Abstract
The advent of modern antimicrobial therapy following the discovery of penicillin during the 1940s yielded remarkable improvements in case fatality rate of serious infections including septic shock. Since then, pathogens have continuously evolved under selective antimicrobial pressure resulting in a lack of significant improvement in clinical effectiveness in the antimicrobial therapy of septic shock despite ever more broad-spectrum and potent drugs. In addition, although substantial effort and money has been expended on the development novel non-antimicrobial therapies of sepsis in the past 30 years, clinical progress in this regard has been limited. This review explores the possibility that the current pathophysiologic paradigm of septic shock fails to appropriately consider the primacy of the microbial burden of infection as the primary driver of septic organ dysfunction. An alternate paradigm is offered that suggests that has substantial implications for optimizing antimicrobial therapy in septic shock. This model of disease progression suggests the key to significant improvement in the outcome of septic shock may lie, in great part, with improvements in delivery of existing antimicrobials and other anti-infectious strategies. Recognition of the role of delays in administration of antimicrobial therapy in the poor outcomes of septic shock is central to this effort. However, therapeutic strategies that improve the degree of antimicrobial cidality likely also have a crucial role.
Keywords: septic shock, antimicrobial, pharmacokinetics, sepsis, infection, antibiotic, life-threatening infection, combination therapy, survival
Introduction
Septic shock and sepsis-associated multiple organ failure remain the most common cause of death in intensive care units of the medically advanced nations. Historically, the mortality associated with sepsis and septic shock has been approximately 50–75%.1-3 The primary advance in the therapy of septic shock was the development of modern (i.e., β-lactam) antibiotic therapy 70 years ago which resulted in a reduction in sepsis-associated mortality to the 30–50% range.1,2 However, the past 50 years has seen a gradual year-to-year increase in the incidence of sepsis.4 As a result, total deaths have increased substantially.4 Current estimates suggest a doubling of total United States cases of severe sepsis from 800 000 annually to 1.6 million cases by 2050 with an increase in population of only 33%.5 Currently, severe sepsis and septic shock cases account for approximately 10–15% of all intensive care unit (ICU) admissions with approximately 25% of cases of sepsis6 and 50–75% of cases of severe sepsis progress to septic shock.7 Septic shock alone represents between 5% and 8% of all ICU admissions.8,9 Despite major advances in technology and constant refinement of our understanding of sepsis pathophysiology, numerous clinical trials have failed to produce any new drugs with consistent beneficial effects on this patient population. Even the efficacy of the only novel non-antimicrobial pharmacotherapy of sepsis and septic shock approved since the advent of modern antimicrobials, activated protein C, has recently been questioned and the product removed from the market.10-12
Current Paradigm: Immunologic Model
Part of the reason for the failure to develop effective novel therapies may be a fundamental misunderstanding of the patho-physiology of septic shock. The currently accepted immunologic paradigm suggests that sepsis is present when systemic activation of inflammatory pathways (i.e., systemic inflammatory response syndrome [SIRS]) is triggered by infection.13 This paradigm holds that the disorder is caused by an infection which initiates an immunologic (inflammatory cytokine and eicosanoid)/coagulation cascade that propagates independently of the underlying infectious trigger.14,15 This view of sepsis as a syndrome that is only indirectly related to the underlying infection is reflected in the classic figure by Bone and colleagues showing the relationships between SIRS and infection (Fig. 1).13 The figure indicates that sepsis is defined by the co-occurrence of infection and SIRS but there is no clear suggestion (in the figure) that uncontrolled infection drives the development of SIRS.
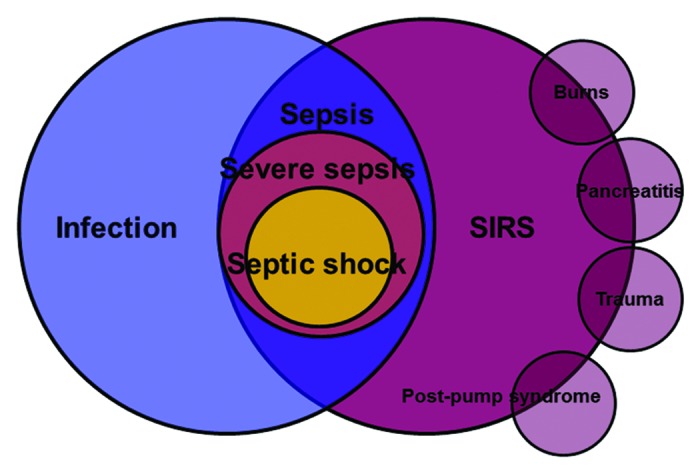
Figure 1. SIRS, sepsis, severe sepsis, and septic shock. SIRS, systemic inflammatory response. Adapted from reference 13. See text for explanation.
That is appropriate given current thinking. In this view, progression of the syndrome (and a counter-inflammatory, “immunoparalytic” phase of illness16,17) will occur as a consequence of inflammatory cellular signaling despite the rapid elimination of the pathogen through administration of cidal antimicrobial therapy14 (Fig. 2). The concept is one of an inflammatory cytokine and coagulation “cascade” that can progress to higher degrees of severity independently of the initial infection trigger. In this model, sepsis, severe sepsis (sepsis with organ failure), and septic shock (sepsis with cardiovascular failure) are considered to be related disorders of increasing severity but sharing a similar basic underlying pathology, one of direct endogenous mediator-driven cellular dysfunction and injury. Septic shock, in particular, is considered an epiphenomenon to the underlying cellular injury induced by these mediators rather than a discrete clinical entity with a distinct pathogenesis and pathophysiology. Overwhelming meningococcemia with septic shock, a condition in which an exquisitely antimicrobial-sensitive organism can be quickly eliminated but where massive tissue damage may still occur is the archetypal infectious syndrome that best fits this paradigm. Notably, the pivotal clinical trial of bactericidal permeability increasing (BPI) protein, an immunomodulatory agent developed as a meningococcal anti-sepsis compound actually exhibited some evidence of benefit in meningococcal sepsis.18 However, other less sensitive pathogenic microorganisms that more frequently cause septic shock fit this archetypical profile less well. Perhaps because of this issue, the variety of immunomodulatory therapies that were developed based on this immunologic paradigm of sepsis have, almost uniformly, failed to improve outcomes in clinical trials.19 The source of the efficacy of drotrecogin-alfa (activated), the one novel product to have shown a potential benefit in sepsis (at least in the initial pivotal study), may have been its potent anticoagulant rather than immunomodulatory activity since heparin also seems to exert a similar benefit.20,21
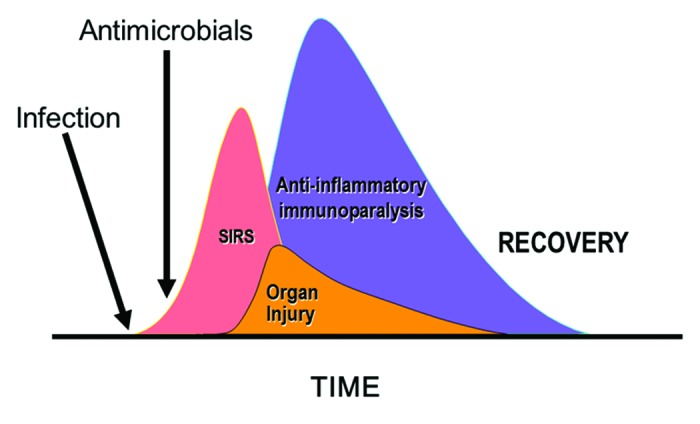
Figure 2. Immunologic view of sepsis and septic shock. SIRS, systemic inflammatory response. Adapted from reference 14. See text for explanation.
The Classic Paradigm: Microbiologic Primacy
The reason for the general failure of immunomodulatory strategies for treatment of sepsis may be that the underlying paradigm fails to accurately reflect the disease process. The primacy of active infection in driving the immunologic “cascade” of sepsis pathophysiology may be underappreciated in the currently accepted immunologic paradigm of sepsis. A key deficiency of this immunologic model of sepsis is that most pathogens cannot be eliminated quickly despite cidal antimicrobial therapy22-27 and likely persist during the period that immunomodulatory therapies (most of which are, in fact, immunosuppressive) might be initiated. A recent autopsy study of sepsis suggested that a persistent septic focus could be found in approximately 75% of 235 surgical ICU patients who died of sepsis/septic shock and in almost 90% of those succumbing in the ICU after at least 7 d of treatment.28
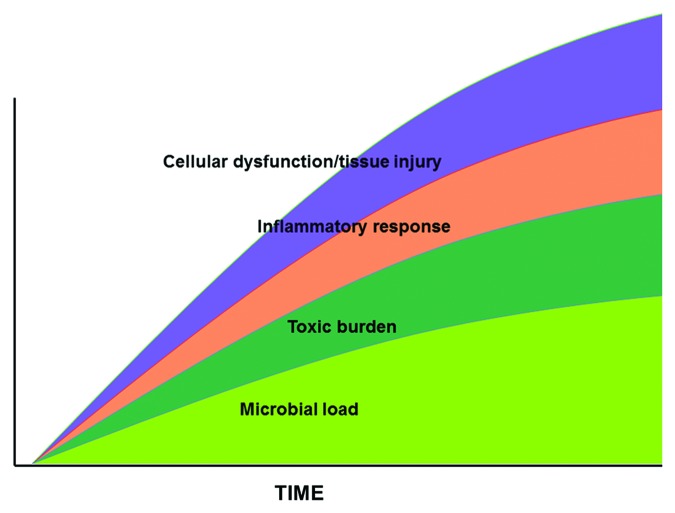
Another view of septic shock derives from the more classic microbiologic paradigm of life-threatening infection and sepsis. In this model, infection is the key driving element of sepsis and septic shock. The septic process begins with a nidus of infection (peritonitis, pneumonia, etc.) (Fig. 3). Within that focus, the organism replicates and, untreated, the microbial infectious load increases over time. Overlying that process, the microbial pathogens release a variety of exo- and endotoxins (toxic burden) which have antigenic properties. These stimulate a further overlay of endogenous mediators including inflammatory cytokines such as TNF-α, IL-1β, IL-6, HMGB1, and eicosanoids such as prostaglandin E2, prostacylin, thromboxanes, leukotrienes (inflammatory response). The result is cellular dysfunction which can be manifested as tissue injury and, ultimately, organ dysfunction including septic shock (cellular dysfunction/tissue injury). The central aspect of this model is that the microbial infectious load substantially drives downstream responses including the development of organ dysfunction and septic shock. This paradigm, which forms the basis of standard antimicrobial therapy of sepsis and septic shock, suggests that elimination of the underlying infection should terminate the downstream inflammatory/coagulant basis for tissue injury and organ dysfunction.
Figure 3. Microbiologic view of sepsis and septic shock. See text for explanation.
A New Composite Model: Integrating Shock
The microbiologic primacy model of sepsis, like the immunologic model, also fails to recognize a key element in mortality of septic states, the concept of irreversible shock as originally described by Wiggers.29 This concept suggests that shock, irrespective of the etiology, can only be tolerated for a limited time. Once present, shock will become irreversible with inevitable progression to death if the condition is not reversed within a short period of time. This concept is directly associated with the idea of the “golden hour” first demonstrated in the context of hemorrhagic/traumatic shock but applicable to various forms of critical injury particularly other shock states. Many studies have now shown that early definitive intervention (i.e., correction of the underlying problem) within a short time of potentially lethal injury has a major impact on survival. Patients with such injury can be maintained for a limited period of time with non-definitive support modalities (e.g., blood products for hemorrhagic shock, intra-aortic balloon pump for myocardial infarction-associated cardiogenic shock, pressors for all forms of shock) but mortality will not be improved without definitive elimination of the underlying source of hemodynamic instability (e.g., thrombolysis,30 angioplasty31 or bypass for cardiogenic shock due to myocardial infarction, embolectomy or thrombolysis of massive pulmonary embolus causing obstructive shock,32 or definitive repair/control of a bleeding lesion causing hypovolemic shock33).
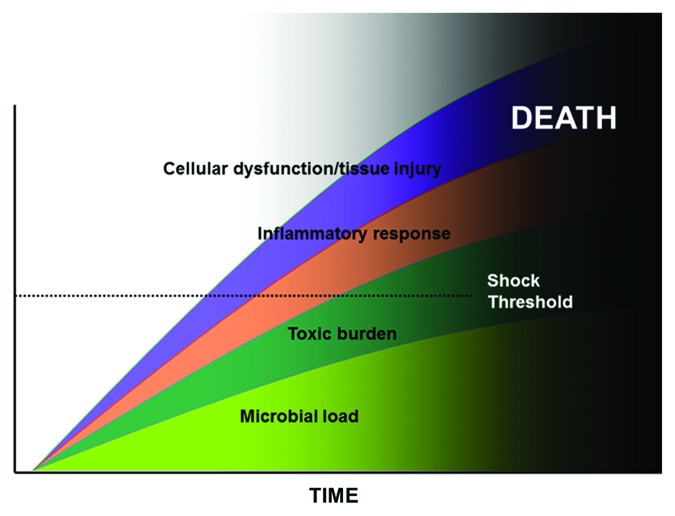
Septic shock can be viewed through a similar prism. In this circumstance, the underlying source of shock is the total microbial load. This paradigm of septic shock implies that the speed with which the inciting infection is reduced to a sub-critical threshold after the onset of persistent or recurrent hypotension (as a marker of shock) will be of paramount importance in survival. The presence of shock becomes a central driver in the genesis of irreversible organ injury rather than an incidental epiphenomenon. A conceptual model that incorporates the key elements of this infectious paradigm of sepsis, immunologic elements from the model described previously and the concept of irreversible shock can be created and used to predict key aspects of pathogenesis of septic shock and develop novel approaches to effective therapy. This construct is similar to the infectious diseases model of septic shock with two major additions (Fig. 4). First, the hatched line indicates the point at which inflammatory mediator-associated cellular dysfunction and tissue injury manifest as septic shock. This threshold will be highly variable between individuals. Those with impaired cardiovascular reserve will go into shock at lower levels of cellular dysfunction/tissue injury. Young, healthy persons may require a substantially greater degree of inflammatory stimulation to reach the same shock threshold. The second new element to the model is that the presence of shock (as commonly manifested by persistent/recurrent hypotension) sets the patient on the path toward irreversible organ injury. At some indeterminate point after onset of hypotension (depending on the degree of hypotension, comorbid contributors, and genotype of the patient), the patient will become irreversibly committed to death. Because of genotypic variations in the host and pathogen and clinical variability in the infection, the exact point at which the injury becomes irreversible for a given patient cannot be determined at present. However, the progression is similar for all patients.
Figure 4. Composite view of sepsis and septic shock. See text for explanation.
