Abstract
We have postulated that the toxic neuropathies associated with neurofilament-filled axonal swellings have a common pathogenesis, the covalent crosslinking of neurofilaments during anterograde transport. The newly described gamma-diketone, 3,4-dimethyl-2,5-hexanedione (DMHD), is a more potent analogue of the toxic metabolite of n-hexane, 2,5-hexanedione. The axonal swellings observed in DMHD toxicity are in the proximal axon, as seen in intoxication with beta, beta'-iminodipropionitrile, rather than in the distal axon, where neurofilamentous swellings are observed in n-hexane, carbon disulfide, and acrylamide neurotoxicity. In these studies, 14C-labeled DMHD and 2-butanone were synthesized and allowed to react with peripheral nerve. Only 14C-labeled DMHD resulted in stable radiolabeled protein polymers, which were retained by nitrocellulose filters with pore sizes as large as 12 microns. More specific evidence for covalent crosslinking of neurofilaments was obtained when DMHD was allowed to react with peripheral nerve in which the neurofilaments had been pulse-labeled with L-[35S]methionine.
Full text
PDF

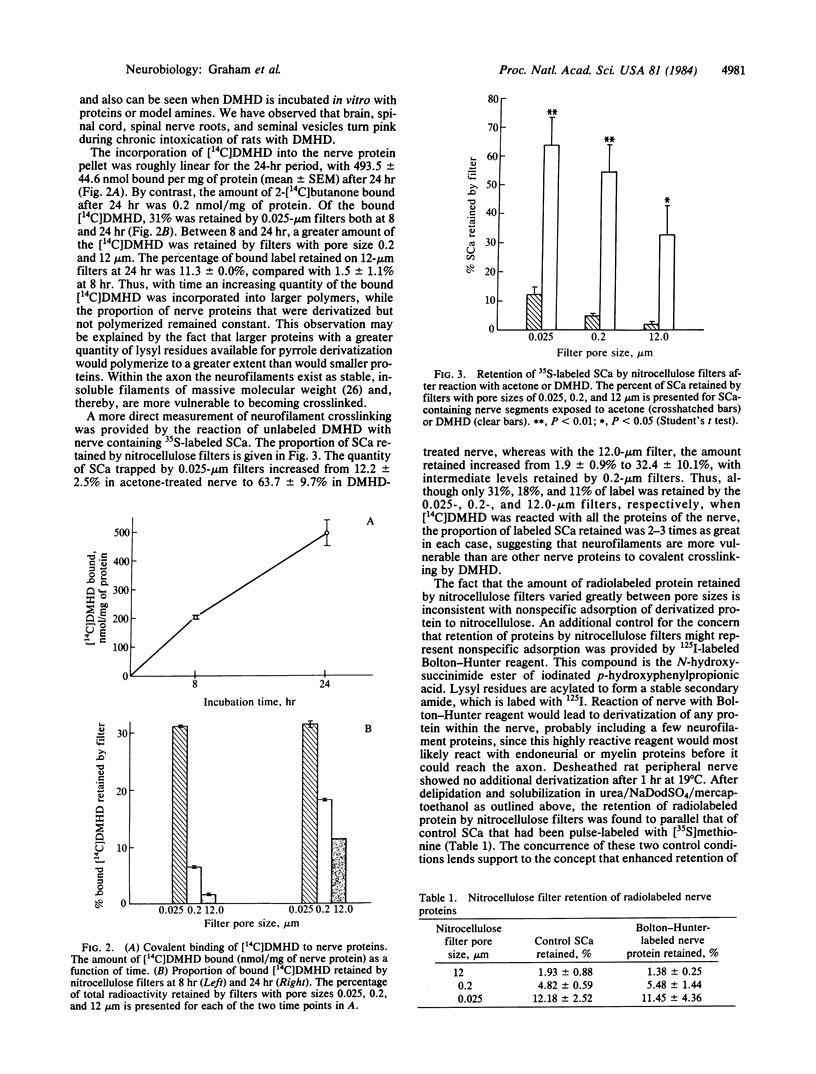

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony D. C., Boekelheide K., Anderson C. W., Graham D. G. The effect of 3,4-dimethyl substitution on the neurotoxicity of 2,5-hexanedione. II. Dimethyl substitution accelerates pyrrole formation and protein crosslinking. Toxicol Appl Pharmacol. 1983 Dec;71(3):372–382. doi: 10.1016/0041-008x(83)90024-8. [DOI] [PubMed] [Google Scholar]
- Anthony D. C., Boekelheide K., Graham D. G. The effect of 3,4-dimethyl substitution on the neurotoxicity of 2,5-hexanedione. I. Accelerated clinical neuropathy is accompanied by more proximal axonal swellings. Toxicol Appl Pharmacol. 1983 Dec;71(3):362–371. doi: 10.1016/0041-008x(83)90023-6. [DOI] [PubMed] [Google Scholar]
- Anthony D. C., Giangaspero F., Graham D. G. The spatio-temporal pattern of the axonopathy associated with the neurotoxicity of 3,4-dimethyl-2,5-hexanedione in the rat. J Neuropathol Exp Neurol. 1983 Sep;42(5):548–560. doi: 10.1097/00005072-198309000-00007. [DOI] [PubMed] [Google Scholar]
- Asbury A. K., Gale M. K., Cox S. C., Baringer J. R., Berg B. O. Giant axonal neuropathy--a unique case with segmental neurofilamentous masses. Acta Neuropathol. 1972;20(3):237–247. doi: 10.1007/BF00686905. [DOI] [PubMed] [Google Scholar]
