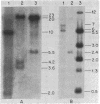
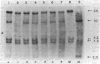
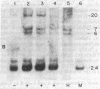
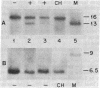
Abstract
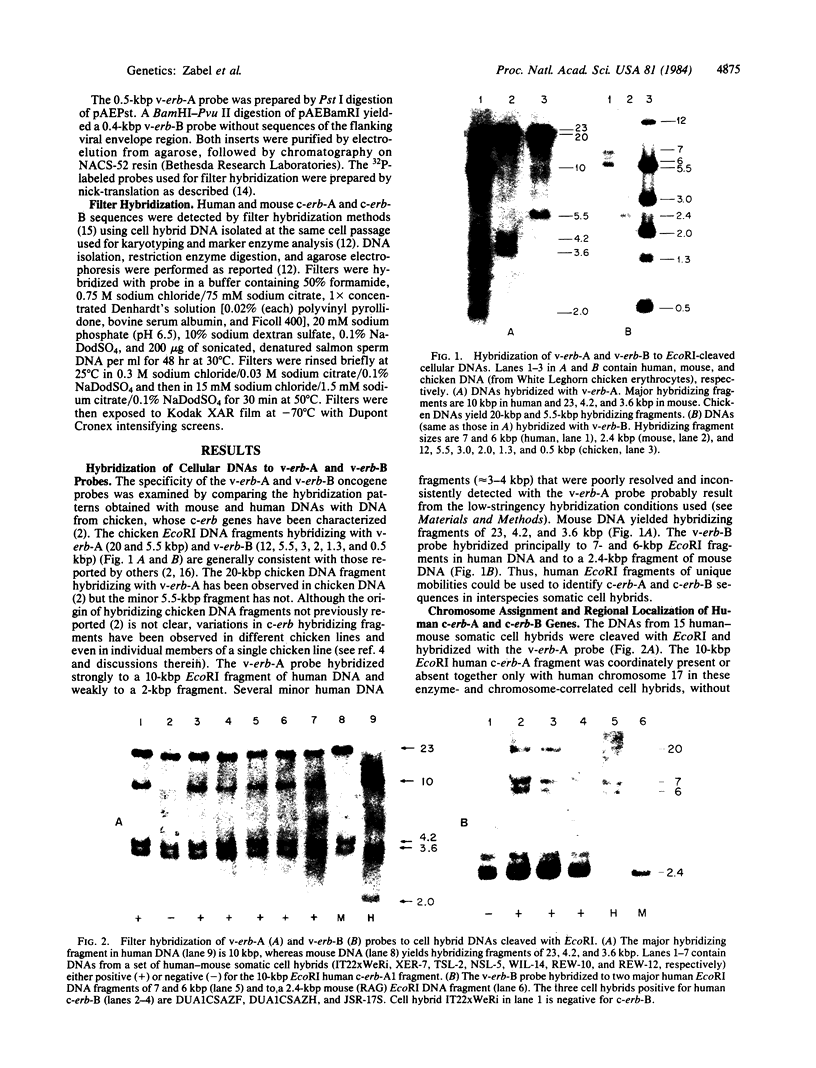
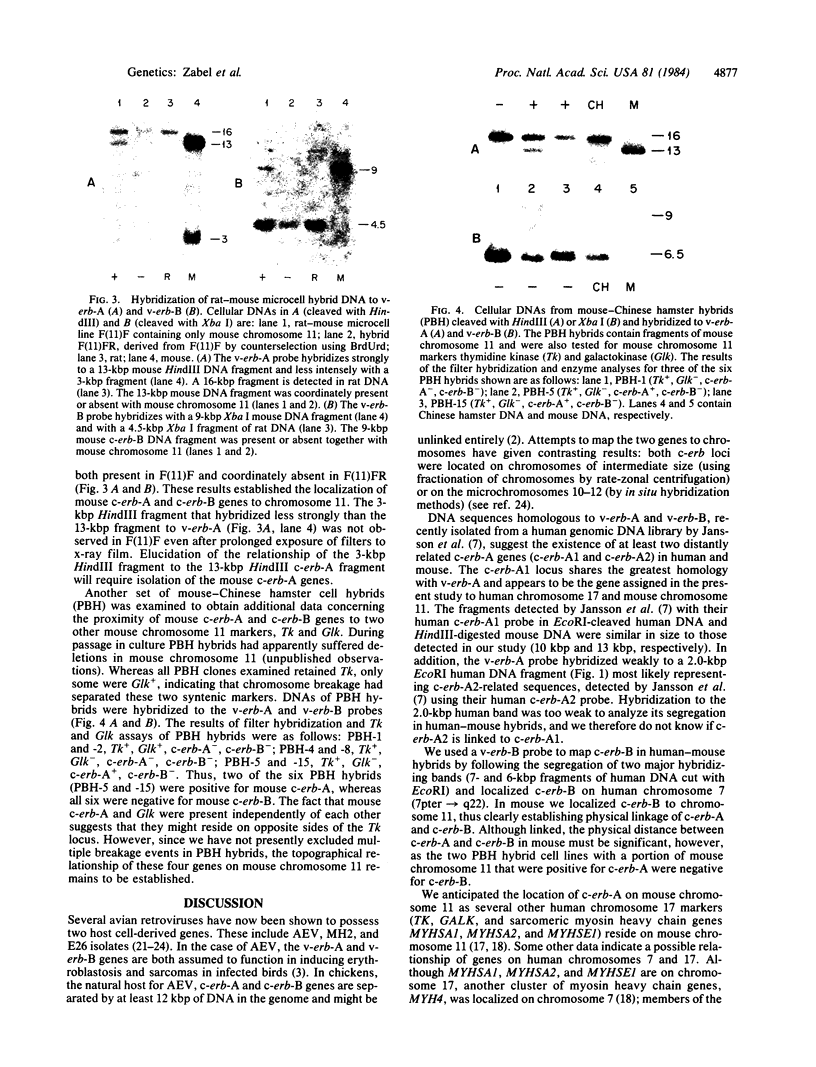
Avian erythroblastosis virus, a retrovirus that causes erythroblastosis and sarcomas in infected birds, possesses two host cell-derived genes [viral (v) erb-A and erb-B]. Although v-erb-B seems to be responsible for oncogenic transformation, v-erb-A might have an enhancing effect on transformation. In chickens, the natural host for avian erythroblastosis virus, cellular (c) erb-A and erb-B genes appear to be unlinked, but their chromosomal locations in other species are unknown. To ascertain the chromosomal location of c-erb genes in man and mouse, we analyzed interspecies somatic cell and microcell hybrids by Southern filter hybridization techniques using specific v-erb-A and v-erb-B probes. We found c-erb-A sequences on human chromosome 17 (17p11----qter) and located c-erb-B on human chromosome 7 (7pter----q22). In contrast, both c-erb-A and c-erb-B reside on mouse chromosome 11.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Borgström G. H., Vuopio P., de la Chapelle A. Abnormalities of chromosome No. 17 in myeloproliferative disorders. Cancer Genet Cytogenet. 1982 Feb;5(2):123–135. doi: 10.1016/0165-4608(82)90003-6. [DOI] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Fournier R. E. A general high-efficiency procedure for production of microcell hybrids. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6349–6353. doi: 10.1073/pnas.78.10.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier R. E., Frelinger J. A. Construction of microcell hybrid clones containing specific mouse chromosomes: application to autosomes 8 and 17. Mol Cell Biol. 1982 May;2(5):526–534. doi: 10.1128/mcb.2.5.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke U., Lalley P. A., Moss W., Ivy J., Minna J. D. Gene mapping in Mus musculus by interspecific cell hybridization: assignment of the genes for tripeptidase-1 to chromosome 10, dipeptidase-2 to chromosome 18, acid phosphatase-1 to chromosome 12, and adenylate kinase-1 to chromosome 2. Cytogenet Cell Genet. 1977;19(2-3):57–84. doi: 10.1159/000130799. [DOI] [PubMed] [Google Scholar]