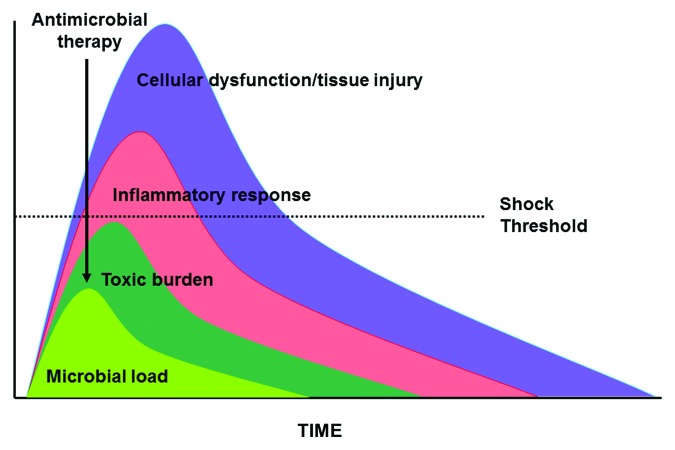
This model suggests that the appropriate approach to therapy of septic shock should be to rapidly reduce the infectious load so that the period of time in shock (irrespective of whether vasopressor are able to maintain blood pressure) before reduction of the microbial load to a subcritical thresh-hold is limited (i.e., minimize the period that sufficient organisms are present to generate shock) (Fig. 5). Based on the model, this should limit the risk that the indeterminate pathophysiologic point at which recovery is no longer possible in septic shock is passed. The issue of time/delay-dependent irreversibility of the injury and irreplaceability of the injured organ are critical to this model. Other conditions where these conditions exist in advanced nations include meningitis/encephalitis, necrotizing pneumonia, and necrotizing soft tissue infections. The mortality risk for other potentially eligible conditions may be somewhat more context-specific. Delays of antimicrobial therapy of endocarditis with valve failure or non-necrotizing pneumonia with respiratory failure may be fatal in areas where valve replacement and mechanical ventilation are unavailable but should be survivable in advanced nations.
Figure 5. Impact of appropriate antimicrobial therapy in sepsis and septic shock. See text for explanation.
There are two major pathophysiologic implications of this model of injury in septic shock. First and foremost, this model suggests that septic shock and sepsis without shock (including sepsis with organ failure other than shock) are fundamentally different diseases rather than a simple continuum of severity of a single syndrome. The difference lies in the time-/delay-dependent risk of irreversible and irreplaceable organ injury. The simplest line of evidence for this proposal is the commonality of the stark clinical features (hypotension, lactic acidosis, substantial exhaustion of compensatory physiologic responses, etc.) and high (>50%) mortality of septic shock and other shock syndromes of any etiology in contrast to the relatively milder clinical features and lower (approximately 15%) mortality of sepsis or severe sepsis.34 A pathophysiologic basis for the proposition that sepsis without shock and septic shock represent distinct clinical entities is suggested in the different profiles of associated endogenous mediators35,36 and evidence of hypotension-associate immune dysfunction in septic shock (compared with sepsis without shock).37 Moreover, if this model correctly describes sepsis and the shock syndromes, there should be a commonality of gene expression responses among all shock syndromes whereas distinct differences would exist between responses of those of sepsis and septic shock. However, this remains to be proven.
The second major implication of this model is that the time delay of effective antimicrobial therapy from onset of hypotension is a surrogate for an increasing microbial burden of organisms. Again, there is evidence to support this contention. We have shown that the onset of shock in a rodent model of E. coli peritonitis/septic shock consistently occurs at a defined microbial organism load in blood.38 Even as varying numbers of organism are implanted into the animal, the time of onset of shock remains constant relative to the density of organisms in the blood. This issue can be difficult to study in humans because of the variability in infecting organism. However, meningococci are remarkably consistent in their growth characteristics. Several studies have demonstrated that earlier antimicrobial therapy is critical in outcome of severe meningococcal disease. One study has demonstrated that increasing severity of the clinical syndrome (fulminant septic shock vs. meningitis or sepsis without shock) is associated with a higher burden of neisserial DNA and LPS in plasma of patients with meningococcal disease.39 In another study, logistic regression analysis demonstrated that blood bacterial load predicted outcome of meningococcal shock.40 Delays in antimicrobial therapy were associated with outcome only in univariate analysis and all deaths were associated with blood bacterial loads of >105 cfu/mL bacteria. Other studies similarly have demonstrated that the increasing organism burden is associated with increased morbidity and mortality in serious infections.41 For example, the risk of septic shock and death in serious pneumococcal and gram-negative infections increases with organism burden42-44 and mortality of S. aureus, E. coli, and K. pneumoniae bacteremia increases with shorter times to blood culture positivity (a surrogate marker of higher bacterial blood counts).45-49 Conversely, appropriate early antimicrobial therapy has been shown to be associated with both improved clinical outcomes and more rapid bacterial clearance in studies of Acinetobacter bacteremia in the critically ill.50 Similarly, the speed of bacterial eradication in 24 h in vitro time kill studies of antibiotic combination therapy have been shown to correlate strongly with clinical and bacteriologic outcomes in severely ill patients with Pseudomonas infections.51
Optimizing Pathogen Clearance
One of the central testable hypotheses that derive from this composite model is that the rapid clearance of pathogens will be the central determinant of outcome in septic shock. In the remainder of this review, we will focus on the application of antimicrobial optimization principles deriving from the concept of microbiologic load/infectious burden as a driver of the pathophysiology of septic shock. Notable among the implications of this model of sepsis progression is that optimization of antimicrobial therapy (i.e., acceleration of microbial clearance) will have the most dramatic impact in septic shock rather than sepsis without shock. Relevant antimicrobial factors to consider are listed in Table 1. Supplemental antimicrobial therapies (source control), antitoxin/immunomodulatory strategies, and supportive measures (fluid and pressor resuscitation) will not be discussed in this review.
Table 1. Antimicrobial determinants of pathogen clearance in septic shock.
| 1) Early antimicrobial therapy |
| a. Initiate microbially-appropriate therapy |
| b. Ensure maximally rapid initiation (avoid delays) |
| c. Utilize a loading dose when possible |
| 2) Antimicrobial potency |
| a. Ensure antimicrobial cidality |
| b. Optimize pharmacokinetic indices |
| i. Time-dependent agents |
| ii. Concentration-dependent agents |
| c. Utilize combination therapy with antimicrobials possessing different mechanisms of action |
| 3) Supplemental therapies |
| a. Source control |
Early Antimicrobial Therapy
Although there are several approaches that may yield accelerated pathogen clearance, the simplest approach involves ensuring that effective antimicrobial therapy is initiated as quickly as possible, particularly once septic shock has developed. In the context of septic shock, early, potent antimicrobial therapy will rapidly reduce the microbial load driving organ injury/dysfunction and hypotension reducing the risk of irreversible shock and death (Fig. 6).
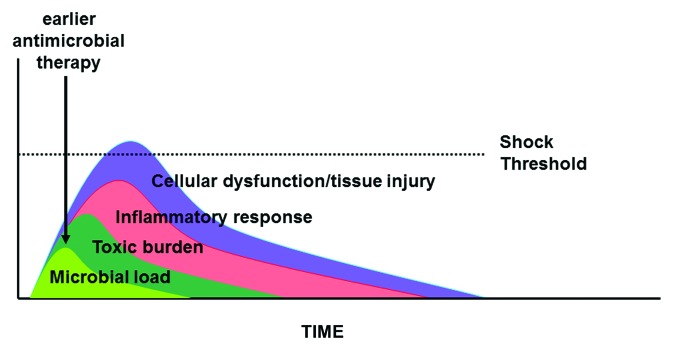
Figure 6. Impact of earlier antimicrobial therapy in sepsis and septic shock. See text for explanation.
In one of the earliest enunciations of this principle as it relates to all serious infections, Paul Ehrlich, in his address to the 17th International Congress of Medicine, 1913 said “Frapper fort et frapper vite” i.e., “hit hard and hit fast” with antimicrobials.52 In the modern context, his advice as it pertains to rapid therapy embodies three distinct elements. First, it is clearly necessary that initial empiric antimicrobial therapy be microbially appropriate (i.e., with in vivo antimicrobial activity for the presumed pathogen). Second, this appropriate empiric therapy must be administered as quickly as possible. Third, therapeutic levels in blood and at the target site should be achieved as quickly as possible following the first antimicrobial dose.
Appropriateness of Antimicrobial Therapy
Although data in sepsis without shock is inconsistent,53-57 most studies suggest that failure to initiate antimicrobial therapy to which the pathogen is sensitive is associated with marked increases in mortality especially in septic shock.3,58-68 For that reason, empiric antibiotic regimens should approach 100% coverage of pathogens for the suspected source of infection. Initiation of inadequate antimicrobial therapy may occur as frequently as 17.1% in community-acquired and 34.3% in nosocomial bacteremia admitted to the ICU.69 Similarly, 18.8% and 28.4% of septic shock cases were initially treated with inadequate antimicrobial therapy in another large study.62
Retrospective studies have shown that the risk of death increases from 30–60% in ICU bacteremia3,70 to 70–100% in gram-negative shock3 when the initial empiric regimen fails to cover the inciting pathogen. More recent data suggests that the initiation of inappropriate empiric antimicrobial therapy (i.e., failing to cover the pathogen) is associated with a reduction in survival of approximately 5-fold (range 2.5- to 10-fold in selected subgroups) from 55% to about 11%.62 These findings of sharply increased mortality risk with initial inadequate antimicrobial therapy apply to serious infections caused by gram-negative and gram-positive bacteria as well as Candida species.3,58-65 Similar findings have been documented with a variety of other serious infections associated with septic shock including community-acquired pneumonia, hospital- and ventilator-associated pneumonia, and bacterial peritonitis (for review, see refs. 66 and 67).
As a consequence of the high mortality associated with inappropriate initial therapy, empiric regimens should err on the side of over-inclusiveness. The most common cause of initiation of inappropriate antimicrobial therapy is a failure of the clinician to appreciate the risk of infection with antibiotic-resistant organisms (either otherwise uncommon organisms with increased native resistance or antibiotic-resistant isolates of common organisms). Selection of an optimal antimicrobial regimen requires knowledge of the probable anatomic site of infection; the patient’s immune status, risk factors, and physical environment; and the local microbiologic flora and organism resistance patterns. Risk factors for infection with resistant organisms include prolonged hospital stay, recent antimicrobial use, prior hospitalization, and prior colonization or infection with multiresistant organisms. Increased severity of illness is associated with risk of inadequate therapy;69 this may imply that the probability of resistant organisms is increased in patients progressing to higher levels of disease severity (i.e., septic shock).
Superior empiric coverage can be obtained through the use of a local and unit-specific antibiograms71,72 or infectious diseases consultation.73-75 Some data suggests improved clinical outcomes with such consultation.76 Although not routinely required, extended spectrum gram-negative regimens, vancomycin, and/or antifungal therapy may be appropriate in specific, high risk cases with severe sepsis. In addition, given that 90–95% of patients with septic shock have co-morbidities or other factors that make them high risk for resistant organisms, it may be appropriate to initially treat all patients with septic shock using a combination of antimicrobials that result in a broadly expanded spectrum of coverage for the first few days. This approach should ensure that high risk patients are not inappropriately categorized as low risk, thereby improving the likelihood of initial adequacy of antimicrobial coverage.
In order to avoid institutional problems with antimicrobial resistance in the long-term, empiric antimicrobial therapy must be adjusted to a narrower regimen within 48–72 h if a plausible pathogen is identified or if the patient stabilizes clinically (i.e., resolution of shock). While several retrospective studies have demonstrated that inappropriate therapy of severe sepsis and septic shock yields increased mortality,3,58-65,70 none have suggested that early narrowing of therapy is detrimental if the organism is identified or if the patient is responding well clinically. To the contrary, some studies have suggested that de-escalation of antimicrobial therapy is associated with improved outcomes.77-80 This approach will maximize appropriate antibiotic coverage of inciting pathogens in septic shock while minimizing selection pressure toward resistant organisms. Although it is tempting to continue a broad spectrum regimen in the 15–20% of patients who are improving but culture negative for a potential pathogen, intensivists must recognize that a strategy of broad spectrum initial antimicrobial therapy will only be sustainable if overuse of these agents can be avoided. Aggressive de-escalation of antimicrobial therapy within 48–72 h after initiation is required.
Antimicrobial Delay
Although available studies indicate that delays in initiation of appropriate antimicrobial therapy of bacteremia/candidemia and sepsis without shock are only inconsistently associated with outcome,64,81-88 such delays appear to have a substantial role in determining the mortality of high-risk infections with a strong association with septic shock.63,89-111 The central role of such delays in septic shock is apparent in the major upward inflection in mortality of antibiotic-treated murine septic shock coincident with the onset of hypotension and lactic acidosis.38 Other animal studies have similarly shown a very rapid inflection in mortality in experimental severe infections absent appropriate antimicrobial therapy.112,113
Human studies pertaining to the impact of delays of antimicrobial therapy on serious infections date back at least to the work of Bodey and colleagues114 who demonstrated increasing mortality risk when appropriate antimicrobials were delayed more than a day following documentation of Pseudomonas bacteremia. Meehan et al. has shown that delays in initial antimicrobial administration greater than 8 h after admission to the emergency room for community-acquired pneumonia is associated with increased mortality in a large cohort of Medicare patients.97 Houck has pushed this boundary lower by demonstrating increased mortality in Medicare patients with community acquired pneumonia who were treated with antimicrobials more than 4 h following ICU admission.102
One major retrospective analysis of septic shock has suggested that the delay to initial administration of effective antimicrobial therapy is the single strongest predictor of survival with significant decreases in projected survival for every hour delay.115 Initiation of effective antimicrobial therapy within the first hour following onset of septic shock-related hypotension was associated with 79.9% survival to hospital discharge. For every additional hour to effective antimicrobial initiation in the first 6 h post-hypotension onset, survival dropped an average of 7.6%. The adjusted odds ratio of death was already significantly increased by the second hour post-hypotension onset and the ratio continued to climb with longer delays. An unpublished analysis of an expanded data set demonstrates that significant decreases in projected survival occur with delays greater than 30 min. Despite these findings, the median time to delivery of effective antimicrobial therapy following initial onset of recurrent/persistent hypotension in septic shock was 6 h.115 Substantial delays before initiation of effective therapy have been shown in several studies of serious infections.97,102,116-119 Additional retrospective studies of human bacteremia, candidemia, septic shock, community-acquired pneumonia, hospital-acquired pneumonia, surgical infections, meningitis with sepsis, and sepsis in solid organ transplants have confirmed that the mortality in these septic conditions is increased with significant delays in antimicrobial administration.61,63,90,93-98,100,101,103-106,109-111,117,120-125
Several studies have now assessed the impact of speed of appropriate empiric antimicrobial therapy on outcome in relationship to other elements of therapy. In our own study of human septic shock,115 we found that 28% of the variance in outcome of septic shock could potentially be explained by variations in speed of delivery of effective antimicrobials while variations in fluid resuscitation could explain <2%. This suggested that greater remediable deficiencies (and greater potential for improvement in care) may lie with the former therapy than with the latter. A recent propensity analysis by Ferrer and colleagues126 of approximately 2800 patients with severe sepsis and septic shock suggested that only rapid antimicrobial therapy (<1 h compared with >6 h of severe sepsis diagnosis) and use of drotrecogin-alfa (activated) among elements of an internationally recommended “sepsis bundle”127 were independently associated with survival. Similarly, Varpula and colleagues128 have shown that only early initiation of antimicrobials (<3 vs >3 h of emergency room admission) among elements of a “sepsis bundle” was associated with improved survival in 92 patients with community-acquired septic shock using logistic regression analysis. Another analysis of the impact of various elements of the bundle demonstrated that only administration of antibiotics within 2 h and obtaining blood cultures before antibiotic administration were associated with improved survival in 316 consecutive patients with severe sepsis or septic shock.129 Likewise, Subramanian and colleagues have shown that only rapid initiation of antimicrobial therapy (<1 h following ICU admission or <3 h following admission to the emergency department) and early restoration of global perfusion indices were independently associated with survival in 95 consecutive patients with septic shock.130 Delays in appropriate antimicrobial therapy have also been associated with development of acute lung injury,131 acute renal failure,132 and worsening of organ failure133 and higher inflammatory cytokines and other inflammatory markers.133,134 Further support for the importance of time to appropriate antimicrobial therapy comes from studies of the impact of bundles of hospital-based interventions which have consistently shown improvement in outcome of sepsis and septic shock.126,135-143 The most consistent element of therapy improved with such bundled quality assurance approaches is timeliness and appropriateness of antimicrobial therapy.144
In view of these data, intravenous administration of broad spectrum antimicrobial should be initiated immediately (preferably <30 min) following the clinical diagnosis of septic shock. Patients with other serious infections are similarly well served with maximally rapid initiation of antimicrobial therapy. Appropriate, intravenous, broad spectrum empiric therapy should be initiated as rapidly as possible in response to clinical suspicion of infection in the presence of persistent hypotension, i.e., presumptive septic shock. An assumption that persistent or recurrent hypotension is caused by anything other than sepsis in the setting of documented or suspected infection should be avoided in the absence of very strong clinical evidence indicating a specific alternate etiology.
