Abstract
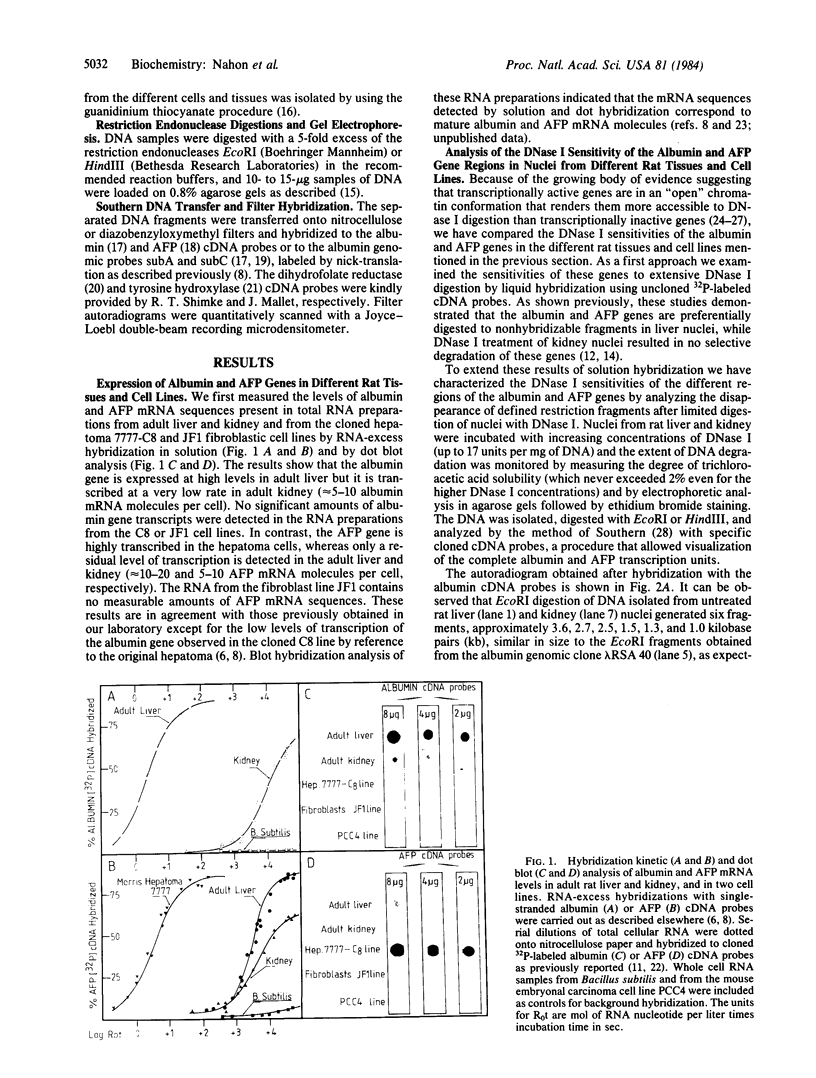
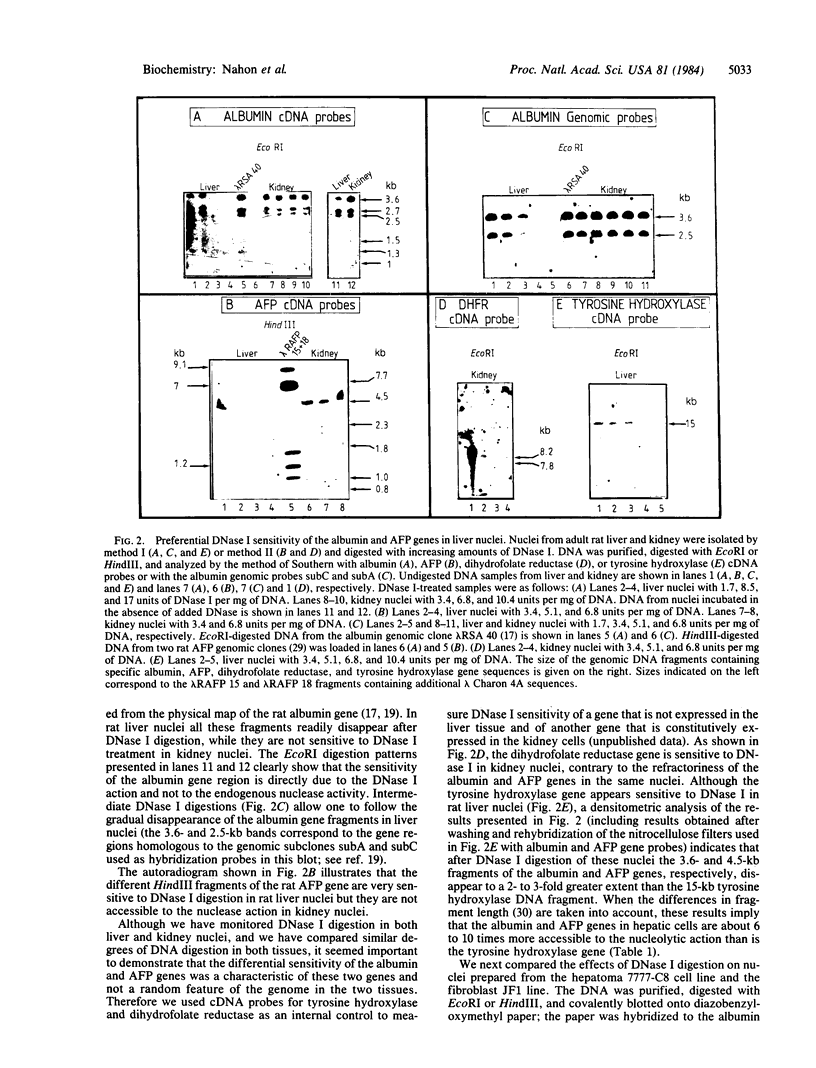
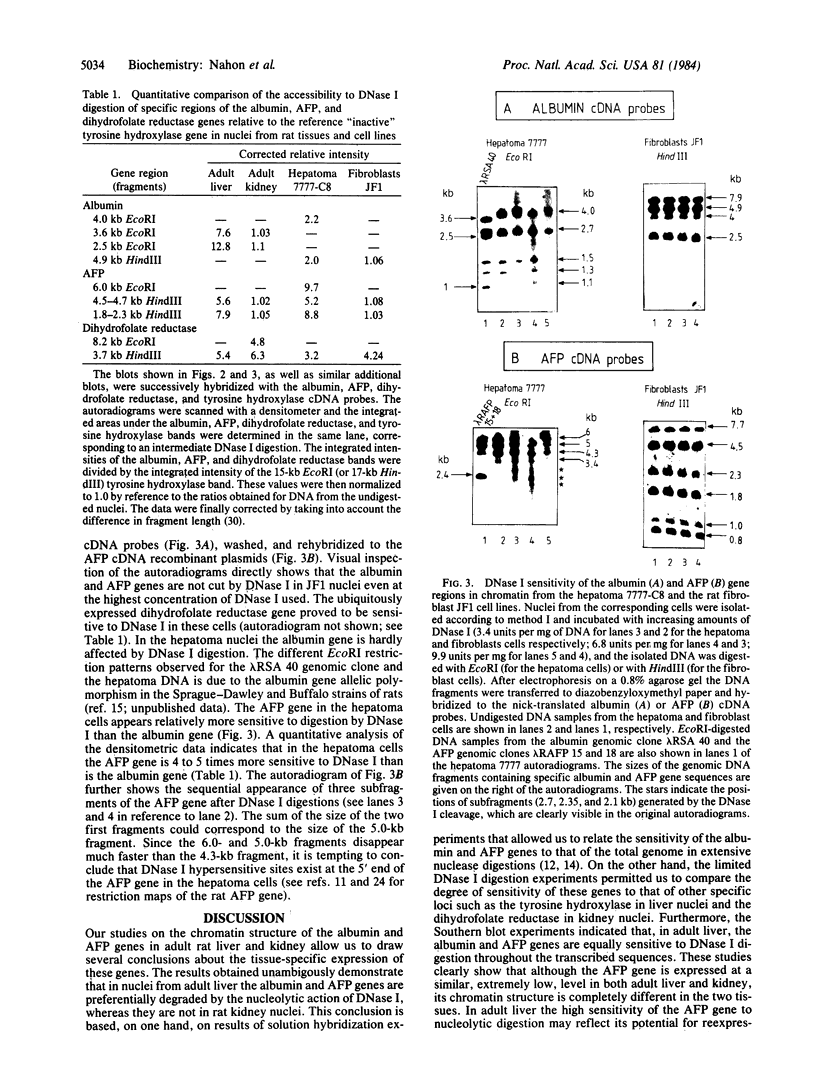
We have examined the DNase I sensitivity of the albumin and alpha-fetoprotein (AFP) genes in different rat tissues (adult liver and kidney) and cloned cell lines (hepatoma 7777-C8, JF1 fibroblasts), which show drastic differences in the level of expression of these two genes. This was done by studying the disappearance of defined restriction endonuclease fragments of these genes as a function of limited DNase I digestion. The sensitivity of these genes was compared to that of a gene not expressed in the hepatic cells and to that of a ubiquitously expressed gene. In nuclei from adult rat liver the albumin and AFP genes were preferentially degraded by the nucleolytic action of DNase I, whereas they were not in rat kidney nuclei. In the hepatoma cells the AFP gene was much more sensitive to DNase I digestion than the albumin gene; both genes were very resistant to DNase I action in fibroblastic nuclei. When analyzed in relation to the level of gene expression our results indicate that alterations in the chromatin structure of the albumin and AFP genes might be involved in the early establishment of the tissue-specific potential of overt gene expression; such alterations reflected in an altered DNase I sensitivity do not appear to be responsible for the changes in gene activity occurring during the terminal differentiation of the hepatocyte; and modifications in the chromatin structure of these genes might occur during oncogenic events; these structural modifications could be related to the changes in gene expression observed in hepatocarcinogenic processes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelev G. I. Alpha-fetoprotein in ontogenesis and its association with malignant tumors. Adv Cancer Res. 1971;14:295–358. doi: 10.1016/s0065-230x(08)60523-0. [DOI] [PubMed] [Google Scholar]
- Bellard M., Kuo M. T., Dretzen G., Chambon P. Differential nuclease sensitivity of the ovalbumin and beta-globin chromatin regions in erythrocytes and oviduct cells of laying hen. Nucleic Acids Res. 1980 Jun 25;8(12):2737–2750. doi: 10.1093/nar/8.12.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch J. B., Weintraub H. Temporal order of chromatin structural changes associated with activation of the major chicken vitellogenin gene. Cell. 1983 May;33(1):65–76. doi: 10.1016/0092-8674(83)90335-5. [DOI] [PubMed] [Google Scholar]
