Abstract
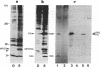
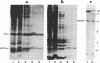
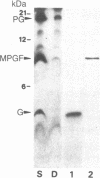
It has previously been shown by biosynthetic labeling studies that glucagon is synthesized in mammalian islets via an 18-kDa precursor, proglucagon, that during processing gives rise to glucagon and a secreted peptide of 10 kDa (the major proglucagon fragment, MPGF). We have now developed a simple procedure for the isolation of this peptide from rat pancreatic islets and have characterized it more fully. On the basis of its amino acid composition, MPGF is identified as the COOH-terminal portion of proglucagon that contains two glucagon-related sequences. These sequences do not appear to be liberated from MPGF in alpha cells of the islets of Langerhans but MPGF may be processed further elsewhere in the body or in other cells of the gastrointestinal tract that produce glucagon precursors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baetens D., Malaisse-Lagae F., Perrelet A., Orci L. Endocrine pancreas: three-dimensional reconstruction shows two types of islets of langerhans. Science. 1979 Dec 14;206(4424):1323–1325. doi: 10.1126/science.390711. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Sanchez-Pescador R., Laybourn P. J., Najarian R. C. Exon duplication and divergence in the human preproglucagon gene. 1983 Jul 28-Aug 3Nature. 304(5924):368–371. doi: 10.1038/304368a0. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Santerre R. F., Mullenbach G. T. Hamster preproglucagon contains the sequence of glucagon and two related peptides. Nature. 1983 Apr 21;302(5910):716–718. doi: 10.1038/302716a0. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Goodman R. H., Lund P. K., Jacobs J. W., Habener J. F. Pre-prosomatostatins. Products of cell-free translations of messenger RNAs from anglerfish islets. J Biol Chem. 1980 Jul 25;255(14):6549–6552. [PubMed] [Google Scholar]
- Katz F. N., Rothman J. E., Lingappa V. R., Blobel G., Lodish H. F. Membrane assembly in vitro: synthesis, glycosylation, and asymmetric insertion of a transmembrane protein. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3278–3282. doi: 10.1073/pnas.74.8.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Rodriguez H. A discontinuous electrophoretic system for separating peptides on polyacrylamide gels. Anal Biochem. 1983 Sep;133(2):515–522. doi: 10.1016/0003-2697(83)90118-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lopez L. C., Frazier M. L., Su C. J., Kumar A., Saunders G. F. Mammalian pancreatic preproglucagon contains three glucagon-related peptides. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5485–5489. doi: 10.1073/pnas.80.18.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund P. K., Goodman R. H., Dee P. C., Habener J. F. Pancreatic preproglucagon cDNA contains two glucagon-related coding sequences arranged in tandem. Proc Natl Acad Sci U S A. 1982 Jan;79(2):345–349. doi: 10.1073/pnas.79.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund P. K., Goodman R. H., Montminy M. R., Dee P. C., Habener J. F. Anglerfish islet pre-proglucagon II. Nucleotide and corresponding amino acid sequence of the cDNA. J Biol Chem. 1983 Mar 10;258(5):3280–3284. [PubMed] [Google Scholar]
- Majzoub J. A., Kronenberg H. M., Potts J. T., Jr, Rich A., Habener J. F. Identification and cell-free translation of mRNA coding for a precursor of parathyroid secretory protein. J Biol Chem. 1979 Aug 10;254(15):7449–7455. [PubMed] [Google Scholar]
- Moody A. J., Holst J. J., Thim L., Jensen S. L. Relationship of glicentin to proglucagon and glucagon in the porcine pancreas. Nature. 1981 Feb 5;289(5797):514–516. doi: 10.1038/289514a0. [DOI] [PubMed] [Google Scholar]
- Obata K., Itoh N., Okamoto H., Yanaihara C., Yanaihara N., Suzuki T. Identification and processing of biosynthetic precursors to vasoactive intestinal polypeptide in human neuroblastoma cells. FEBS Lett. 1981 Dec 21;136(1):123–126. doi: 10.1016/0014-5793(81)81228-8. [DOI] [PubMed] [Google Scholar]
- Patzelt C., Labrecque A. D., Duguid J. R., Carroll R. J., Keim P. S., Heinrikson R. L., Steiner D. F. Detection and kinetic behavior of preproinsulin in pancreatic islets. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1260–1264. doi: 10.1073/pnas.75.3.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzelt C., Schug G. The major proglucagon fragment: an abundant islet protein and secretory product. FEBS Lett. 1981 Jun 29;129(1):127–130. doi: 10.1016/0014-5793(81)80772-7. [DOI] [PubMed] [Google Scholar]
- Patzelt C., Tager H. S., Carroll R. J., Steiner D. F. Identification and processing of proglucagon in pancreatic islets. Nature. 1979 Nov 15;282(5736):260–266. doi: 10.1038/282260a0. [DOI] [PubMed] [Google Scholar]
- Patzelt C., Tager H. S., Carroll R. J., Steiner D. F. Identification of prosomatostatin in pancreatic islets. Proc Natl Acad Sci U S A. 1980 May;77(5):2410–2414. doi: 10.1073/pnas.77.5.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ravazzola M., Orci L. Glucagon and glicentin immunoreactivity are topologically segregated in the alpha granule of the human pancreatic A cell. Nature. 1980 Mar 6;284(5751):66–67. doi: 10.1038/284066a0. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Quinn P. S., Chan S. J., Marsh J., Tager H. S. Processing mechanisms in the biosynthesis of proteins. Ann N Y Acad Sci. 1980;343:1–16. doi: 10.1111/j.1749-6632.1980.tb47238.x. [DOI] [PubMed] [Google Scholar]
- Sundby F., Jacobsen H., Moody A. J. Purification and characterization of a protein from porcine gut with glucagon-like immunoreactivity. Horm Metab Res. 1976 Sep;8(5):366–371. doi: 10.1055/s-0028-1093615. [DOI] [PubMed] [Google Scholar]
- Tager H. S., Markese J. Intestinal and pancreatic glucagon-like peptides. Evidence for identity of higher molecular weight forms. J Biol Chem. 1979 Apr 10;254(7):2229–2233. [PubMed] [Google Scholar]
- Tager H. S., Steiner D. F. Isolation of a glucagon-containing peptide: primary structure of a possible fragment of proglucagon. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2321–2325. doi: 10.1073/pnas.70.8.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thim L., Moody A. J. Purification and chemical characterization of a glicentin-related pancreatic peptide (proglucagon fragment) from porcine pancreas. Biochim Biophys Acta. 1982 May 3;703(2):134–141. doi: 10.1016/0167-4838(82)90041-3. [DOI] [PubMed] [Google Scholar]
- Thim L., Moody A. J. The primary structure of porcine glicentin (proglucagon). Regul Pept. 1981 May;2(2):139–150. doi: 10.1016/0167-0115(81)90007-0. [DOI] [PubMed] [Google Scholar]