Laboratory tests congruent with sepsis or septic shock should be considered supportive of the diagnosis but obtaining such tests should never delay antimicrobial therapy. For septic shock, the presumptive diagnosis should be made on clinical criteria. A potential survival advantage may exist if a pathogenic organism can be isolated in severe infections including septic shock.62,145 Every effort should be made to obtain appropriate site-specific cultures in order to allow identification and susceptibility testing of the pathogenic organism; however, as with other laboratory testing, such efforts should not delay antimicrobial therapy.
Loading Doses
Although administration of early appropriate antimicrobial therapy is the central element in management of septic shock, clearance of pathogens will not begin until therapeutic levels of the antimicrobials in the circulation are achieved. The pharmacokinetic principles underlying drug absorption and distribution for optimization of antimicrobial dosing in non-critically ill patients are well established. However, an equivalent broad understanding of dosing issues in critically ill patients has lagged.
Patients with critical illness are known to have major alternations in antimicrobial pharmacokinetics. In particular, many antimicrobials exhibit markedly increased volume of distribution (Vd) especially those agents that are primarily distributed in the extracellular space. Included in this group are β-lactams, aminoglycosides, vancomycin, teicoplanin, and colistin.146-148 This increased Vd can result in inadequate levels of these antimicrobials during the initial treatment phase.149-152 Failure to achieve therapeutic levels early after initiation of antimicrobials may result in suboptimal organism clearance and inferior clinical results.
Therapeutic serum levels of a variety of antimicrobials fail to be consistently achieved using standard dosing regimens in critically ill patients with sepsis and septic shock. β-lactams represent a particularly important example of the problem given their frequency of use in the ICU. In septic shock patients, inadequate serum concentrations for coverage of Pseudomonas during the first dosing interval have been shown for piperacillin–tazobactam, ceftazidime, cefepime, and meropenem using standard intermittent dosing.150 Similarly, initial serum levels of aminoglycosides in septic patients are often inadequate resulting in inferior clinical outcomes.153,154 Comparable finding have been reported for fluoroquinolones,155,156 vancomycin,152,157 teicoplanin,158 and colistin.149,159
An emerging body of literature suggests that loading doses of some antimicrobials can potentially yield improved clinical outcomes.149-151,154,157-162 Supportive data exists in severely ill patients including those with septic shock for aminoglycosides,162 fluoroquinolones,161 vancomycin,152,157 teicoplanin,158 and colistin.149,159 Loading doses may be particularly important when β-lactams are administered as continuous or extended infusions (see later section on this subject). In this circumstance, failure to use a loading dose can substantially delay achievement of therapeutic levels and may be responsible for less than optimal clinical responses in some studies of extended/continuous infusion of β-lactams in critically ill patients.163 Loading doses are also recommended for a broad range of other antimicrobials including tigecycline, fluconazole, anidulafungin, and caspofungin.
Potency of Antimicrobial Therapy
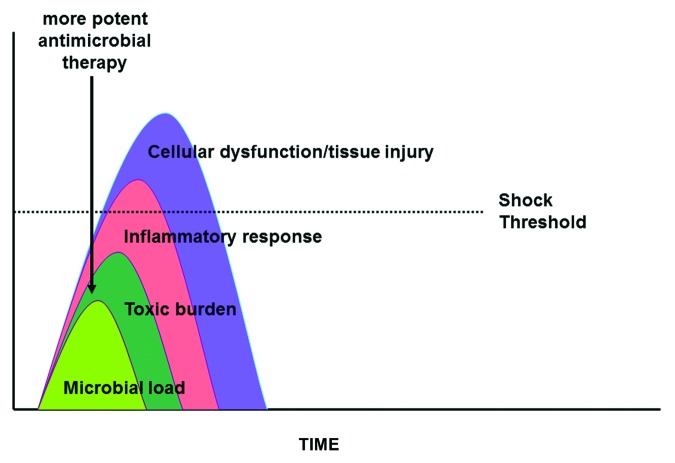
According to Ehrlich’s dictate on optimizing therapy of serious infections, the first principle was to “hit hard”.52 In the simplest terms, this can be understood to mean that, all other things being equal, highly potent antimicrobial regimens providing the most rapid clearance of pathogens are preferred (Fig. 7). This principle has many implications in regards to antimicrobial strategy. It would suggest that cidal therapy may be preferred over static since the terms “static” and “cidal” intrinsically define the rate of pathogen clearance. This principle also suggests that pharmacokinetic optimization of antimicrobial dosing is a requisite element of ideal therapy since “optimization” refers to dosing regimens that clear pathogens more effectively. Further, to the extent that combination therapy has been shown to accelerate pathogen clearance in some models of infection,164 this principle implies that improved survival should result.
Figure 7. Impact of more potent antimicrobial therapy in sepsis and septic shock. See text for explanation.
Rapid pathogen clearance should, in theory, lead to improved clinical response in those infections where a persistent organism burden leads to a time-dependent risk of irreversible and irreplaceable organ injury. The classic example is bacterial meningitis where persistence of organism after initiation of therapy is associated with poor outcome.165,166 In the case of septic shock, more rapid pathogen clearance can similarly be expected to lead to less release of endogenous mediators, more rapid resolution of hemodynamic instability, and improved survival.
Cidal vs. Static Therapy
Although cidal therapy, by definition, should provide more rapid clearance of pathogens, clinical studies generally suggest a lack of clinical superiority over static therapy in most infections.167-169 However, there is a paucity of data on this issue in conditions with time-dependent risk particularly septic shock.
The best known study that has addressed the issue of the importance of cidality in life-threatening infections is the classic study of bacterial meningitis by Lepper and colleagues.170 Meningitis, like septic shock is a condition where the speed of pathogen clearance would be expected to be associated with outcome given that delays of therapy can be expected to result in a progressively increasing risk of irreversible organ (brain) injury and, therefore, death. Lepper’s 1951 study demonstrated that the addition of tetracycline, a bacteriostatic antibiotic, to penicillin (a bactericidal drug dependent on active bacterial replication for killing activity) for therapy of pneumococcal meningitis was associated with worse survival compared with penicillin alone. The basis of this outcome has been thought to be potential antagonism of penicillin-driven bacterial clearance by tetracycline. Similar results have been described when β-lactams are combined with chloramphenicol for treatment of pediatric gram-negative (excluding Haemophilus influenzae) meningitis.171-174 Chloramphenicol, though a cidal drug for most meningeal pathogens is static for gram-negatives (other than H. influenzae). Supportive data in experimental rabbit meningitis suggests a similar need for cidal therapy.175
In recent years, relatively few studies have compared the efficacy of well-established cidal vs static agents in serious infections that may be associated with sepsis and septic shock. One randomized controlled study of anidulafungin (a cidal antifungal echinocandin176) demonstrated superiority over a static triazole, fluconazole in invasive candida infections.26 As with bacterial endocarditis177-179 and osteomyelitis,180 higher cidal activity of antibiotic regimens is associated with better clinical cure rates in neutropenic gram-negative bacteremia.181
With respect to specific bacteriostatic antibiotics, at least one study has suggested inferior microbiological clearance of pathogens in association with a trend to inferior clinical response in clinically evaluable subset in patients with skin and soft tissue infection treated with tigecycline (a static drug) compared with a combination of aztreonam and vancomycin182 though other studies have failed to show similar results.183,184 A metaanalysis of tigecycline non-inferiority trials has shown evidence of increased mortality in the tigecycline arm.185 The use of chloramphenicol for intra-abdominal infections yielded similar initial clinical outcomes compared with penicillin with gentamicin for community-acquired pneumonia in third-world children.186 However, its use was associated with a significantly higher relapse rate at one month suggesting suboptimal microbiologic cure.
Although nominally a cidal agent, vancomycin has relatively weak bacterial killing activity relative to anti-staphylococcal penicillins for methicillin-sensitive S. aureus (MSSA) in time kill studies.187 Accordingly, retrospective studies have shown that vancomycin yields inferior clinical responses and/or survival than anti-staphylococcal β-lactams in patients with MSSA bacteremic infections including pneumonia.92,187-191 Notably, the bacteriostatic agents quinipristin/dalfopristin (a streptogramin) and linezolid (an oxazolidinone) appear to be no more effective than vancomycin for therapy of serious S. aureus infections.192-194 The cidal lipopeptide daptomycin in contrast tends to be superior to vancomycin and comparable to β-lactams in the treatment of bacteremic S. aureus infections.195,196
Overall, the available evidence supports the potential superiority of cidal therapy in life-threatening infections where a time-dependent risk of irreversible and irreplaceable organ injury exists. However, additional studies will be required to definitively address this question in septic shock where the difference should be most profound.
Pharmacokinetic Optimization
A substantial body of literature suggests that optimization of dosing strategies can improve pathogen clearance and clinical responses in infection. However, to date, data on the impact of pharmacokinetic (PK) optimization on mortality in serious infections, particularly septic shock, remains sparse. This review will focus on the two groups of agents used commonly as monotherapy. However, similar principles regarding PK optimization and ideal antimicrobial therapy apply to other agents.
Time-dependent killing agents
For β-lactam antibiotics, the key PK parameter for optimization of pathogen clearance is the fractional time above the minimal inhibitory concentration (fT > MIC) of the pathogen. This refers to the amount of time (relative to the dosing interval) that the concentration of free antibiotic in the plasma exceeds the minimal inhibitory concentration (MIC) of the organism. Experimental studies including murine neutropenic thigh infection and pneumonia models suggests that a fT > MIC of >40–70% (depending on the class of β-lactam) during the course of therapy yields maximal rate of pathogen clearance and clinical response/cure rate.197 There are relatively few studies that examine the role of fT > MIC in serious human infections.
In one study, the rate of clearance of gram-negative bacteria from a total of 32 cases of nosocomial pneumonia among the critically ill was correlated with the fractional time above the dynamic response concentration (similar to fT > MIC) of the pathogen to the treatment antibiotic, cefmenoxime.198 Higher values were also associated with decreased durations of therapy. More recently, the same group has shown that a fT > MIC of 100% in 76 patients enrolled in comparative RCTs of ceftazidime and cefepime for sepsis with bacteremia, lower respiratory tract infection, or complicated urinary tract infection was associated with better bacterial eradication and clinical cure rate than patients with an fT > MIC < 100%.199 In another retrospective study of non-urinary tract infections caused by P. aeruginosa, achievement of cefepime exposures of > 60% fT > MIC minimized the possibility of a poor microbiological response.200 In another study, continuous infusion (which generates 100% fT > MIC for sensitive pathogens) rather than intermittent cefmandole (both with intermittent carbenicillin) was resulted in improved clinical cure in the neutropenic (ANC < 100) and cefmandole sensitive pathogen groups of a group of 235 randomized patients.201 The use of continuous infusion as opposed to intermittent administration of piperacillin has also been shown to be associated with a more rapid decrease in APACHE II score at days 2–4 of the ICU stay in another randomized trial of 40 septic critically ill patients with serious infections.202 Continuous infusion of meropenem has further been shown to be superior to intermittent dosing with respect to microbiologic cure in a randomized, open label study in severe infections admitted to ICU (with a trend favoring improved clinical cure) compared with standard intermittent dosing.203
Among the sickest of the approximately 200 patients (with an APACHE II score of ≥17), extended infusion of piperacillin/tazobactam (4 h rather than a standard 30 min bolus which also increases fT > MIC) for serious Pseudomonas infections has been found to be associated with a shorter hospitalization and lower 14 d mortality.204 This finding is particularly noteworthy in that a mortality effect was noted in only the most critically ill patients; presumably, a large proportion of these would have had septic shock. Similarly, others have shown that continuous infusion of meropenem, piperacillin/tazobactam, and ceftazidime are each associated with a higher rate of clinical cures in high risk gram-negative ventilator-associated pneumonia than is intermittent dosing.205-207 This applied particularly to organisms with higher MIC values including Pseudomonas. A recent double-blind, randomized controlled study of severe sepsis has shown that continuous infusion of β-lactams yields a higher clinical cure rate than does intermittent infusion of the same dose of the antibiotic.208
At least two metaanalyses of continuous infusion of β-lactams in human infection have been published.209,210 Neither showed an overall beneficial effect of continuous infusion; however, both yielded intriguing insights. Each study commented on the trend toward greater beneficial effects in those studies with high baseline mortality risk, an observation that is congruent with our underlying hypothesis that the benefit of pharmacokinetic optimization of dosing strategies on mortality should exist primarily in septic shock and other conditions where a time-dependent risk of irreversible and irreplaceable organ failure exists. In totality, these data support the use of high-end daily dosing at short intervals, extended infusions if necessary and continuous infusions where possible. These data also suggest the need for studies of continuous infusion β-lactam therapy in the highest risk septic shock patients who are most likely to benefit.