- Frykberg L., Palmieri S., Beug H., Graf T., Hayman M. J., Vennström B. Transforming capacities of avian erythroblastosis virus mutants deleted in the erbA or erbB oncogenes. Cell. 1983 Jan;32(1):227–238. doi: 10.1016/0092-8674(83)90513-5. [DOI] [PubMed] [Google Scholar]
- Fung Y. K., Lewis W. G., Crittenden L. B., Kung H. J. Activation of the cellular oncogene c-erbB by LTR insertion: molecular basis for induction of erythroblastosis by avian leukosis virus. Cell. 1983 Jun;33(2):357–368. doi: 10.1016/0092-8674(83)90417-8. [DOI] [PubMed] [Google Scholar]
- Goff S. P., D'Eustachio P., Ruddle F. H., Baltimore D. Chromosomal assignment of the endogenous proto-oncogene C-abl. Science. 1982 Dec 24;218(4579):1317–1319. doi: 10.1126/science.6293057. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H. Avian leukemia viruses: interaction with their target cells in vivo and in vitro. Biochim Biophys Acta. 1978 Nov 17;516(3):269–299. doi: 10.1016/0304-419x(78)90011-2. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H. Role of the v-erbA and v-erbB oncogenes of avian erythroblastosis virus in erythroid cell transformation. Cell. 1983 Aug;34(1):7–9. doi: 10.1016/0092-8674(83)90130-7. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N., Groffen J., Stephenson J. R., Spurr N. K., Goodfellow P. N., Solomon E., Carritt B., Bodmer W. F. Chromosomal localization of human cellular homologues of two viral oncogenes. Nature. 1982 Oct 21;299(5885):747–749. doi: 10.1038/299747a0. [DOI] [PubMed] [Google Scholar]
- Jansson M., Philipson L., Vennström B. Isolation and characterization of multiple human genes homologous to the oncogenes of avian erythroblastosis virus. EMBO J. 1983;2(4):561–565. doi: 10.1002/j.1460-2075.1983.tb01463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan N. C., Flordellis C. S., Garon C. F., Duesberg P. H., Papas T. S. Avian carcinoma virus MH2 contains a transformation-specific sequence, mht, and shares the myc sequence with MC29, CMII, and OK10 viruses. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6566–6570. doi: 10.1073/pnas.80.21.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak C. A., Sears J. F., Hoggan M. D. Genetic mapping of the mouse oncogenes c-Ha-ras-1 and c-fes to chromosome 7. J Virol. 1983 Jul;47(1):217–220. doi: 10.1128/jvi.47.1.217-220.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinwand L. A., Fournier R. E., Nadal-Ginard B., Shows T. B. Multigene family for sarcomeric myosin heavy chain in mouse and human DNA: localization on a single chromosome. Science. 1983 Aug 19;221(4612):766–769. doi: 10.1126/science.6879174. [DOI] [PubMed] [Google Scholar]