- CHOU S. M., HARTMANN H. A. AXONAL LESIONS AND WALTZING SYNDROME AFTER IDPN ADMINISTRATION IN RATS. WITH A CONCEPT--"AXOSTASIS". Acta Neuropathol. 1964 May 5;3:428–450. doi: 10.1007/BF00688453. [DOI] [PubMed] [Google Scholar]
- Carpenter S. Proximal axonal enlargement in motor neuron disease. Neurology. 1968 Sep;18(9):841–851. doi: 10.1212/wnl.18.9.841. [DOI] [PubMed] [Google Scholar]
- Cavanagh J. B., Bennetts R. J. On the pattern of changes in the rat nervous system produced by 2,5 hexanediol. A topographical study by light microscopy. Brain. 1981 Jun;104(2):297–318. doi: 10.1093/brain/104.2.297. [DOI] [PubMed] [Google Scholar]
- Chiu F. C., Norton W. T. Bulk preparation of CNS cytoskeleton and the separation of individual neurofilament proteins by gel filtration: dye-binding characteristics and amino acid compositions. J Neurochem. 1982 Nov;39(5):1252–1260. doi: 10.1111/j.1471-4159.1982.tb12562.x. [DOI] [PubMed] [Google Scholar]
- Chou S. M., Hartmann H. A. Electron microscopy of focal neuroaxonal lesions produced by beta-beta-iminodipropionitrile (IDPN) in rats. I. The advanced lesions. Acta Neuropathol. 1965 Jul 1;4(6):590–603. doi: 10.1007/BF00691211. [DOI] [PubMed] [Google Scholar]
- Clark A. W., Griffin J. W., Price D. L. The axonal pathology in chronic IDPN intoxication. J Neuropathol Exp Neurol. 1980 Jan;39(1):42–55. doi: 10.1097/00005072-198001000-00004. [DOI] [PubMed] [Google Scholar]
- Graham D. G., Anthony D. C., Boekelheide K. In vitro and in vivo studies of the molecular pathogenesis of n-Hexane neuropathy. Neurobehav Toxicol Teratol. 1982 Nov-Dec;4(6):629–634. [PubMed] [Google Scholar]
- Graham D. G., Anthony D. C., Boekelheide K., Maschmann N. A., Richards R. G., Wolfram J. W., Shaw B. R. Studies of the molecular pathogenesis of hexane neuropathy. II. Evidence that pyrrole derivatization of lysyl residues leads to protein crosslinking. Toxicol Appl Pharmacol. 1982 Jul;64(3):415–422. doi: 10.1016/0041-008x(82)90237-x. [DOI] [PubMed] [Google Scholar]
- Griffin J. W., Drachman D. B., Price D. L. Fast axonal transport in motor nerve regeneration. J Neurobiol. 1976 Jul;7(4):355–370. doi: 10.1002/neu.480070407. [DOI] [PubMed] [Google Scholar]
- HESS A., YOUNG J. Z. The nodes of Ranvier. Proc R Soc Lond B Biol Sci. 1952 Nov 20;140(900):301–320. doi: 10.1098/rspb.1952.0063. [DOI] [PubMed] [Google Scholar]
- Herskowitz A., Ishii N., Schaumburg H. N-hexane neuropathy. A syndrome occurring as a result of industrial exposure. N Engl J Med. 1971 Jul 8;285(2):82–85. doi: 10.1056/NEJM197107082850204. [DOI] [PubMed] [Google Scholar]