Concentration-dependent killing agents
For fluoroquinolones and aminoglycoside antibiotics, the key PK parameter for optimization of pathogen clearance is the area under the curve divided by the MIC relative to the dosing interval and normalized to 24 h (AUC24/MIC) although peak/maximum concentration divided by the MIC of the pathogen (Cmax/MIC) is a closely related value.211-215 These refer again to free drug values. Experimental animal models and human studies suggest that a AUC24/MIC of >87–125 (depending on the individual drug and clinical syndrome) during the course of therapy yields optimal pathogen clearance and clinical cure.197,211-215
In one study of ciprofloxacin therapy of serious infections in 74 critically ill patients, an AUC24/MIC of >125 (total drug) was associated with a significantly better clinical response and bacterial clearance than values below that cut-off.215 Time to bacterial eradication sequentially fell with AUC24/MIC values of <125 (32 d), 125–250 (6.6 d), and >250 (2.0 d). Another study found that clinical cure rates in excess of 90% could be achieved in cases of Pseudomonas bacteremia with AUC24/MIC values of >123 and Cmax/MIC values >8 for ciprofloxacin and/or aminoglycoside therapy.213 The same authors found cure rates of >90% above and <30% below a threshold AUC24/MIC of 250.216 Similarly, levofloxacin Cmax/MIC of >12.2 was found to be associated with a favorable clinical response and pathogen eradication in a prospective open-label study of hospitalized infections requiring at least 3 d of intravenous therapy.217 Other have found that patients with VAP experienced more consistent eradication of the pathogen when the AUC24/MIC was ≥87.218 Similarly, others have shown more effective elimination of P. aeruginosa when levofloxacin AUC24/MIC was ≥88 (90% vs 43% if <88).219 Unfortunately, no human data linking fluoroquinolone PK indices to survival or mortality surrogates and no studies of septic shock have yet been reported.
Nonetheless, given that several studies have shown that inadequate plasma concentrations of fluoroquinolones can be anticipated in critically ill patients, an approach that maximizes the dose within a non-toxic range (e.g., 600 every 12 h for ciprofloxacin or 750 mg daily for levofloxacin assuming preserved renal function) is likely to provide maximally rapid pathogen clearance.156,212,216 Such a high dose approach is prudent particularly for critically ill patients with severe sepsis and/or septic shock where rapid clearance of pathogen would be expected to improve survival.
Vancomycin is another antibiotic whose efficacy is most closely related to concentration-dependent pharmacokinetic indices. One retrospective study of methicillin-resistant S. aureus bacteremia reported fewer vancomycin treatment failures in those who had AUC24/MIC values ≥421.220 In another retrospective study in patients with MRSA pneumonia, better microbiological and clinical outcomes in patients who had vancomycin AUC24/MIC values ≥400.221 The author has recently demonstrated that AUC24/MIC is independently associated with survival in a retrospective study of MRSA septic shock.222
Antimicrobial pharmacokinetic indices have been linked clinical and microbiologic response in a variety of studies but studies showing an association with survival are more limited. To the extent that such studies exist, they tend to show a survival advantage in critically ill patients, particularly those with septic shock.
Combination vs. Monotherapy
Assuming that a pathogen is sensitive to one antibiotic in a regimen, the incremental contribution of a second antibiotic to which the pathogen is sensitive is uncertain. Combination therapy using antibiotics of different classes can generate an additive or even synergistic antimicrobial killing effect leading to more rapid pathogen clearance.164,223-226 Antimicrobial synergy with increased bacterial clearance appears to be best established for β-lactam/aminoglycoside combinations.227-230 However, similar data on synergistic activity has emerged for combinations of a β-lactam and fluoroquinolone.231-237 There is even some data suggesting additive effects238 or even potential synergism239-242 for β-lactam/macrolides combinations in certain circumstances.
Despite animal models164,243,244 and clinical studies of infection including endocarditis, gram negative bacteremia, cryptococcal meningitis, and neutropenic sepsis225,245-247 suggesting a potential therapeutic role of combination therapy, the clinical benefit with respect to outcome of severe infections associated with sepsis has been uncertain. One metaanalysis of randomized controlled trials failed to demonstrate a benefit of β-lactam/aminoglycoside combination therapy in a wide variety of infections.248 Two separate metaanalyses have also failed to demonstrate evidence of benefit with combination therapy in immunocompetent patients with sepsis and/or gram-negative bacteremia.249,250 Other metaanalyses of neutropenic sepsis have similarly suggested little incremental benefit of combination therapy of a β-lactam ± an aminoglycoside.251
A few studies have suggested the possibility that a mortality benefit of combination therapy may exist in sicker patients, particularly those with severe pneumococcal pneumonia/bacteremia252-255 and gram-negative bacteremia.256-258 Based on these data and the view that increased bacterial clearance would be beneficial in septic shock, we performed a stratified metaanalysis/metaregression of 60 sepsis data sets. This analysis showed that combination therapy using 2 drugs (primarily β-lactams with a second agent) yielded a consistent benefit in terms of clinical cure and survival only in patient groups with septic shock or otherwise at high baseline (monotherapy) risk of death.259 Although a pooled odds ratio indicated no overall mortality/clinical response benefit in sepsis (including both sepsis without shock and septic shock) with combination therapy (odds ratio 0.856, 95% confidence interval 0.71–1.03, P = 0.0943, I2 = 45.1%), stratification of data sets by monotherapy mortality risk demonstrated substantial benefit in the most severely ill subset (monotherapy risk of death >25%, OR 0.51, 95% CI 0.41–0.64, I2 = 8.6%). Of those 24 data sets that could be stratified by the presence or absence of shock/critical illness, the more severely ill group with shock consistently demonstrated increased efficacy of a combination therapy strategy (OR 0.49 95% CI 0.35–0.70, P < 0.0001, I2 = 0%). Metaregression indicated that efficacy of combination therapy was dependent only on the risk of death in the monotherapy group. A consistent benefit of combination therapy was found in groups with a baseline (monotherapy) mortality risk of >25% and/or characterized by the presence of septic shock. Of note, these findings held even when the analysis was restricted to only randomized controlled trials.
Our recent study of 2446 propensity-matched septic shock patients has expanded this observation.260 We found that combination therapy using two antibiotics of different antimicrobial classes (i.e., a β-lactam with a fluoroquinolone, aminoglycoside, or in the case of sensitive gram-positives, macrolide/clindamycin) to which the pathogen was sensitive in vitro resulted in an improved 28 d (63.7% vs 71%, hazard ratio, 0.77; 95% confidence interval, 0.67–0.88; P = 0.0002) and hospital survival (52.2% vs 62.6%, odds ratio, 0.69; 95% confidence interval, 0.59–0.81; P < 0.0001). This beneficial effect was independent of organism group and clinical syndrome and was restricted to β-lactams in combination with fluoroquinolones, aminoglycosides, or a macrolide/clindamycin. Interestingly, the most potent β-lactams, β-lactamase inhibitor combinations, and carbapenems (which have 100% t > MIC and therefore maximal cidality for most pathogens) failed to demonstrate evidence of benefit with combination therapy. As predicted by our model of the pathogenesis of septic shock, duration of hypotension was also significantly shorter with combination therapy.
While a randomized controlled trial of therapy of severe sepsis using meropenem ± moxifloxacin failed to demonstrate similar benefit,261 this outcome was predicted by the failure in our propensity study260 for carbapenem combinations to yield a survival advantage for the reason noted above. Several additional recent retrospective studies have similarly shown a benefit of combination therapy using antibiotics with different mechanisms of action in septic shock and related conditions.262-264
Although highly suggestive, these recent retrospective analyses cannot be considered definitive. However, pending the publication of appropriate randomized trials, a strategy of several days of combination therapy for cases of septic shock may be advisable. If our model of microbial load-driven pathogenesis of septic shock accurately reflects the pathophysiology of septic shock, only a few days of combination therapy (until hemodynamic stabilization) is required since at that point, the organism burden has been reduced to a level which no longer drives septic shock and significant ongoing organ injury.
Conclusion
There has been little improvement in the mortality of septic shock since the advent of modern antimicrobial therapy over 60 years ago. The development of ever more broad-spectrum and potent antimicrobials has predictably resulted in evolutionary pressure on microbial pathogens resulting in selection toward resistant organisms. One consequence of this phenomenon may be the lack of progress in efficacy of antimicrobial therapy of septic shock over the ensuing decades.
This review presents a novel model of septic shock pathogenesis that integrates microbiologic, immunologic, and physiologic aspects of the disorder. This alternate paradigm of septic shock pathogenesis differentiates between the pathophysiologic processes that underlie sepsis without shock and septic shock. The model can be used to understand and predict key determinants of antimicrobial therapy response in septic shock. In particular, this model implies that improved outcomes in severe infections with septic shock may be easily achieved through better use of the antimicrobials already in our armamentarium.
In the past, resuscitative elements have taken priority in the management of septic shock. Optimal use of antimicrobial therapy has not been emphasized. However, the reviewed data suggest that empiric, broad spectrum antimicrobial administration should be considered an intrinsic component of initial resuscitation of septic shock. In addition, early optimization of pathogen clearance using highly cidal agents, optimization of antimicrobial pharmacokinetics, and use of appropriate combination therapy appears to increase survival. Available evidence suggests that an approach that combines rapid and potent therapy (i.e., hitting “fast and hard”) should result in significant reductions of septic shock mortality.
Disclosure of Potential Conflicts of Interest
A.K. holds investigator-initiated research grants for the study of septic shock from Astellas and Pfizer. He also holds additional unrelated research grants from GSK and Roche.
References
- 1.Finland M, Jones WF, Barnes MW. Occurence of serious bacterial infections since the introduction of antibacterial agents. JAMA. 1959;84:2188–97. doi: 10.1001/jama.1959.63010180008012. [DOI] [PubMed] [Google Scholar]
- 2.Hemminki E, Paakkulainen A. The effect of antibiotics on mortality from infectious diseases in Sweden and Finland. Am J Public Health. 1976;66:1180–4. doi: 10.2105/AJPH.66.12.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kreger BE, Craven DE, McCabe WR. Gram-negative bacteremia. IV. Re-evaluation of clinical features and treatment in 612 patients. Am J Med. 1980;68:344–55. doi: 10.1016/0002-9343(80)90102-3. [DOI] [PubMed] [Google Scholar]
- 4.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 5.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Sands KE, Bates DW, Lanken PN, Graman PS, Hibberd PL, Kahn KL, Parsonnet J, Panzer R, Orav EJ, Snydman DR, et al. Academic Medical Center Consortium Sepsis Project Working Group Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA. 1997;278:234–40. doi: 10.1001/jama.1997.03550030074038. [DOI] [PubMed] [Google Scholar]
- 7.Brun-Buisson C, Doyon F, Carlet J, Dellamonica P, Gouin F, Lepoutre A, Mercier JC, Offenstadt G, Régnier B, French ICU Group for Severe Sepsis Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. JAMA. 1995;274:968–74. doi: 10.1001/jama.1995.03530120060042. [DOI] [PubMed] [Google Scholar]
- 8.Annane D, Aegerter P, Jars-Guincestre MC, Guidet B, CUB-Réa Network Current epidemiology of septic shock: the CUB-Réa Network. Am J Respir Crit Care Med. 2003;168:165–72. doi: 10.1164/rccm.2201087. [DOI] [PubMed] [Google Scholar]
- 9.Pakhale S, Roberts D, Light B, Sharma S, Kumar A. A geographically and temporally comprehensive analysis of septic shock: Impact of age, sex and socioeconomic status. Crit Care Med. 2005;33:103-M. [Google Scholar]
- 10.Eichacker PQ, Natanson C. Increasing evidence that the risks of rhAPC may outweigh its benefits. Intensive Care Med. 2007;33:396–9. doi: 10.1007/s00134-007-0556-8. [DOI] [PubMed] [Google Scholar]
- 11.Gårdlund B. Activated protein C (Xigris) treatment in sepsis: a drug in trouble. Acta Anaesthesiol Scand. 2006;50:907–10. doi: 10.1111/j.1399-6576.2006.01086.x. [DOI] [PubMed] [Google Scholar]
- 12.Ranieri VM, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, Finfer S, Gårdlund B, Marshall JC, Rhodes A, Artigas A, et al. PROWESS-SHOCK Study Group Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055–64. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 13.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ, The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 14.van der Poll T, van Deventer SJ. Cytokines and anticytokines in the pathogenesis of sepsis. Infect Dis Clin North Am. 1999;13:413–26, ix. doi: 10.1016/S0891-5520(05)70083-0. [DOI] [PubMed] [Google Scholar]
