Abstract
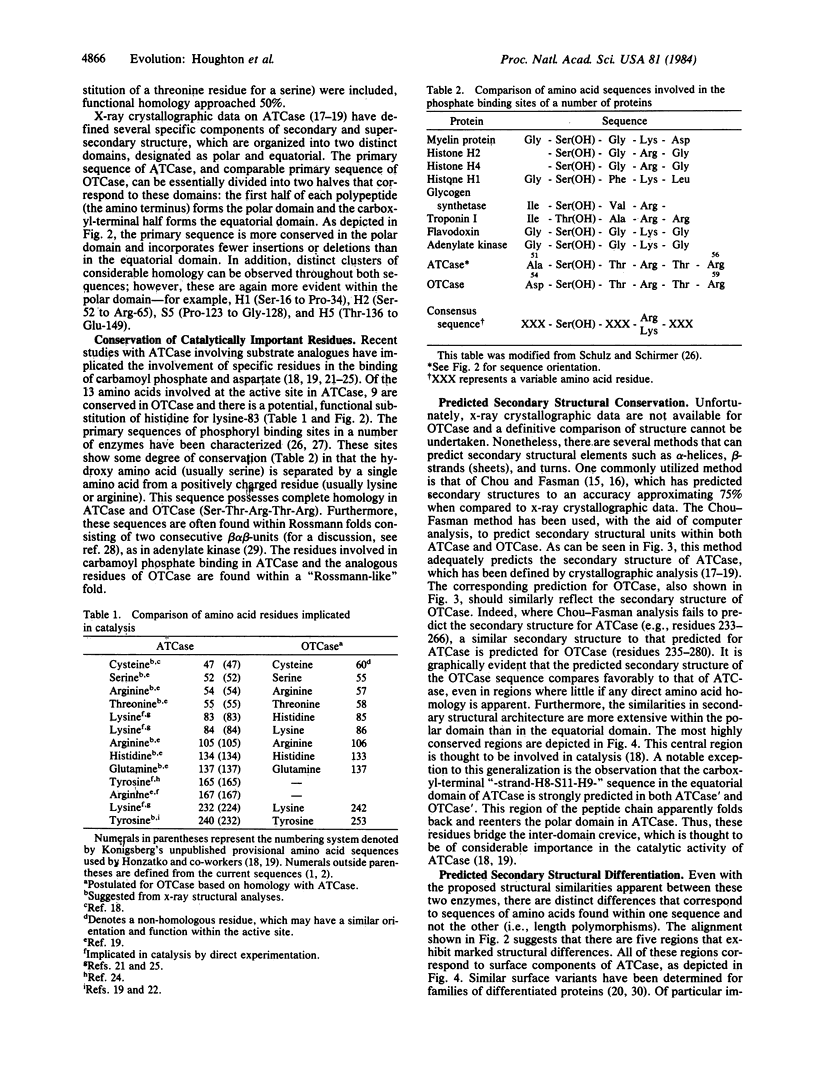
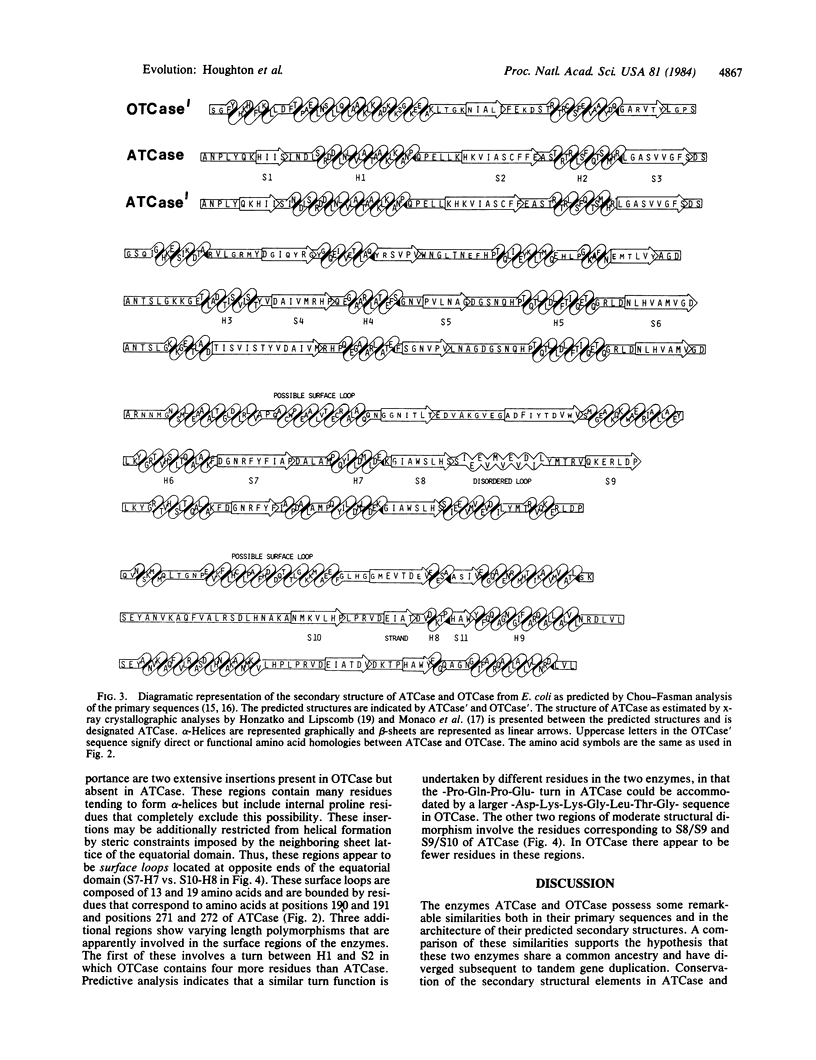
The amino acid sequence of aspartate transcarbamoylase (carbamoylphosphate:L-aspartate carbamoyltransferase, EC 2.1.3.2) has been compared with that of ornithine transcarbamoylase (carbamoylphosphate:L-ornithine carbamoyltransferase, EC 2.1.3.3). The primary sequence homology is 25-40%, depending upon the alignment of homologous residues. The homologies are incorporated into discrete clusters and are interrupted by regions of length polymorphism. The most striking homologies correspond to regions putatively involved in the binding of the common substrate, carbamoyl phosphate. Chou-Fasman predictive analysis [Chou, P. Y. & Fasman, G. D. (1974) Biochemistry 13, 211-222; 222-245] indicates substantial conservation of secondary structural elements within the two enzymes, even in regions whose primary sequence is quite divergent. The results reported herein demonstrate that the two enzymes, aspartate transcarbamoylase and ornithine transcarbamoylase, share a common evolutionary origin and appear to have retained similar structural conformations throughout their evolutionary development.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencini D. A., Houghton J. E., Hoover T. A., Foltermann K. F., Wild J. R., O'Donovan G. A. The DNA sequence of argI from Escherichia coli K12. Nucleic Acids Res. 1983 Dec 10;11(23):8509–8518. doi: 10.1093/nar/11.23.8509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Craik C. S., Rutter W. J., Fletterick R. Splice junctions: association with variation in protein structure. Science. 1983 Jun 10;220(4602):1125–1129. doi: 10.1126/science.6344214. [DOI] [PubMed] [Google Scholar]
- Delbaere L. T., Hutcheon W. L., James M. N., Thiessen W. E. Tertiary structural differences between microbial serine proteases and pancreatic serine enzymes. Nature. 1975 Oct 30;257(5529):758–763. doi: 10.1038/257758a0. [DOI] [PubMed] [Google Scholar]
- Gerhart J. C., Schachman H. K. Distinct subunits for the regulation and catalytic activity of aspartate transcarbamylase. Biochemistry. 1965 Jun;4(6):1054–1062. doi: 10.1021/bi00882a012. [DOI] [PubMed] [Google Scholar]
- Gigot D., Glansdorff N., Legrain C., Piérard A., Stalon V., Konigsberg W., Caplier I., Strosberg A. D., Hervé G. Comparison of the N-terminal sequences of aspartate and ornithine carbamoyltransferases of Escherichia coli. FEBS Lett. 1977 Sep 1;81(1):28–32. doi: 10.1016/0014-5793(77)80920-4. [DOI] [PubMed] [Google Scholar]
- Glansdorff N., Sand G., Verhoef C. The dual genetic control of ornithine transcarbamylase synthesis in Escherichia coli K12. Mutat Res. 1967 Nov-Dec;4(6):743–751. doi: 10.1016/0027-5107(67)90083-8. [DOI] [PubMed] [Google Scholar]