- Hoffman P. N., Lasek R. J. The slow component of axonal transport. Identification of major structural polypeptides of the axon and their generality among mammalian neurons. J Cell Biol. 1975 Aug;66(2):351–366. doi: 10.1083/jcb.66.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. B., Cavanagh J. B. Cytochemical staining characteristics of peripheral nodes of Ranvier in hexacarbon intoxication. J Neurocytol. 1983 Jun;12(3):459–473. doi: 10.1007/BF01159385. [DOI] [PubMed] [Google Scholar]
- Jones H. B., Cavanagh J. B. Distortions of the nodes of Ranvier from axonal distension by filamentous masses in hexacarbon intoxication. J Neurocytol. 1983 Jun;12(3):439–458. doi: 10.1007/BF01159384. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Schaumburg H. H., Spencer P. S. Degeneration in central and peripheral nervous systems produced by pure n-hexane: an experimental study. Brain. 1976 Jun;99(2):183–192. doi: 10.1093/brain/99.2.183. [DOI] [PubMed] [Google Scholar]
- Schaumburg H. H., Wiśniewski H. M., Spencer P. S. Ultrastructural studies of the dying-back process. I. Peripheral nerve terminal and axon degeneration in systemic acrylamide intoxication. J Neuropathol Exp Neurol. 1974 Apr;33(2):260–284. doi: 10.1097/00005072-197404000-00006. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Abraham C., Ihara Y. Brain transglutaminase: in vitro crosslinking of human neurofilament proteins into insoluble polymers. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6070–6074. doi: 10.1073/pnas.79.19.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D. J., Ihara Y., Salazar F. J. Alzheimer's disease: insolubility of partially purified paired helical filaments in sodium dodecyl sulfate and urea. Science. 1982 Mar 5;215(4537):1243–1245. doi: 10.1126/science.6120571. [DOI] [PubMed] [Google Scholar]
- Spencer P. S., Sabri M. I., Schaumburg H. H., Moore C. L. Does a defect of energy metabolism in the nerve fiber underlie axonal degeneration in polyneuropathies? Ann Neurol. 1979 Jun;5(6):501–507. doi: 10.1002/ana.410050602. [DOI] [PubMed] [Google Scholar]
- Spencer P. S., Schaumburg H. H. Ultrastructural studies of the dying-back process. III. The evolution of experimental peripheral giant axonal degeneration. J Neuropathol Exp Neurol. 1977 Mar-Apr;36(2):276–299. doi: 10.1097/00005072-197703000-00005. [DOI] [PubMed] [Google Scholar]
- Towfighi J., Gonatas N. K., Pleasure D., Cooper H. S., McCree L. Glue sniffer's neuropathy. Neurology. 1976 Mar;26(3):238–243. doi: 10.1212/wnl.26.3.238. [DOI] [PubMed] [Google Scholar]