- 15.van der Poll T. Coagulation and inflammation. J Endotoxin Res. 2001;7:301–4. doi: 10.1177/09680519010070040301. [DOI] [PubMed] [Google Scholar]
- 16.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–50. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 17.Remick DG. Pathophysiology of sepsis. Am J Pathol. 2007;170:1435–44. doi: 10.2353/ajpath.2007.060872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levin M, Quint PA, Goldstein B, Barton P, Bradley JS, Shemie SD, Yeh T, Kim SS, Cafaro DP, Scannon PJ, et al. Recombinant bactericidal/permeability-increasing protein (rBPI21) as adjunctive treatment for children with severe meningococcal sepsis: a randomised trial. rBPI21 Meningococcal Sepsis Study Group. Lancet. 2000;356:961–7. doi: 10.1016/S0140-6736(00)02712-4. [DOI] [PubMed] [Google Scholar]
- 19.Freeman BD, Natanson C. Anti-inflammatory therapies in sepsis and septic shock. Expert Opin Investig Drugs. 2000;9:1651–63. doi: 10.1517/13543784.9.7.1651. [DOI] [PubMed] [Google Scholar]
- 20.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, et al. Recombinant human protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 21.Zarychanski R, Doucette S, Fergusson D, Roberts D, Houston DS, Sharma S, Gulati H, Kumar A. Early intravenous unfractionated heparin and mortality in septic shock. Crit Care Med. 2008;36:2973–9. doi: 10.1097/CCM.0b013e31818b8c6b. [DOI] [PubMed] [Google Scholar]
- 22.Khatib R, Johnson LB, Fakih MG, Riederer K, Khosrovaneh A, Shamse Tabriz M, Sharma M, Saeed S. Persistence in Staphylococcus aureus bacteremia: incidence, characteristics of patients and outcome. Scand J Infect Dis. 2006;38:7–14. doi: 10.1080/00365540500372846. [DOI] [PubMed] [Google Scholar]
- 23.Hawkins C, Huang J, Jin N, Noskin GA, Zembower TR, Bolon M. Persistent Staphylococcus aureus bacteremia: an analysis of risk factors and outcomes. Arch Intern Med. 2007;167:1861–7. doi: 10.1001/archinte.167.17.1861. [DOI] [PubMed] [Google Scholar]
- 24.Chowers MY, Gottesman B, Paul M, Weinberger M, Pitlik S, Leibovici L. Persistent bacteremia in the absence of defined intravascular foci: clinical significance and risk factors. Eur J Clin Microbiol Infect Dis. 2003;22:592–6. doi: 10.1007/s10096-003-0999-y. [DOI] [PubMed] [Google Scholar]
- 25.Kullberg BJ, Sobel JD, Ruhnke M, Pappas PG, Viscoli C, Rex JH, Cleary JD, Rubinstein E, Church LW, Brown JM, et al. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomised non-inferiority trial. Lancet. 2005;366:1435–42. doi: 10.1016/S0140-6736(05)67490-9. [DOI] [PubMed] [Google Scholar]
- 26.Reboli AC, Rotstein C, Pappas PG, Chapman SW, Kett DH, Kumar D, Betts R, Wible M, Goldstein BP, Schranz J, et al. Anidulafungin Study Group Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med. 2007;356:2472–82. doi: 10.1056/NEJMoa066906. [DOI] [PubMed] [Google Scholar]
- 27.Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med. 1991;115:674–80. doi: 10.7326/0003-4819-115-9-674. [DOI] [PubMed] [Google Scholar]
- 28.Torgersen C, Moser P, Luckner G, Mayr V, Jochberger S, Hasibeder WR, Dünser MW. Macroscopic postmortem findings in 235 surgical intensive care patients with sepsis. Anesth Analg. 2009;108:1841–7. doi: 10.1213/ane.0b013e318195e11d. [DOI] [PubMed] [Google Scholar]
- 29.Wiggers CJ. Experimental Haemorrhage Shoc k. Physiology of Shock: The Commonwealth Fund New York, NY: Harvard University Press, 1950:121 -43. [Google Scholar]
- 30.De Luca G, Suryapranata H, Ottervanger JP, Antman EM. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts. Circulation. 2004;109:1223–5. doi: 10.1161/01.CIR.0000121424.76486.20. [DOI] [PubMed] [Google Scholar]
- 31.Simoons ML, Serruys PW, vd Brand M, Bär F, de Zwaan C, Res J, Verheugt FW, Krauss XH, Remme WJ, Vermeer F, et al. Improved survival after early thrombolysis in acute myocardial infarction. A randomised trial by the Interuniversity Cardiology Institute in The Netherlands. Lancet. 1985;2:578–82. doi: 10.1016/S0140-6736(85)90584-7. [DOI] [PubMed] [Google Scholar]
- 32.Wood KE. The presence of shock defines the threshold to initiate thrombolytic therapy in patients with pulmonary embolism. Intensive Care Med. 2002;28:1537–46. doi: 10.1007/s00134-002-1486-0. [DOI] [PubMed] [Google Scholar]
- 33.Sampalis JS, Lavoie A, Williams JI, Mulder DS, Kalina M. Impact of on-site care, prehospital time, and level of in-hospital care on survival in severely injured patients. J Trauma. 1993;34:252–61. doi: 10.1097/00005373-199302000-00014. [DOI] [PubMed] [Google Scholar]
- 34.Vallés J, Rello J, Ochagavía A, Garnacho J, Alcalá MA. Community-acquired bloodstream infection in critically ill adult patients: impact of shock and inappropriate antibiotic therapy on survival. Chest. 2003;123:1615–24. doi: 10.1378/chest.123.5.1615. [DOI] [PubMed] [Google Scholar]
- 35.Ulloa L, Tracey KJ. The “cytokine profile”: a code for sepsis. Trends Mol Med. 2005;11:56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Czura CJ, Tracey KJ. Lipid unites disparate syndromes of sepsis. Nat Med. 2004;10:124–5. doi: 10.1038/nm0204-124. [DOI] [PubMed] [Google Scholar]
- 37.Simon PM, Delude RL, Lee M, Kong L, Guzik LJ, Huang DT, Angus DC, Kellum JA, GenIMS Investigators Duration and magnitude of hypotension and monocyte deactivation in patients with community-acquired pneumonia. Shock. 2011;36:553–9. doi: 10.1097/SHK.0b013e318235331e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar A, Haery C, Paladugu B, Kumar A, Symeoneides S, Taiberg L, Osman J, Trenholme G, Opal SM, Goldfarb R, et al. The duration of hypotension before the initiation of antibiotic treatment is a critical determinant of survival in a murine model of Escherichia coli septic shock: association with serum lactate and inflammatory cytokine levels. J Infect Dis. 2006;193:251–8. doi: 10.1086/498909. [DOI] [PubMed] [Google Scholar]
- 39.Øvstebø R, Brandtzaeg P, Brusletto B, Haug KB, Lande K, Høiby EA, Kierulf P. Use of robotized DNA isolation and real-time PCR to quantify and identify close correlation between levels of Neisseria meningitidis DNA and lipopolysaccharides in plasma and cerebrospinal fluid from patients with systemic meningococcal disease. J Clin Microbiol. 2004;42:2980–7. doi: 10.1128/JCM.42.7.2980-2987.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lala HM, Mills GD, Barratt K, Bonning J, Manikkam NE, Martin D. Meningococcal disease deaths and the frequency of antibiotic administration delays. J Infect. 2007;54:551–7. doi: 10.1016/j.jinf.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 41.Yagupsky P, Nolte FS. Quantitative aspects of septicemia. Clin Microbiol Rev. 1990;3:269–79. doi: 10.1128/cmr.3.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rello J, Lisboa T, Lujan M, Gallego M, Kee C, Kay I, Lopez D, Waterer GW, DNA-Neumococo Study Group Severity of pneumococcal pneumonia associated with genomic bacterial load. Chest. 2009;136:832–40. doi: 10.1378/chest.09-0258. [DOI] [PubMed] [Google Scholar]
- 43.Kreger BE, Craven DE, Carling PC, McCabe WR. Gram-negative bacteremia. III. Reassessment of etiology, epidemiology and ecology in 612 patients. Am J Med. 1980;68:332–43. doi: 10.1016/0002-9343(80)90101-1. [DOI] [PubMed] [Google Scholar]
- 44.DuPont HL, Spink WW. Infections due to gram-negative organisms: an analysis of 860 patients with bacteremia at the University of Minnesota Medical Center, 1958-1966. Medicine (Baltimore) 1969;48:307–32. doi: 10.1097/00005792-196907000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Marra AR, Edmond MB, Forbes BA, Wenzel RP, Bearman GML. Time to blood culture positivity as a predictor of clinical outcome of Staphylococcus aureus bloodstream infection. J Clin Microbiol. 2006;44:1342–6. doi: 10.1128/JCM.44.4.1342-1346.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khatib R, Riederer K, Saeed S, Johnson LB, Fakih MG, Sharma M, Tabriz MS, Khosrovaneh A. Time to positivity in Staphylococcus aureus bacteremia: possible correlation with the source and outcome of infection. Clin Infect Dis. 2005;41:594–8. doi: 10.1086/432472. [DOI] [PubMed] [Google Scholar]
- 47.Liao CH, Lai CC, Hsu MS, Huang YT, Chu FY, Hsu HS, Hsueh PR. Correlation between time to positivity of blood cultures with clinical presentation and outcomes in patients with Klebsiella pneumoniae bacteraemia: prospective cohort study. Clin Microbiol Infect. 2009;15:1119–25. doi: 10.1111/j.1469-0691.2009.02720.x. [DOI] [PubMed] [Google Scholar]
- 48.Peralta G, Roiz MP, Sánchez MB, Garrido JC, Ceballos B, Rodríguez-Lera MJ, Mateos F, De Benito I. Time-to-positivity in patients with Escherichia coli bacteraemia. Clin Microbiol Infect. 2007;13:1077–82. doi: 10.1111/j.1469-0691.2007.01817.x. [DOI] [PubMed] [Google Scholar]
- 49.Martínez JA, Soto S, Fabrega A, Almela M, Mensa J, Soriano A, Marco F, Jimenez de Anta MT, Vila J. Relationship of phylogenetic background, biofilm production, and time to detection of growth in blood culture vials with clinical variables and prognosis associated with Escherichia coli bacteremia. J Clin Microbiol. 2006;44:1468–74. doi: 10.1128/JCM.44.4.1468-1474.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chuang YC, Chang SC, Wang WK. Using the rate of bacterial clearance determined by real-time polymerase chain reaction as a timely surrogate marker to evaluate the appropriateness of antibiotic usage in critical patients with Acinetobacter baumannii bacteremia. Crit Care Med. 2012;40:2273–80. doi: 10.1097/CCM.0b013e3182515190. [DOI] [PubMed] [Google Scholar]
- 51.Chandrasekar PH, Crane LR, Bailey EJ. Comparison of the activity of antibiotic combinations in vitro with clinical outcome and resistance emergence in serious infection by Pseudomonas aeruginosa in non-neutropenic patients. J Antimicrob Chemother. 1987;19:321–9. doi: 10.1093/jac/19.3.321. [DOI] [PubMed] [Google Scholar]
- 52.Ehrlich P. Address in Pathology, ON CHEMIOTHERAPY: Delivered before the Seventeenth International Congress of Medicine. Br Med J. 1913;2:353–9. doi: 10.1136/bmj.2.2746.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ammerlaan H, Seifert H, Harbarth S, Brun-Buisson C, Torres A, Antonelli M, Kluytmans J, Bonten M, European Practices of Infections with Staphylococcus aureus (SEPIA) Study Group Adequacy of antimicrobial treatment and outcome of Staphylococcus aureus bacteremia in 9 Western European countries. Clin Infect Dis. 2009;49:997–1005. doi: 10.1086/605555. [DOI] [PubMed] [Google Scholar]
- 54.Haddy RI, Nadkarni DD, Mann BL, Little DR, Domers TD, Clover RD, Silvers MJ. Clostridial bacteremia in the community hospital. Scand J Infect Dis. 2000;32:27–30. doi: 10.1080/00365540050164173. [DOI] [PubMed] [Google Scholar]
- 55.Elhanan G, Sarhat M, Raz R. Empiric antibiotic treatment and the misuse of culture results and antibiotic sensitivities in patients with community-acquired bacteraemia due to urinary tract infection. J Infect. 1997;35:283–8. doi: 10.1016/S0163-4453(97)93194-7. [DOI] [PubMed] [Google Scholar]
- 56.Blot S, Vandewoude K, De Bacquer D, Colardyn F. Nosocomial bacteremia caused by antibiotic-resistant gram-negative bacteria in critically ill patients: clinical outcome and length of hospitalization. Clin Infect Dis. 2002;34:1600–6. doi: 10.1086/340616. [DOI] [PubMed] [Google Scholar]
- 57.Blot S, Depuydt P, Vogelaers D, Decruyenaere J, De Waele J, Hoste E, Peleman R, Claeys G, Verschraegen G, Colardyn F, et al. Colonization status and appropriate antibiotic therapy for nosocomial bacteremia caused by antibiotic-resistant gram-negative bacteria in an intensive care unit. Infect Control Hosp Epidemiol. 2005;26:575–9. doi: 10.1086/502575. [DOI] [PubMed] [Google Scholar]
- 58.Young LS, Martin WJ, Meyer RD, Weinstein RJ, Anderson ET. Gram-negative rod bacteremia: microbiologic, immunologic, and therapeutic considerations. Ann Intern Med. 1977;86:456–71. doi: 10.7326/0003-4819-86-4-456. [DOI] [PubMed] [Google Scholar]
- 59.Romero-Vivas J, Rubio M, Fernandez C, Picazo JJ. Mortality associated with nosocomial bacteremia due to methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 1995;21:1417–23. doi: 10.1093/clinids/21.6.1417. [DOI] [PubMed] [Google Scholar]
- 60.Nguyen MH, Peacock JE, Jr., Tanner DC, Morris AJ, Nguyen ML, Snydman DR, Wagener MM, Yu VL. Therapeutic approaches in patients with candidemia. Evaluation in a multicenter, prospective, observational study. Arch Intern Med. 1995;155:2429–35. doi: 10.1001/archinte.1995.00430220087009. [DOI] [PubMed] [Google Scholar]
- 61.Vergis EN, Hayden MK, Chow JW, Snydman DR, Zervos MJ, Linden PK, Wagener MM, Schmitt B, Muder RR. Determinants of vancomycin resistance and mortality rates in enterococcal bacteremia. a prospective multicenter study. Ann Intern Med. 2001;135:484–92. doi: 10.7326/0003-4819-135-7-200110020-00007. [DOI] [PubMed] [Google Scholar]
- 62.Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE, Dodek P, Wood G, Kumar A, Simon D, et al. Cooperative Antimicrobial Therapy of Septic Shock Database Research Group Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest. 2009;136:1237–48. doi: 10.1378/chest.09-0087. [DOI] [PubMed] [Google Scholar]
- 63.Garnacho-Montero J, García-Cabrera E, Diaz-Martín A, Lepe-Jiménez JA, Iraurgi-Arcarazo P, Jiménez-Alvarez R, Revuelto-Rey J, Aznar-Martín J. Determinants of outcome in patients with bacteraemic pneumococcal pneumonia: importance of early adequate treatment. Scand J Infect Dis. 2010;42:185–92. doi: 10.3109/00365540903418522. [DOI] [PubMed] [Google Scholar]