- Honzatko R. B., Crawford J. L., Monaco H. L., Ladner J. E., Ewards B. F., Evans D. R., Warren S. G., Wiley D. C., Ladner R. C., Lipscomb W. N. Crystal and molecular structures of native and CTP-liganded aspartate carbamoyltransferase from Escherichia coli. J Mol Biol. 1982 Sep 15;160(2):219–263. doi: 10.1016/0022-2836(82)90175-9. [DOI] [PubMed] [Google Scholar]
- Honzatko R. B., Lipscomb W. N. Interactions of phosphate ligands with Escherichia coli aspartate carbamoyltransferase in the crystalline state. J Mol Biol. 1982 Sep 15;160(2):265–286. doi: 10.1016/0022-2836(82)90176-0. [DOI] [PubMed] [Google Scholar]
- Hoover T. A., Roof W. D., Foltermann K. F., O'Donovan G. A., Bencini D. A., Wild J. R. Nucleotide sequence of the structural gene (pyrB) that encodes the catalytic polypeptide of aspartate transcarbamoylase of Escherichia coli. Proc Natl Acad Sci U S A. 1983 May;80(9):2462–2466. doi: 10.1073/pnas.80.9.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempe T. D., Stark G. R. Pyridoxal 5'-phosphate, a fluorescent probe in the active site of aspartate transcarbamylase. J Biol Chem. 1975 Sep 10;250(17):6861–6869. [PubMed] [Google Scholar]
- Konigsberg W. H., Henderson L. Amino acid sequence of the catalytic subunit of aspartate transcarbamoylase from Escherichia coli. Proc Natl Acad Sci U S A. 1983 May;80(9):2467–2471. doi: 10.1073/pnas.80.9.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landfear S. M., Evans D. R., Lipscomb W. N. Elimination of cooperativity in aspartate transcarbamylase by nitration of a single tyrosine residue. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2654–2658. doi: 10.1073/pnas.75.6.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen A. M., Landfear S. M., Lipscomb W. N. Inactivation of the catalytic subunit of aspartate transcarbamylase by nitration with tetranitromethane. J Biol Chem. 1980 Jan 25;255(2):602–607. [PubMed] [Google Scholar]
- Lauritzen A. M., Lipscomb W. N. Modification of three active site lysine residues in the catalytic subunit of aspartate transcarbamylase by D- and L-bromosuccinate. J Biol Chem. 1982 Feb 10;257(3):1312–1319. [PubMed] [Google Scholar]
- Lebioda L., Hatada M. H., Tulinsky A., Mavridis I. M. Comparison of the folding of 2-keto-3-deoxy-6-phosphogluconate aldolase, triosephosphate isomerase and pyruvate kinase. Implications in molecular evolution. J Mol Biol. 1982 Dec 5;162(2):445–458. doi: 10.1016/0022-2836(82)90537-x. [DOI] [PubMed] [Google Scholar]
- Legrain C., Halleux P., Stalon V., Glansdorff N. The dual genetic control of ornithine carbamolytransferase in Escherichia coli. A case of bacterial hybrid enzymes. Eur J Biochem. 1972 May;27(1):93–102. doi: 10.1111/j.1432-1033.1972.tb01814.x. [DOI] [PubMed] [Google Scholar]
