Abstract
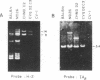
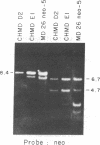
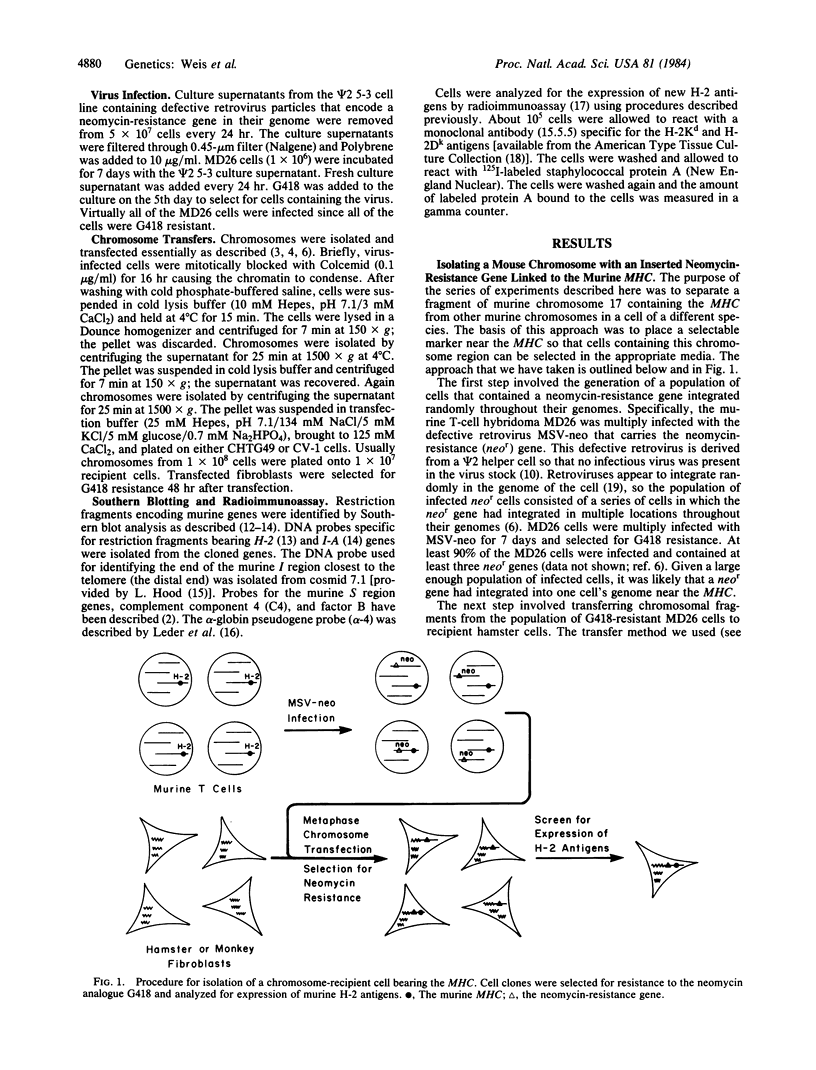
We have developed an approach for genetic analysis of the murine H-2 complex that has broad general applicability to the study of eukaryotic genome organization. We have used a retroviral vector to introduce a selectable marker into the mouse genome close to the major histocompatibility complex (MHC). Chromosomal segments containing large portions of the MHC from these donor cells have been transferred both to hamster and monkey cell recipients. The procedure involved the following steps. First, a murine cell line was multiply infected with a defective recombinant murine leukemia virus that contains the neomycin-resistance gene (a gene that confers resistance to G418). In this way, the neomycin-resistance gene was introduced at multiple sites in the mouse genome. Second, metaphase chromosomes, prepared from this infected cell population, were transferred to hamster cell recipients. Third, two G418-resistant transferents were identified that expressed murine H-2 antigens on their cell surface. These transferents were shown to contain a large segment of the murine MHC (H-2K and I regions) by DNA hybridization. The neomycin-resistance gene and the mouse MHC genes must be physically linked in these cells since they could be cotransferred from the hamster cells to monkey cells. Fourth, the murine cell carrying the neomycin-resistance gene near the MHC was identified from the original donor cell population. This cell will serve as a useful source of chromosome fragments for analysis of larger portions of the MHC. This series of steps can serve as a paradigm for the first steps in a detailed genetic analysis of any specific region of a mammalian genome to which one or more genes have already been mapped.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Athwal R. S., McBride O. W. Serial transfer of a human gene to rodent cells by sequential chromosome-mediated gene transfer. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2943–2947. doi: 10.1073/pnas.74.7.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin D. D., Woods D. E., Whitehead A. S., Goldberger G., Colten H. R., Seidman J. G. Molecular map of the murine S region. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6947–6951. doi: 10.1073/pnas.80.22.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Eustachio P., Fein B., Michaelson J., Taylor B. A. The alpha-globin pseudogene on mouse chromosome 17 is closely linked to H-2. J Exp Med. 1984 Mar 1;159(3):958–963. doi: 10.1084/jem.159.3.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G. A., Margulies D. H., Camerini-Otero R. D., Ozato K., Seidman J. G. Structure and expression of a mouse major histocompatibility antigen gene, H-2Ld. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1994–1998. doi: 10.1073/pnas.79.6.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G. A., Margulies D. H., Shykind B., Seidman J. G., Ozato K. Exon shuffling: mapping polymorphic determinants on hybrid mouse transplantation antigens. Nature. 1982 Dec 23;300(5894):755–757. doi: 10.1038/300755a0. [DOI] [PubMed] [Google Scholar]