- 64.Lueangarun S, Leelarasamee A. Impact of inappropriate empiric antimicrobial therapy on mortality of septic patients with bacteremia: A retrospective study. Interdiscip Perspect Infect Dis. 2012;2012:765205. doi: 10.1155/2012/765205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Micek ST, Welch EC, Khan J, Pervez M, Doherty JA, Reichley RM, Kollef MH. Empiric combination antibiotic therapy is associated with improved outcome against sepsis due to Gram-negative bacteria: a retrospective analysis. Antimicrob Agents Chemother. 2010;54:1742–8. doi: 10.1128/AAC.01365-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McGregor JC, Rich SE, Harris AD, Perencevich EN, Osih R, Lodise TP, Jr., Miller RR, Furuno JP. A systematic review of the methods used to assess the association between appropriate antibiotic therapy and mortality in bacteremic patients. Clin Infect Dis. 2007;45:329–37. doi: 10.1086/519283. [DOI] [PubMed] [Google Scholar]
- 67.Kollef MH. Broad-spectrum antimicrobials and the treatment of serious bacterial infections: getting it right up front. Clin Infect Dis. 2008;47(Suppl 1):S3–13. doi: 10.1086/590061. [DOI] [PubMed] [Google Scholar]
- 68.Paul M, Shani V, Muchtar E, Kariv G, Robenshtok E, Leibovici L. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother. 2010;54:4851–63. doi: 10.1128/AAC.00627-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kollef MH, Sherman G, Ward S, Fraser VJ. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest. 1999;115:462–74. doi: 10.1378/chest.115.2.462. [DOI] [PubMed] [Google Scholar]
- 70.Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118:146–55. doi: 10.1378/chest.118.1.146. [DOI] [PubMed] [Google Scholar]
- 71.Kaufman D, Haas CE, Edinger R, Hollick G. Antibiotic susceptibility in the surgical intensive care unit compared with the hospital-wide antibiogram. Arch Surg. 1998;133:1041–5. doi: 10.1001/archsurg.133.10.1041. [DOI] [PubMed] [Google Scholar]
- 72.Green DL. Selection of an empiric antibiotic regimen for hospital-acquired pneumonia using a unit and culture-type specific antibiogram. J Intensive Care Med. 2005;20:296–301. doi: 10.1177/0885066605278650. [DOI] [PubMed] [Google Scholar]
- 73.Byl B, Clevenbergh P, Jacobs F, Struelens MJ, Zech F, Kentos A, Thys JP. Impact of infectious diseases specialists and microbiological data on the appropriateness of antimicrobial therapy for bacteremia. Clin Infect Dis. 1999;29:60–6, discussion 67-8. doi: 10.1086/520182. [DOI] [PubMed] [Google Scholar]
- 74.Raineri E, Pan A, Mondello P, Acquarolo A, Candiani A, Crema L. Role of the infectious diseases specialist consultant on the appropriateness of antimicrobial therapy prescription in an intensive care unit. Am J Infect Control. 2008;36:283–90. doi: 10.1016/j.ajic.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 75.Kerremans JJ, Verbrugh HA, Vos MC. Frequency of microbiologically correct antibiotic therapy increased by infectious disease consultations and microbiological results. J Clin Microbiol. 2012;50:2066–8. doi: 10.1128/JCM.06051-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fowler VG, Jr., Sanders LL, Sexton DJ, Kong L, Marr KA, Gopal AK, Gottlieb G, McClelland RS, Corey GR. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis. 1998;27:478–86. doi: 10.1086/514686. [DOI] [PubMed] [Google Scholar]
- 77.Kollef MH, Morrow LE, Niederman MS, Leeper KV, Anzueto A, Benz-Scott L, Rodino FJ. Clinical characteristics and treatment patterns among patients with ventilator-associated pneumonia. Chest. 2006;129:1210–8. doi: 10.1378/chest.129.5.1210. [DOI] [PubMed] [Google Scholar]
- 78.Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162:505–11. doi: 10.1164/ajrccm.162.2.9909095. [DOI] [PubMed] [Google Scholar]
- 79.Aarts MAW, Brun-Buisson C, Cook DJ, Kumar A, Opal S, Rocker G, Smith T, Vincent JL, Marshall JC. Antibiotic management of suspected nosocomial ICU-acquired infection: does prolonged empiric therapy improve outcome? Intensive Care Med. 2007;33:1369–78. doi: 10.1007/s00134-007-0723-y. [DOI] [PubMed] [Google Scholar]
- 80.Joung MK, Lee JA, Moon SY, Cheong HS, Joo EJ, Ha YE, Sohn KM, Chung SM, Suh GY, Chung DR, et al. Impact of de-escalation therapy on clinical outcomes for intensive care unit-acquired pneumonia. Crit Care. 2011;15:R79. doi: 10.1186/cc10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin MY, Weinstein RA, Hota B. Delay of active antimicrobial therapy and mortality among patients with bacteremia: impact of severe neutropenia. Antimicrob Agents Chemother. 2008;52:3188–94. doi: 10.1128/AAC.01553-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fang CT, Shau WY, Hsueh PR, Chen YC, Wang JT, Hung CC, Huang LY, Chang SC. Early empirical glycopeptide therapy for patients with methicillin-resistant Staphylococcus aureus bacteraemia: impact on the outcome. J Antimicrob Chemother. 2006;57:511–9. doi: 10.1093/jac/dkl006. [DOI] [PubMed] [Google Scholar]
- 83.Giner AM, Kuster SP, Zbinden R, Ruef C, Ledergerber B, Weber R. Initial management of and outcome in patients with pneumococcal bacteremia: a retrospective study at a Swiss university hospital, 2003-2009. Infection. 2011;39:519–26. doi: 10.1007/s15010-011-0218-1. [DOI] [PubMed] [Google Scholar]
- 84.Corona A, Bertolini G, Lipman J, Wilson AP, Singer M. Antibiotic use and impact on outcome from bacteraemic critical illness: the BActeraemia Study in Intensive Care (BASIC) J Antimicrob Chemother. 2010;65:1276–85. doi: 10.1093/jac/dkq088. [DOI] [PubMed] [Google Scholar]
- 85.Silber SH, Garrett C, Singh R, Sweeney A, Rosenberg C, Parachiv D, Okafo T. Early administration of antibiotics does not shorten time to clinical stability in patients with moderate-to-severe community-acquired pneumonia. Chest. 2003;124:1798–804. doi: 10.1378/chest.124.5.1798. [DOI] [PubMed] [Google Scholar]
- 86.Dedier J, Singer DE, Chang Y, Moore M, Atlas SJ. Processes of care, illness severity, and outcomes in the management of community-acquired pneumonia at academic hospitals. Arch Intern Med. 2001;161:2099–104. doi: 10.1001/archinte.161.17.2099. [DOI] [PubMed] [Google Scholar]
- 87.Cheng AC, Buising KL. Delayed administration of antibiotics and mortality in patients with community-acquired pneumonia. Ann Emerg Med. 2009;53:618–24. doi: 10.1016/j.annemergmed.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 88.Kaasch AJ, Rieg S, Kuetscher J, Brodt HR, Widmann T, Herrmann M, Meyer C, Welte T, Kern P, Haars U, et al. preSABATO study group* Delay in the administration of appropriate antimicrobial therapy in Staphylococcus aureus bloodstream infection: a prospective multicenter hospital-based cohort study. Infection. 2013;41:979–85. doi: 10.1007/s15010-013-0428-9. [DOI] [PubMed] [Google Scholar]
- 89.Anderson DJ, Engemann JJ, Harrell LJ, Carmeli Y, Reller LB, Kaye KS. Predictors of mortality in patients with bloodstream infection due to ceftazidime-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 2006;50:1715–20. doi: 10.1128/AAC.50.5.1715-1720.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Whitelaw DA, Rayner BL, Willcox PA. Community-acquired bacteremia in the elderly: a prospective study of 121 cases. J Am Geriatr Soc. 1992;40:996–1000. doi: 10.1111/j.1532-5415.1992.tb04475.x. [DOI] [PubMed] [Google Scholar]
- 91.Erbay A, Idil A, Gözel MG, Mumcuoğlu I, Balaban N. Impact of early appropriate antimicrobial therapy on survival in Acinetobacter baumannii bloodstream infections. Int J Antimicrob Agents. 2009;34:575–9. doi: 10.1016/j.ijantimicag.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 92.Khatib R, Saeed S, Sharma M, Riederer K, Fakih MG, Johnson LB. Impact of initial antibiotic choice and delayed appropriate treatment on the outcome of Staphylococcus aureus bacteremia. Eur J Clin Microbiol Infect Dis. 2006;25:181–5. doi: 10.1007/s10096-006-0096-0. [DOI] [PubMed] [Google Scholar]
- 93.Lodise TP, Jr., Patel N, Kwa A, Graves J, Furuno JP, Graffunder E, Lomaestro B, McGregor JC. Predictors of 30-day mortality among patients with Pseudomonas aeruginosa bloodstream infections: impact of delayed appropriate antibiotic selection. Antimicrob Agents Chemother. 2007;51:3510–5. doi: 10.1128/AAC.00338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lodise TP, McKinnon PS, Swiderski L, Rybak MJ. Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clin Infect Dis. 2003;36:1418–23. doi: 10.1086/375057. [DOI] [PubMed] [Google Scholar]
- 95.Kang CI, Kim SH, Kim HB, Park SW, Choe YJ, Oh MD, Kim EC, Choe KW. Pseudomonas aeruginosa bacteremia: risk factors for mortality and influence of delayed receipt of effective antimicrobial therapy on clinical outcome. Clin Infect Dis. 2003;37:745–51. doi: 10.1086/377200. [DOI] [PubMed] [Google Scholar]
- 96.Menéndez R, Torres A, Reyes S, Zalacain R, Capelastegui A, Aspa J, Borderías L, Martín-Villasclaras JJ, Bello S, Alfageme I, et al. Initial management of pneumonia and sepsis: factors associated with improved outcome. Eur Respir J. 2012;39:156–62. doi: 10.1183/09031936.00188710. [DOI] [PubMed] [Google Scholar]
- 97.Meehan TP, Fine MJ, Krumholz HM, Scinto JD, Galusha DH, Mockalis JT, Weber GF, Petrillo MK, Houck PM, Fine JM. Quality of care, process, and outcomes in elderly patients with pneumonia. JAMA. 1997;278:2080–4. doi: 10.1001/jama.1997.03550230056037. [DOI] [PubMed] [Google Scholar]
- 98.Mathevon T, Souweine B, Traoré O, Aublet B, Caillaud D. ICU-acquired nosocomial infection: impact of delay of adequate antibiotic treatment. Scand J Infect Dis. 2002;34:831–5. doi: 10.1080/0036554021000026934. [DOI] [PubMed] [Google Scholar]
- 99.Heath CH, Grove DI, Looke DFM. Delay in appropriate therapy of Legionella pneumonia associated with increased mortality. Eur J Clin Microbiol Infect Dis. 1996;15:286–90. doi: 10.1007/BF01695659. [DOI] [PubMed] [Google Scholar]
- 100.Iregui M, Ward S, Sherman G, Fraser VJ, Kollef MH. Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest. 2002;122:262–8. doi: 10.1378/chest.122.1.262. [DOI] [PubMed] [Google Scholar]
- 101.Gacouin A, Le Tulzo Y, Lavoue S, Camus C, Hoff J, Bassen R, Arvieux C, Heurtin C, Thomas R. Severe pneumonia due to Legionella pneumophila: prognostic factors, impact of delayed appropriate antimicrobial therapy. Intensive Care Med. 2002;28:686–91. doi: 10.1007/s00134-002-1304-8. [DOI] [PubMed] [Google Scholar]
- 102.Houck PM, Bratzler DW, Nsa W, Ma A, Bartlett JG. Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community-acquired pneumonia. Arch Intern Med. 2004;164:637–44. doi: 10.1001/archinte.164.6.637. [DOI] [PubMed] [Google Scholar]
- 103.Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, Bearden DT. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis. 2006;43:25–31. doi: 10.1086/504810. [DOI] [PubMed] [Google Scholar]
- 104.Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother. 2005;49:3640–5. doi: 10.1128/AAC.49.9.3640-3645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hsu DI, Nguyen M, Nguyen L, Law A, Wong-Beringer A. A multicentre study to evaluate the impact of timing of caspofungin administration on outcomes of invasive candidiasis in non-immunocompromised adult patients. J Antimicrob Chemother. 2010;65:1765–70. doi: 10.1093/jac/dkq216. [DOI] [PubMed] [Google Scholar]
- 106.Patel GP, Simon D, Scheetz M, Crank CW, Lodise T, Patel N. The effect of time to antifungal therapy on mortality in Candidemia associated septic shock. Am J Ther. 2009;16:508–11. doi: 10.1097/MJT.0b013e3181a1afb7. [DOI] [PubMed] [Google Scholar]
- 107.Taur Y, Cohen N, Dubnow S, Paskovaty A, Seo SK. Effect of antifungal therapy timing on mortality in cancer patients with candidemia. Antimicrob Agents Chemother. 2010;54:184–90. doi: 10.1128/AAC.00945-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zahar JR, Azoulay E, Klement E, De Lassence A, Lucet JC, Regnier B, Schlemmer B, Bedos JP. Delayed treatment contributes to mortality in ICU patients with severe active pulmonary tuberculosis and acute respiratory failure. Intensive Care Med. 2001;27:513–20. doi: 10.1007/s001340000849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kethireddy S, Light RB, Miranejad Y, Maki D, Arabi Y, Lapinsky S, Simon D, Kumar A, Parrillo JE, Kumar A. Mycobacterium tuberculosis Septic Shock. Chest. 2013;144:474–82. doi: 10.1378/chest.12-1286/. [DOI] [PubMed] [Google Scholar]
- 110.Gaieski DF, Mikkelsen ME, Band RA, Pines JM, Massone R, Furia FF, Shofer FS, Goyal M. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010;38:1045–53. doi: 10.1097/CCM.0b013e3181cc4824. [DOI] [PubMed] [Google Scholar]
- 111.Barie PS, Hydo LJ, Shou J, Larone DH, Eachempati SR. Influence of antibiotic therapy on mortality of critical surgical illness caused or complicated by infection. Surg Infect (Larchmt) 2005;6:41–54. doi: 10.1089/sur.2005.6.41. [DOI] [PubMed] [Google Scholar]
- 112.Jamieson WG, Pliagus G, Marchuk S, DeRose G, Moffat D, Stafford L, Finley RJ, Sibbald W, Taylor BM, Duff J. Effect of antibiotic and fluid resuscitation upon survival time in experimental intestinal ischemia. Surg Gynecol Obstet. 1988;167:103–8. [PubMed] [Google Scholar]
- 113.Greisman SE, DuBuy JB, Woodward CL. Experimental gram-negative bacterial sepsis: prevention of mortality not preventable by antibiotics alone. Infect Immun. 1979;25:538–57. doi: 10.1128/iai.25.2.538-557.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bodey GP, Jadeja L, Elting L. Pseudomonas bacteremia. Retrospective analysis of 410 episodes. Arch Intern Med. 1985;145:1621–9. doi: 10.1001/archinte.1985.00360090089015. [DOI] [PubMed] [Google Scholar]
- 115.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–96. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 116.Natsch S, Kullberg BJ, van der Meer JW, Meis JF. Delay in administering the first dose of antibiotics in patients admitted to hospital with serious infections. Eur J Clin Microbiol Infect Dis. 1998;17:681–4. doi: 10.1007/s100960050160. [DOI] [PubMed] [Google Scholar]