- Cassio D., Weiss M. C., Ott M. O., Sala-Trepat J. M., Friès J., Erdos T. Expression of the albumin gene in rat hepatoma cells and their dedifferentiated variants. Cell. 1981 Dec;27(2 Pt 1):351–358. doi: 10.1016/0092-8674(81)90418-9. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Felber B. K., Gerber-Huber S., Meier C., May F. E., Westley B., Weber R., Ryffel G. U. Quantitation of DNase I sensitivity in Xenopus chromatin containing active and inactive globin, albumin and vitellogenin genes. Nucleic Acids Res. 1981 Jun 11;9(11):2455–2474. doi: 10.1093/nar/9.11.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal A., Nahon J. L., Lucotte G., Sala-Trepat J. M. Structural variants of the alpha-fetoprotein gene in different inbred strains of rat. Mol Gen Genet. 1984;195(1-2):153–158. doi: 10.1007/BF00332738. [DOI] [PubMed] [Google Scholar]
- Gal A., Nahon J. L., Sala-Trepat J. M. Detection of rare mRNA species in a complex RNA population by blot hybridization techniques: a comparative survey. Anal Biochem. 1983 Jul 1;132(1):190–194. doi: 10.1016/0003-2697(83)90446-3. [DOI] [PubMed] [Google Scholar]
- Gitlin D., Perricelli A. Synthesis of serum albumin, prealbumin, alpha-foetoprotein, alpha-1-antitrypsin and transferrin by the human yolk sac. Nature. 1970 Dec 5;228(5275):995–997. doi: 10.1038/228995a0. [DOI] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. The calcium dependent endonuclease activity of isolated nuclear preparations. Relationships between its occurrence and the occurrence of other classes of enzymes found in nuclear preparations. Biochem Biophys Res Commun. 1973 May 15;52(2):475–481. doi: 10.1016/0006-291x(73)90736-5. [DOI] [PubMed] [Google Scholar]
- Jagodzinski L. L., Sargent T. D., Yang M., Glackin C., Bonner J. Sequence homology between RNAs encoding rat alpha-fetoprotein and rat serum albumin. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3521–3525. doi: 10.1073/pnas.78.6.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Szybalski W. Orientation of transcription of the lac operon and its repressor gene in Escherichia coli. J Mol Biol. 1969 Feb 28;40(1):145–151. doi: 10.1016/0022-2836(69)90303-9. [DOI] [PubMed] [Google Scholar]