- Gusella J. F., Keys C., VarsanyiBreiner A., Kao F. T., Jones C., Puck T. T., Housman D. Isolation and localization of DNA segments from specific human chromosomes. Proc Natl Acad Sci U S A. 1980 May;77(5):2829–2833. doi: 10.1073/pnas.77.5.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann Y., Golstein P., Pierres M., Springer T. A., Eshhar Z. LFA-1 but not Lyt-2 is associated with killing activity of cytotoxic T lymphocyte hybridomas. Nature. 1982 Nov 25;300(5890):357–360. doi: 10.1038/300357a0. [DOI] [PubMed] [Google Scholar]
- Leder A., Swan D., Ruddle F., D'Eustachio P., Leder P. Dispersion of alpha-like globin genes of the mouse to three different chromosomes. Nature. 1981 Sep 17;293(5829):196–200. doi: 10.1038/293196a0. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- McBride O. W., Ozer H. L. Transfer of genetic information by purified metaphase chromosomes. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1258–1262. doi: 10.1073/pnas.70.4.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor A. L., Golden L., Weiss E., Bullman H., Hurst J., Simpson E., James R. F., Townsend A. R., Taylor P. M., Schmidt W. Expression of murine H-2Kb histocompatibility antigen in cells transformed with cloned H-2 genes. Nature. 1982 Aug 5;298(5874):529–534. doi: 10.1038/298529a0. [DOI] [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Rao R. N., Rogers S. G. Plasmid pKC7: a vector containing ten restriction endonuclease sites suitable for cloning DNA segments. Gene. 1979 Sep;7(1):79–82. doi: 10.1016/0378-1119(79)90044-1. [DOI] [PubMed] [Google Scholar]
- Robinson R. R., Germain R. N., McKean D. J., Mescher M., Seidman J. G. Extensive polymorphism surrounding the murine Ia A beta chain gene. J Immunol. 1983 Oct;131(4):2025–2031. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Steinmetz M., Hood L. Genes of the major histocompatibility complex in mouse and man. Science. 1983 Nov 18;222(4625):727–733. doi: 10.1126/science.6356354. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Minard K., Horvath S., McNicholas J., Srelinger J., Wake C., Long E., Mach B., Hood L. A molecular map of the immune response region from the major histocompatibility complex of the mouse. Nature. 1982 Nov 4;300(5887):35–42. doi: 10.1038/300035a0. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Winoto A., Minard K., Hood L. Clusters of genes encoding mouse transplantation antigens. Cell. 1982 Mar;28(3):489–498. doi: 10.1016/0092-8674(82)90203-3. [DOI] [PubMed] [Google Scholar]
- Winoto A., Steinmetz M., Hood L. Genetic mapping in the major histocompatibility complex by restriction enzyme site polymorphisms: most mouse class I genes map to the Tla complex. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3425–3429. doi: 10.1073/pnas.80.11.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]