- 117.Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM. 2005;98:291–8. doi: 10.1093/qjmed/hci047. [DOI] [PubMed] [Google Scholar]
- 118.van Tuijn CF, Luitse JS, van der Valk M, van Wissen S, Prins M, Rosmulder R, Geerlings SE, et al. Reduction of the door-to-needle time for administration of antibiotics in patients with a severe infection: a tailored intervention project. Quicker, faster, better? Neth J Med. 2010;68:123–7. [PubMed] [Google Scholar]
- 119.Clarke RT, Warnick J, Stretton K, Littlewood TJ. Improving the immediate management of neutropenic sepsis in the UK: lessons from a national audit. Br J Haematol. 2011;153:773–9. doi: 10.1111/j.1365-2141.2011.08693.x. [DOI] [PubMed] [Google Scholar]
- 120.Aronin SI, Peduzzi P, Quagliarello VJ. Community-acquired bacterial meningitis: risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann Intern Med. 1998;129:862–9. doi: 10.7326/0003-4819-129-11_Part_1-199812010-00004. [DOI] [PubMed] [Google Scholar]
- 121.Miner JR, Heegaard W, Mapes A, Biros M. Presentation, time to antibiotics, and mortality of patients with bacterial meningitis at an urban county medical center. J Emerg Med. 2001;21:387–92. doi: 10.1016/S0736-4679(01)00407-3. [DOI] [PubMed] [Google Scholar]
- 122.Larché J, Azoulay E, Fieux F, Mesnard L, Moreau D, Thiery G, Darmon M, Le Gall JR, Schlemmer B. Improved survival of critically ill cancer patients with septic shock. Intensive Care Med. 2003;29:1688–95. doi: 10.1007/s00134-003-1957-y. [DOI] [PubMed] [Google Scholar]
- 123.Hamandi B, Holbrook AM, Humar A, Brunton J, Papadimitropoulos EA, Wong GG, Thabane L. Delay of adequate empiric antibiotic therapy is associated with increased mortality among solid-organ transplant patients. Am J Transplant. 2009;9:1657–65. doi: 10.1111/j.1600-6143.2009.02664.x. [DOI] [PubMed] [Google Scholar]
- 124.Gurnani PK, Patel GP, Crank CW, Vais D, Lateef O, Akimov S, Balk R, Simon D. Impact of the implementation of a sepsis protocol for the management of fluid-refractory septic shock: A single-center, before-and-after study. Clin Ther. 2010;32:1285–93. doi: 10.1016/j.clinthera.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 125.Grim SA, Berger K, Teng C, Gupta S, Layden JE, Janda WM, Clark NM. Timing of susceptibility-based antifungal drug administration in patients with Candida bloodstream infection: correlation with outcomes. J Antimicrob Chemother. 2012;67:707–14. doi: 10.1093/jac/dkr511. [DOI] [PubMed] [Google Scholar]
- 126.Ferrer R, Artigas A, Suarez D, Palencia E, Levy MM, Arenzana A, Pérez XL, Sirvent JM, Edusepsis Study Group Effectiveness of treatments for severe sepsis: a prospective, multicenter, observational study. Am J Respir Crit Care Med. 2009;180:861–6. doi: 10.1164/rccm.200812-1912OC. [DOI] [PubMed] [Google Scholar]
- 127.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, et al. International Surviving Sepsis Campaign Guidelines Committee. American Association of Critical-Care Nurses. American College of Chest Physicians. American College of Emergency Physicians. Canadian Critical Care Society. European Society of Clinical Microbiology and Infectious Diseases. European Society of Intensive Care Medicine. European Respiratory Society. International Sepsis Forum. Japanese Association for Acute Medicine. Japanese Society of Intensive Care Medicine. Society of Critical Care Medicine. Society of Hospital Medicine. Surgical Infection Society. World Federation of Societies of Intensive and Critical Care Medicine Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 128.Varpula M, Karlsson S, Parviainen I, Ruokonen E, Pettilä V, Finnsepsis Study Group Community-acquired septic shock: early management and outcome in a nationwide study in Finland. Acta Anaesthesiol Scand. 2007;51:1320–6. doi: 10.1111/j.1399-6576.2007.01439.x. [DOI] [PubMed] [Google Scholar]
- 129.de Sousa AG, Junior CJF, Santos GPD, Laselva CR, Polessi J, Lisboa LF, et al. The impact of each action in the Surviving Sepsis Campaign measures on hospital mortality of patients with severe sepsis/septic shock. Einstein. 2008;6:323–7. [Google Scholar]
- 130.Subramanian S, Yilmaz M, Rehman A, Hubmayr RD, Afessa B, Gajic O. Liberal vs. conservative vasopressor use to maintain mean arterial blood pressure during resuscitation of septic shock: an observational study. Intensive Care Med. 2008;34:157–62. doi: 10.1007/s00134-007-0862-1. [DOI] [PubMed] [Google Scholar]
- 131.Iscimen R, Cartin-Ceba R, Yilmaz M, Khan H, Hubmayr RD, Afessa B, Gajic O. Risk factors for the development of acute lung injury in patients with septic shock: an observational cohort study. Crit Care Med. 2008;36:1518–22. doi: 10.1097/CCM.0b013e31816fc2c0. [DOI] [PubMed] [Google Scholar]
- 132.Bagshaw SM, Lapinsky S, Dial S, Arabi Y, Dodek P, Wood G, Ellis P, Guzman J, Marshall J, Parrillo JE, et al. Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group Acute kidney injury in septic shock: clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med. 2009;35:871–81. doi: 10.1007/s00134-008-1367-2. [DOI] [PubMed] [Google Scholar]
- 133.Garnacho-Montero J, Aldabo-Pallas T, Garnacho-Montero C, Cayuela A, Jiménez R, Barroso S, Ortiz-Leyba C. Timing of adequate antibiotic therapy is a greater determinant of outcome than are TNF and IL-10 polymorphisms in patients with sepsis. Crit Care. 2006;10:R111. doi: 10.1186/cc4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Calbo E, Alsina M, Rodríguez-Carballeira M, Lite J, Garau J. The impact of time on the systemic inflammatory response in pneumococcal pneumonia. Eur Respir J. 2010;35:614–8. doi: 10.1183/09031936.00052709. [DOI] [PubMed] [Google Scholar]
- 135.Thiel SW, Asghar MF, Micek ST, Reichley RM, Doherty JA, Kollef MH. Hospital-wide impact of a standardized order set for the management of bacteremic severe sepsis. Crit Care Med. 2009;37:819–24. doi: 10.1097/CCM.0b013e318196206b. [DOI] [PubMed] [Google Scholar]
- 136.Nguyen HB, Corbett SW, Steele R, Banta J, Clark RT, Hayes SR, Edwards J, Cho TW, Wittlake WA. Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality. Crit Care Med. 2007;35:1105–12. doi: 10.1097/01.CCM.0000259463.33848.3D. [DOI] [PubMed] [Google Scholar]
- 137.Sebat F, Musthafa AA, Johnson D, Kramer AA, Shoffner D, Eliason M, Henry K, Spurlock B. Effect of a rapid response system for patients in shock on time to treatment and mortality during 5 years. Crit Care Med. 2007;35:2568–75. doi: 10.1097/01.CCM.0000287593.54658.89. [DOI] [PubMed] [Google Scholar]
- 138.Castellanos-Ortega A, Suberviola B, García-Astudillo LA, Holanda MS, Ortiz F, Llorca J, Delgado-Rodríguez M. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three-year follow-up quasi-experimental study. Crit Care Med. 2010;38:1036–43. doi: 10.1097/CCM.0b013e3181d455b6. [DOI] [PubMed] [Google Scholar]
- 139.Lefrant JY, Muller L, Raillard A, Jung B, Beaudroit L, Favier L, Masson B, Dingemans G, Thévenot F, Selcer D, et al. Sepsi d’Oc study Group in the AzuRéa Group Reduction of the severe sepsis or septic shock associated mortality by reinforcement of the recommendations bundle: a multicenter study. Ann Fr Anesth Reanim. 2010;29:621–8. doi: 10.1016/j.annfar.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 140.Cardoso T, Carneiro AH, Ribeiro O, Teixeira-Pinto A, Costa-Pereira A. Reducing mortality in severe sepsis with the implementation of a core 6-hour bundle: results from the Portuguese community-acquired sepsis study (SACiUCI study) Crit Care. 2010;14:R83. doi: 10.1186/cc9008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Phua J, Koh Y, Du B, Tang YQ, Divatia JV, Tan CC, Gomersall CD, Faruq MO, Shrestha BR, Gia Binh N, et al. MOSAICS Study Group Management of severe sepsis in patients admitted to Asian intensive care units: prospective cohort study. BMJ. 2011;342:d3245. doi: 10.1136/bmj.d3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Patel GW, Roderman N, Gehring H, Saad J, Bartek W. Assessing the effect of the Surviving Sepsis Campaign treatment guidelines on clinical outcomes in a community hospital. Ann Pharmacother. 2010;44:1733–8. doi: 10.1345/aph.1P251. [DOI] [PubMed] [Google Scholar]
- 143.Pestaña D, Espinosa E, Sangüesa-Molina JR, Ramos R, Pérez-Fernández E, Duque M, Martínez-Casanova E, REASEP Sepsis Study Group Compliance with a sepsis bundle and its effect on intensive care unit mortality in surgical septic shock patients. J Trauma. 2010;69:1282–7. doi: 10.1097/TA.0b013e3181c4539f. [DOI] [PubMed] [Google Scholar]
- 144.Barochia AV, Cui X, Vitberg D, Suffredini AF, O’Grady NP, Banks SM, Minneci P, Kern SJ, Danner RL, Natanson C, et al. Bundled care for septic shock: an analysis of clinical trials. Crit Care Med. 2010;38:668–78. doi: 10.1097/CCM.0b013e3181cb0ddf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Weinstein MP, Towns ML, Quartey SM, Mirrett S, Reimer LG, Parmigiani G, Reller LB. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997;24:584–602. doi: 10.1093/clind/24.4.584. [DOI] [PubMed] [Google Scholar]
- 146.Ulldemolins M, Rello J. The relevance of drug volume of distribution in antibiotic dosing. Curr Pharm Biotechnol. 2011;12:1996–2001. doi: 10.2174/138920111798808365. [DOI] [PubMed] [Google Scholar]
- 147.Varghese JM, Roberts JA, Lipman J. Antimicrobial pharmacokinetic and pharmacodynamic issues in the critically ill with severe sepsis and septic shock. Crit Care Clin. 2011;27:19–34. doi: 10.1016/j.ccc.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 148.del Mar Fernández de Gatta Garcia M, Revilla N, Calvo MV, Domínguez-Gil A, Sánchez Navarro A. Pharmacokinetic/pharmacodynamic analysis of vancomycin in ICU patients. Intensive Care Med. 2007;33:279–85. doi: 10.1007/s00134-006-0470-5. [DOI] [PubMed] [Google Scholar]
- 149.Mohamed AF, Karaiskos I, Plachouras D, Karvanen M, Pontikis K, Jansson B, Papadomichelakis E, Antoniadou A, Giamarellou H, Armaganidis A, et al. Application of a loading dose of colistin methanesulfonate in critically ill patients: population pharmacokinetics, protein binding, and prediction of bacterial kill. Antimicrob Agents Chemother. 2012;56:4241–9. doi: 10.1128/AAC.06426-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Taccone FS, Laterre PF, Dugernier T, Spapen H, Delattre I, Wittebole X, De Backer D, Layeux B, Wallemacq P, Vincent JL, et al. Insufficient β-lactam concentrations in the early phase of severe sepsis and septic shock. Crit Care. 2010;14:R126. doi: 10.1186/cc9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Taccone FS, Laterre PF, Spapen H, Dugernier T, Delattre I, Layeux B, De Backer D, Wittebole X, Wallemacq P, Vincent JL, et al. Revisiting the loading dose of amikacin for patients with severe sepsis and septic shock. Crit Care. 2010;14:R53. doi: 10.1186/cc8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ocampos-Martinez E, Penaccini L, Scolletta S, Abdelhadii A, Devigili A, Cianferoni S, de Backer D, Jacobs F, Cotton F, Vincent JL, et al. Determinants of early inadequate vancomycin concentrations during continuous infusion in septic patients. Int J Antimicrob Agents. 2012;39:332–7. doi: 10.1016/j.ijantimicag.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 153.Moore RD, Smith CR, Lietman PS. The association of aminoglycoside plasma levels with mortality in patients with gram-negative bacteremia. J Infect Dis. 1984;149:443–8. doi: 10.1093/infdis/149.3.443. [DOI] [PubMed] [Google Scholar]
- 154.Chelluri L, Jastremski MS. Inadequacy of standard aminoglycoside loading doses in acutely ill patients. Crit Care Med. 1987;15:1143–5. doi: 10.1097/00003246-198712000-00015. [DOI] [PubMed] [Google Scholar]
- 155.Pletz MW, Bloos F, Burkhardt O, Brunkhorst FM, Bode-Böger SM, Martens-Lobenhoffer J, Greer MW, Stass H, Welte T. Pharmacokinetics of moxifloxacin in patients with severe sepsis or septic shock. Intensive Care Med. 2010;36:979–83. doi: 10.1007/s00134-010-1864-y. [DOI] [PubMed] [Google Scholar]
- 156.van Zanten ARH, Polderman KH, van Geijlswijk IM, van der Meer GYG, Schouten MA, Girbes ARJ. Ciprofloxacin pharmacokinetics in critically ill patients: a prospective cohort study. J Crit Care. 2008;23:422–30. doi: 10.1016/j.jcrc.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 157.Wang J-T, Fang C-T, Chen Y-C, Chang S-C. Necessity of a loading dose when using vancomycin in critically ill patients. J Antimicrob Chemother. 2001;47:246. doi: 10.1093/jac/47.2.246. [DOI] [PubMed] [Google Scholar]
- 158.Pea F, Brollo L, Viale P, Pavan F, Furlanut M. Teicoplanin therapeutic drug monitoring in critically ill patients: a retrospective study emphasizing the importance of a loading dose. J Antimicrob Chemother. 2003;51:971–5. doi: 10.1093/jac/dkg147. [DOI] [PubMed] [Google Scholar]
- 159.Plachouras D, Karvanen M, Friberg LE, Papadomichelakis E, Antoniadou A, Tsangaris I, Karaiskos I, Poulakou G, Kontopidou F, Armaganidis A, et al. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob Agents Chemother. 2009;53:3430–6. doi: 10.1128/AAC.01361-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pea F, Viale P. Bench-to-bedside review: Appropriate antibiotic therapy in severe sepsis and septic shock--does the dose matter? Crit Care. 2009;13:214. doi: 10.1186/cc7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shorr AF, Khashab MM, Xiang JX, Tennenberg AM, Kahn JB. Levofloxacin 750-mg for 5 days for the treatment of hospitalized Fine Risk Class III/IV community-acquired pneumonia patients. Respir Med. 2006;100:2129–36. doi: 10.1016/j.rmed.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 162.Oparaoji EC, Siram S, Shoheiber O, Cornwell EE, 3rd, Mezghebe HM. Appropriateness of a 4 mg/kg gentamicin or tobramycin loading dose in post-operative septic shock patients. J Clin Pharm Ther. 1998;23:185–90. doi: 10.1046/j.1365-2710.1998.00126.x. [DOI] [PubMed] [Google Scholar]