- Legrain C., Stalon V., Glansdorff N. Escherichia coli ornithine carbamolytransferase isoenzymes: evolutionary significance and the isolation of lambdaargF and lambdaargI transducing bacteriophages. J Bacteriol. 1976 Oct;128(1):35–38. doi: 10.1128/jb.128.1.35-38.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco H. L., Crawford J. L., Lipscomb W. N. Three-dimensional structures of aspartate carbamoyltransferase from Escherichia coli and of its complex with cytidine triphosphate. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5276–5280. doi: 10.1073/pnas.75.11.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K. T., Iwahashi K., Yamamoto Y., Iitaka Y., Yoshida N., Mitsui Y. Crystal structure of a microbial ribonuclease, RNase St. Nature. 1982 Oct 7;299(5883):564–566. doi: 10.1038/299564a0. [DOI] [PubMed] [Google Scholar]
- Pai E. F., Sachsenheimer W., Schirmer R. H., Schulz G. E. Substrate positions and induced-fit in crystalline adenylate kinase. J Mol Biol. 1977 Jul;114(1):37–45. doi: 10.1016/0022-2836(77)90281-9. [DOI] [PubMed] [Google Scholar]
- Piette J., Cunin R., Van Vliet F., Charlier D., Crabeel M., Ota Y., Glansdorff N. Homologous control sites and DNA transcription starts in the related argF and argI genes of Escherichia coli K12. EMBO J. 1982;1(7):853–857. doi: 10.1002/j.1460-2075.1982.tb01259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S. T., Rossmann M. G. Comparison of super-secondary structures in proteins. J Mol Biol. 1973 May 15;76(2):241–256. doi: 10.1016/0022-2836(73)90388-4. [DOI] [PubMed] [Google Scholar]
- Riley M., Anilionis A. Evolution of the bacterial genome. Annu Rev Microbiol. 1978;32:519–560. doi: 10.1146/annurev.mi.32.100178.002511. [DOI] [PubMed] [Google Scholar]
- Roof W. D., Foltermann K. F., Wild J. R. The organization and regulation of the pyrBI operon in E. coli includes a rho-independent attenuator sequence. Mol Gen Genet. 1982;187(3):391–400. doi: 10.1007/BF00332617. [DOI] [PubMed] [Google Scholar]
- Sachsenheimer W., Schulz G. E. Two conformations of crystalline adenylate kinase. J Mol Biol. 1977 Jul;114(1):23–36. doi: 10.1016/0022-2836(77)90280-7. [DOI] [PubMed] [Google Scholar]
- Wall K. A., Flatgaard J. E., Schachman H. K., Gibbons I. Purification and characterization of a mutant aspartate transcarbamoylase lacking enzyme activity. J Biol Chem. 1979 Dec 10;254(23):11910–11916. [PubMed] [Google Scholar]
- York M. K., Stodolsky M. Characterization of P1argF derivatives from Escherichia coli K12 transduction. I. IS1 elements flank the argF gene segment. Mol Gen Genet. 1981;181(2):230–240. doi: 10.1007/BF00268431. [DOI] [PubMed] [Google Scholar]


