axthelm MK, Picker LJ, Urba WJ. Anti-OX40 (CD134) administration to nonhuman primates: immunostimulatory effects and toxicokinetic study. J Immunother. 2006;29:575–585. doi: 10.1097/01.cji.0000211319.00031.fc. [DOI] [PubMed] [Google Scholar]