- Lamouroux A., Faucon Biguet N., Samolyk D., Privat A., Salomon J. C., Pujol J. F., Mallet J. Identification of cDNA clones coding for rat tyrosine hydroxylase antigen. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3881–3885. doi: 10.1073/pnas.79.12.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucotte G., Gal A., Nahon J. L., Sala-Trepat J. M. Eco RI restriction-site polymorphism of the albumin gene in different inbred strains of rat. Biochem Genet. 1982 Dec;20(11-12):1105–1115. doi: 10.1007/BF00498935. [DOI] [PubMed] [Google Scholar]
- Mathis D., Oudet P., Chambon P. Structure of transcribing chromatin. Prog Nucleic Acid Res Mol Biol. 1980;24:1–55. doi: 10.1016/s0079-6603(08)60670-4. [DOI] [PubMed] [Google Scholar]
- Nahon J. L., Gal A., Frain M., Sell S., Sala-Trepat J. M. No evidence for post-transcriptional control of albumin and alpha-fetoprotein gene expression in developing rat liver neoplasia. Nucleic Acids Res. 1982 Mar 25;10(6):1895–1911. doi: 10.1093/nar/10.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunberg J. H., Kaufman R. J., Chang A. C., Cohen S. N., Schimke R. T. Structure and genomic organization of the mouse dihydrofolate reductase gene. Cell. 1980 Feb;19(2):355–364. doi: 10.1016/0092-8674(80)90510-3. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Seppälä M. alpha-Fetoprotein in cancer and fetal development. Adv Cancer Res. 1979;29:275–346. doi: 10.1016/s0065-230x(08)60849-0. [DOI] [PubMed] [Google Scholar]
- Sala-Trepat J. M., Dever J., Sargent T. D., Thomas K., Sell S., Bonner J. Changes in expression of albumin and alpha-fetoprotein genes during rat liver development and neoplasia. Biochemistry. 1979 May 29;18(11):2167–2178. doi: 10.1021/bi00578a006. [DOI] [PubMed] [Google Scholar]
- Sala-Trepat J. M., Sargent T. D., Sell S., Bonner J. alpha-Fetoprotein and albumin genes of rats: no evidence for amplification-deletion or rearrangement in rat liver carcinogenesis. Proc Natl Acad Sci U S A. 1979 Feb;76(2):695–699. doi: 10.1073/pnas.76.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent T. D., Jagodzinski L. L., Yang M., Bonner J. Fine structure and evolution of the rat serum albumin gene. Mol Cell Biol. 1981 Oct;1(10):871–883. doi: 10.1128/mcb.1.10.