- Leprince D., Gegonne A., Coll J., de Taisne C., Schneeberger A., Lagrou C., Stehelin D. A putative second cell-derived oncogene of the avian leukaemia retrovirus E26. Nature. 1983 Nov 24;306(5941):395–397. doi: 10.1038/306395a0. [DOI] [PubMed] [Google Scholar]
- Minna J. D., Marshall T. H., Shaffer-Berman P. V. Chinese hamster X mouse hybrid cells segregating mouse chromosomes and isozymes. Somatic Cell Genet. 1975 Oct;1(4):355–369. doi: 10.1007/BF01538667. [DOI] [PubMed] [Google Scholar]
- Mitelman F., Levan G. Clustering of aberrations to specific chromosomes in human neoplasms. IV. A survey of 1,871 cases. Hereditas. 1981;95(1):79–139. doi: 10.1111/j.1601-5223.1981.tb01331.x. [DOI] [PubMed] [Google Scholar]
- Nowell P., Wilmoth D., Lange B. Cytogenetics of childhood preleukemia. Cancer Genet Cytogenet. 1983 Nov;10(3):261–266. doi: 10.1016/0165-4608(83)90054-7. [DOI] [PubMed] [Google Scholar]
- Nunn M. F., Seeburg P. H., Moscovici C., Duesberg P. H. Tripartite structure of the avian erythroblastosis virus E26 transforming gene. Nature. 1983 Nov 24;306(5941):391–395. doi: 10.1038/306391a0. [DOI] [PubMed] [Google Scholar]
- Prakash K., McBride O. W., Swan D. C., Devare S. G., Tronick S. R., Aaronson S. A. Molecular cloning and chromosomal mapping of a human locus related to the transforming gene of Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5210–5214. doi: 10.1073/pnas.79.17.5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rowley J. D., Golomb H. M., Dougherty C. 15/17 translocation, a consistent chromosomal change in acute promyelocytic leukaemia. Lancet. 1977 Mar 5;1(8010):549–550. doi: 10.1016/s0140-6736(77)91415-5. [DOI] [PubMed] [Google Scholar]
- Rowley J. D. Human oncogene locations and chromosome aberrations. Nature. 1983 Jan 27;301(5898):290–291. doi: 10.1038/301290a0. [DOI] [PubMed] [Google Scholar]
- Sakaguchi A. Y., Lalley P. A., Naylor S. L. Human and mouse cellular myc protooncogenes reside on chromosomes involved in numerical and structural aberrations in cancer. Somatic Cell Genet. 1983 May;9(3):391–405. doi: 10.1007/BF01539146. [DOI] [PubMed] [Google Scholar]