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent T. D., Wu J. R., Sala-Trepat J. M., Wallace R. B., Reyes A. A., Bonner J. The rat serum albumin gene: analysis of cloned sequences. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3256–3260. doi: 10.1073/pnas.76.7.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S., Becker F. F., Leffert H. L., Watabe L. Expression of an oncodevelopmental gene product (alpha-fetoprotein) during fetal development and adult oncogenesis. Cancer Res. 1976 Nov;36(11 Pt 2):4239–4249. [PubMed] [Google Scholar]
- Sell S., Thomas K., Michalson M., Salatrepat J., Bonner J. Control of albumin and alpha-fetoprotein expression in rat liver and in some transplantable hepatocellular carcinomas. Biochim Biophys Acta. 1979 Aug 29;564(1):173–178. doi: 10.1016/0005-2787(79)90198-9. [DOI] [PubMed] [Google Scholar]
- Sellem C. H., Frain M., Erdos T., Sala-Trepat J. M. Differential expression of albumin and alpha-fetoprotein genes in fetal tissues of mouse and rat. Dev Biol. 1984 Mar;102(1):51–60. doi: 10.1016/0012-1606(84)90174-x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tilghman S. M., Belayew A. Transcriptional control of the murine albumin/alpha-fetoprotein locus during development. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5254–5257. doi: 10.1073/pnas.79.17.5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedel M., Gomez-Garcia M., Sala M., Sala-Trepat J. M. Changes in methylation pattern of albumin and alpha-fetoprotein genes in developing rat liver and neoplasia. Nucleic Acids Res. 1983 Jul 11;11(13):4335–4354. doi: 10.1093/nar/11.13.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Larsen A., Groudine M. Alpha-Globin-gene switching during the development of chicken embryos: expression and chromosome structure. Cell. 1981 May;24(2):333–344. doi: 10.1016/0092-8674(81)90323-8. [DOI] [PubMed] [Google Scholar]
- Wu C., Gilbert W. Tissue-specific exposure of chromatin structure at the 5' terminus of the rat preproinsulin II gene. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1577–1580. doi: 10.1073/pnas.78.3.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. The 5' ends of Drosophila heat shock genes in chromatin are hypersensitive to DNase I. Nature. 1980 Aug 28;286(5776):854–860. doi: 10.1038/286854a0. [DOI] [PubMed] [Google Scholar]