- Sakaguchi A. Y., Lalley P. A., Zabel B. U., Ellis R. W., Scolnick E. M., Naylor S. L. Chromosome assignments of four mouse cellular homologs of sarcoma and leukemia virus oncogenes. Proc Natl Acad Sci U S A. 1984 Jan;81(2):525–529. doi: 10.1073/pnas.81.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi A. Y., Naylor S. L., Shows T. B. A sequence homologous to Rous sarcoma virus v-src Is on human chromosome 20. Prog Nucleic Acid Res Mol Biol. 1983;29:279–283. doi: 10.1016/s0079-6603(08)60460-2. [DOI] [PubMed] [Google Scholar]
- Sealy L., Moscovici G., Moscovici C., Bishop J. M. Site-specific mutagenesis of avian erythroblastosis virus: v-erb-A is not required for transformation of fibroblasts. Virology. 1983 Oct 15;130(1):179–194. doi: 10.1016/0042-6822(83)90126-5. [DOI] [PubMed] [Google Scholar]
- Sergeant A., Saule S., Leprince D., Begue A., Rommens C., Stehelin D. Molecular cloning and characterization of the chicken DNA locus related to the oncogene erbB of avian erythroblastosis virus. EMBO J. 1982;1(2):237–242. doi: 10.1002/j.1460-2075.1982.tb01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilo B. Z., Weinberg R. A. DNA sequences homologous to vertebrate oncogenes are conserved in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6789–6792. doi: 10.1073/pnas.78.11.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spurr N. K., Solomon E., Jansson M., Sheer D., Goodfellow P. N., Bodmer W. F., Vennstrom B. Chromosomal localisation of the human homologues to the oncogenes erbA and B. EMBO J. 1984 Jan;3(1):159–163. doi: 10.1002/j.1460-2075.1984.tb01777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan D., Oskarsson M., Keithley D., Ruddle F. H., D'Eustachio P., Vande Woude G. F. Chromosomal localization of the Moloney sarcoma virus mouse cellular (c-mos) sequence. J Virol. 1982 Nov;44(2):752–754. doi: 10.1128/jvi.44.2.752-754.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennström B., Bishop J. M. Isolation and characterization of chicken DNA homologous to the two putative oncogenes of avian erythroblastosis virus. Cell. 1982 Jan;28(1):135–143. doi: 10.1016/0092-8674(82)90383-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Nishida T., Miyajima N., Kawai S., Ooi T., Toyoshima K. The erbB gene of avian erythroblastosis virus is a member of the src gene family. Cell. 1983 Nov;35(1):71–78. doi: 10.1016/0092-8674(83)90209-x. [DOI] [PubMed] [Google Scholar]