- 163.Kollef MH, Chastre J, Clavel M, Restrepo MI, Michiels B, Kaniga K, Cirillo I, Kimko H, Redman R. A randomized trial of 7-day doripenem versus 10-day imipenem-cilastatin for ventilator-associated pneumonia. Crit Care. 2012;16:R218. doi: 10.1186/cc11862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Calandra T, Glauser MP. Immunocompromised animal models for the study of antibiotic combinations. Am J Med. 1986;80(5C):45–52. [PubMed] [Google Scholar]
- 165.Lebel MH, McCracken GH., Jr. Delayed cerebrospinal fluid sterilization and adverse outcome of bacterial meningitis in infants and children. Pediatrics. 1989;83:161–7. [PubMed] [Google Scholar]
- 166.Lebel MH. Adverse outcome of bacterial meningitis due to delayed sterilization of cerebrospinal fluid. Antibiot Chemother (1971) 1992;45:226–38. doi: 10.1159/000421013. [DOI] [PubMed] [Google Scholar]
- 167.Finberg RW, Moellering RC, Tally FP, Craig WA, Pankey GA, Dellinger EP, West MA, Joshi M, Linden PK, Rolston KV, et al. The importance of bactericidal drugs: future directions in infectious disease. Clin Infect Dis. 2004;39:1314–20. doi: 10.1086/425009. [DOI] [PubMed] [Google Scholar]
- 168.French GL. Bactericidal agents in the treatment of MRSA infections--the potential role of daptomycin. J Antimicrob Chemother. 2006;58:1107–17. doi: 10.1093/jac/dkl393. [DOI] [PubMed] [Google Scholar]
- 169.Pankey GA, Sabath LD. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin Infect Dis. 2004;38:864–70. doi: 10.1086/381972. [DOI] [PubMed] [Google Scholar]
- 170.Lepper MH, Dowling HF. Treatment of pneumoccic meningitis with penicillin compared with penicillin plus aureomycin. Studies including observations on an apparent antagonism between penicillin and aureomycin. Arch Intern Med. 1951;88:489–94. doi: 10.1001/archinte.1951.03810100073006. [DOI] [PubMed] [Google Scholar]
- 171.French GL, Ling TKW, Davies DP, Leung DTY. Antagonism of ceftazidime by chloramphenicol in vitro and in vivo during treatment of gram negative meningitis. Br Med J (Clin Res Ed) 1985;291:636–7. doi: 10.1136/bmj.291.6496.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mathies AW, Jr., Leedom JM, Ivler D, Wehrle PF, Portnoy B. Antibiotic antagonism in bacterial meningitis. Antimicrob Agents Chemother (Bethesda) 1967;7:218–24. [PubMed] [Google Scholar]
- 173.Brown TH, Alford RH. Failure of chloramphenicol and cefotaxime therapy in Klebsiella meningitis: possible role of antibiotic antagonism. South Med J. 1985;78:869–71. doi: 10.1097/00007611-198507000-00025. [DOI] [PubMed] [Google Scholar]
- 174.Cherubin CE, Marr JS, Sierra MF, Becker S. Listeria and gram-negative bacillary meningitis in New York City, 1972-1979. Frequent causes of meningitis in adults. Am J Med. 1981;71:199–209. doi: 10.1016/0002-9343(81)90106-6. [DOI] [PubMed] [Google Scholar]
- 175.Scheld WM, Sande MA. Bactericidal versus bacteriostatic antibiotic therapy of experimental pneumococcal meningitis in rabbits. J Clin Invest. 1983;71:411–9. doi: 10.1172/JCI110785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhanel GG, Saunders DG, Hoban DJ, Karlowsky JA. Influence of human serum on antifungal pharmacodynamics with Candida albicans. Antimicrob Agents Chemother. 2001;45:2018–22. doi: 10.1128/AAC.45.7.2018-2022.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Denny AE, Peterson LR, Gerding DN, Hall WH. Serious staphylococcal infections with strains tolerant to bactericidal antibiotics. Arch Intern Med. 1979;139:1026–31. doi: 10.1001/archinte.1979.03630460058018. [DOI] [PubMed] [Google Scholar]
- 178.Weinstein MP, Stratton CW, Ackley A, Hawley HB, Robinson PA, Fisher BD, Alcid DV, Stephens DS, Reller LB. Multicenter collaborative evaluation of a standardized serum bactericidal test as a prognostic indicator in infective endocarditis. Am J Med. 1985;78:262–9. doi: 10.1016/0002-9343(85)90436-X. [DOI] [PubMed] [Google Scholar]
- 179.Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345:1318–30. doi: 10.1056/NEJMra010082. [DOI] [PubMed] [Google Scholar]
- 180.Weinstein MP, Stratton CW, Hawley HB, Ackley A, Reller LB. Multicenter collaborative evaluation of a standardized serum bactericidal test as a predictor of therapeutic efficacy in acute and chronic osteomyelitis. Am J Med. 1987;83:218–22. doi: 10.1016/0002-9343(87)90688-7. [DOI] [PubMed] [Google Scholar]
- 181.Sculier JP, Klastersky J. Significance of serum bactericidal activity in gram-negative bacillary bacteremia in patients with and without granulocytopenia. Am J Med. 1984;76:429–35. doi: 10.1016/0002-9343(84)90662-4. [DOI] [PubMed] [Google Scholar]
- 182.Breedt J, Teras J, Gardovskis J, Maritz FJ, Vaasna T, Ross DP, Gioud-Paquet M, Dartois N, Ellis-Grosse EJ, Loh E, Tigecycline 305 cSSSI Study Group Safety and efficacy of tigecycline in treatment of skin and skin structure infections: results of a double-blind phase 3 comparison study with vancomycin-aztreonam. Antimicrob Agents Chemother. 2005;49:4658–66. doi: 10.1128/AAC.49.11.4658-4666.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sacchidanand S, Penn RL, Embil JM, Campos ME, Curcio D, Ellis-Grosse E, Loh E, Rose G. Efficacy and safety of tigecycline monotherapy compared with vancomycin plus aztreonam in patients with complicated skin and skin structure infections: Results from a phase 3, randomized, double-blind trial. Int J Infect Dis. 2005;9:251–61. doi: 10.1016/j.ijid.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 184.Oliva ME, Rekha A, Yellin A, Pasternak J, Campos M, Rose GM, Babinchak T, Ellis-Grosse EJ, Loh E, 301 Study Group A multicenter trial of the efficacy and safety of tigecycline versus imipenem/cilastatin in patients with complicated intra-abdominal infections [Study ID Numbers: 3074A1-301-WW; ClinicalTrials.gov Identifier: NCT00081744] BMC Infect Dis. 2005;5:88. doi: 10.1186/1471-2334-5-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Prasad P, Sun J, Danner RL, Natanson C. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis. 2012;54:1699–709. doi: 10.1093/cid/cis270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Duke T, Poka H, Dale F, Michael A, Mgone J, Wal T. Chloramphenicol versus benzylpenicillin and gentamicin for the treatment of severe pneumonia in children in Papua New Guinea: a randomised trial. Lancet. 2002;359:474–80. doi: 10.1016/S0140-6736(02)07677-8. [DOI] [PubMed] [Google Scholar]
- 187.Small PM, Chambers HF. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob Agents Chemother. 1990;34:1227–31. doi: 10.1128/AAC.34.6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.González C, Rubio M, Romero-Vivas J, González M, Picazo JJ. Bacteremic pneumonia due to Staphylococcus aureus: A comparison of disease caused by methicillin-resistant and methicillin-susceptible organisms. Clin Infect Dis. 1999;29:1171–7. doi: 10.1086/313440. [DOI] [PubMed] [Google Scholar]
- 189.Kim SH, Kim KH, Kim HB, Kim NJ, Kim EC, Oh MD, Choe KW. Outcome of vancomycin treatment in patients with methicillin-susceptible Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2008;52:192–7. doi: 10.1128/AAC.00700-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Stryjewski ME, Szczech LA, Benjamin DK, Jr., Inrig JK, Kanafani ZA, Engemann JJ, Chu VH, Joyce MJ, Reller LB, Corey GR, et al. Use of vancomycin or first-generation cephalosporins for the treatment of hemodialysis-dependent patients with methicillin-susceptible Staphylococcus aureus bacteremia. Clin Infect Dis. 2007;44:190–6. doi: 10.1086/510386. [DOI] [PubMed] [Google Scholar]
- 191.Chan KE, Warren HS, Thadhani RI, Steele DJ, Hymes JL, Maddux FW, Hakim RM. Prevalence and outcomes of antimicrobial treatment for Staphylococcus aureus bacteremia in outpatients with ESRD. J Am Soc Nephrol. 2012;23:1551–9. doi: 10.1681/ASN.2012010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rubinstein E, Cammarata S, Oliphant T, Wunderink R, Linezolid Nosocomial Pneumonia Study Group Linezolid (PNU-100766) versus vancomycin in the treatment of hospitalized patients with nosocomial pneumonia: a randomized, double-blind, multicenter study. Clin Infect Dis. 2001;32:402–12. doi: 10.1086/318486. [DOI] [PubMed] [Google Scholar]
- 193.Raad I, Bompart F, Hachem R. Prospective, randomized dose-ranging open phase II pilot study of quinupristin/dalfopristin versus vancomycin in the treatment of catheter-related staphylococcal bacteremia. Eur J Clin Microbiol Infect Dis. 1999;18:199–202. doi: 10.1007/s100960050258. [DOI] [PubMed] [Google Scholar]
- 194.Fagon J, Patrick H, Haas DW, Torres A, Gibert C, Cheadle WG, Falcone RE, Anholm JD, Paganin F, Fabian TC, et al. Nosocomial Pneumonia Group Treatment of gram-positive nosocomial pneumonia. Prospective randomized comparison of quinupristin/dalfopristin versus vancomycin. Am J Respir Crit Care Med. 2000;161:753–62. doi: 10.1164/ajrccm.161.3.9904115. [DOI] [PubMed] [Google Scholar]
- 195.Fowler VG, Jr., Boucher HW, Corey GR, Abrutyn E, Karchmer AW, Rupp ME, Levine DP, Chambers HF, Tally FP, Vigliani GA, et al. S. aureus Endocarditis and Bacteremia Study Group Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355:653–65. doi: 10.1056/NEJMoa053783. [DOI] [PubMed] [Google Scholar]
- 196.Alder J. The use of daptomycin for Staphylococcus aureus infections in critical care medicine. Crit Care Clin. 2008;24:349–63, ix-x. doi: 10.1016/j.ccc.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 197.Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–10, quiz 11-2. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 198.Schentag JJ, Smith IL, Swanson DJ, DeAngelis C, Fracasso JE, Vari A, Vance JW. Role for dual individualization with cefmenoxime. Am J Med. 1984;77(6A):43–50. doi: 10.1016/s0002-9343(84)80074-1. [DOI] [PubMed] [Google Scholar]
- 199.McKinnon PS, Paladino JA, Schentag JJ. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents. 2008;31:345–51. doi: 10.1016/j.ijantimicag.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 200.Crandon JL, Bulik CC, Kuti JL, Nicolau DP. Clinical pharmacodynamics of cefepime in patients infected with Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2010;54:1111–6. doi: 10.1128/AAC.01183-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bodey GP, Ketchel SJ, Rodriguez V. A randomized study of carbenicillin plus cefamandole or tobramycin in the treatment of febrile episodes in cancer patients. Am J Med. 1979;67:608–16. doi: 10.1016/0002-9343(79)90242-0. [DOI] [PubMed] [Google Scholar]
- 202.Rafati MR, Rouini MR, Mojtahedzadeh M, Najafi A, Tavakoli H, Gholami K, Fazeli MR. Clinical efficacy of continuous infusion of piperacillin compared with intermittent dosing in septic critically ill patients. Int J Antimicrob Agents. 2006;28:122–7. doi: 10.1016/j.ijantimicag.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 203.Chytra I, Stepan M, Benes J, Pelnar P, Zidkova A, Bergerova T, Pradl R, Kasal E. Clinical and microbiological efficacy of continuous versus intermittent application of meropenem in critically ill patients: a randomized open-label controlled trial. Crit Care. 2012;16:R113. doi: 10.1186/cc11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lodise TP, Jr., Lomaestro BM, Drusano GL. Piperacillin-tazobactam for Pseudomonas aeruginosa infection: clinical implications of an extended-infusion dosing strategy. Clin Infect Dis. 2007;44:357–63. doi: 10.1086/510590. [DOI] [PubMed] [Google Scholar]
- 205.Lorente L, Lorenzo L, Martín MM, Jiménez A, Mora ML. Meropenem by continuous versus intermittent infusion in ventilator-associated pneumonia due to gram-negative bacilli. Ann Pharmacother. 2006;40:219–23. doi: 10.1345/aph.1G467. [DOI] [PubMed] [Google Scholar]
- 206.Lorente L, Jiménez A, Palmero S, Jiménez JJ, Iribarren JL, Santana M, Martín MM, Mora ML. Comparison of clinical cure rates in adults with ventilator-associated pneumonia treated with intravenous ceftazidime administered by continuous or intermittent infusion: a retrospective, nonrandomized, open-label, historical chart review. Clin Ther. 2007;29:2433–9. doi: 10.1016/j.clinthera.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 207.Lorente L, Jiménez A, Martín MM, Iribarren JL, Jiménez JJ, Mora ML. Clinical cure of ventilator-associated pneumonia treated with piperacillin/tazobactam administered by continuous or intermittent infusion. Int J Antimicrob Agents. 2009;33:464–8. doi: 10.1016/j.ijantimicag.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 208.Dulhunty JM, Roberts JA, Davis JS, Webb SA, Bellomo R, Gomersall C, Shirwadkar C, Eastwood GM, Myburgh J, Paterson DL, et al. Continuous infusion of beta-lactam antibiotics in severe sepsis: a multicenter double-blind, randomized controlled trial. Clin Infect Dis. 2013;56:236–44. doi: 10.1093/cid/cis856. [DOI] [PubMed] [Google Scholar]
- 209.Kasiakou SK, Sermaides GJ, Michalopoulos A, Soteriades ES, Falagas ME. Continuous versus intermittent intravenous administration of antibiotics: a meta-analysis of randomised controlled trials. Lancet Infect Dis. 2005;5:581–9. doi: 10.1016/S1473-3099(05)70218-8. [DOI] [PubMed] [Google Scholar]
- 210.Roberts JA, Webb S, Paterson D, Ho KM, Lipman J. A systematic review on clinical benefits of continuous administration of β-lactam antibiotics. Crit Care Med. 2009;37:2071–8. doi: 10.1097/CCM.0b013e3181a0054d. [DOI] [PubMed] [Google Scholar]
- 211.Scaglione F, Mouton JW, Mattina R, Fraschini F. Pharmacodynamics of levofloxacin and ciprofloxacin in a murine pneumonia model: peak concentration/MIC versus area under the curve/MIC ratios. Antimicrob Agents Chemother. 2003;47:2749–55. doi: 10.1128/AAC.47.9.2749-2755.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Winterboer TM, Lecci KA, Olsen KM. Continuing education: alternative approaches to optimizing antimicrobial pharmacodynamics in critically ill patients. J Pharm Pract. 2010;23:6–18. doi: 10.1177/0897190009356550. [DOI] [PubMed] [Google Scholar]
- 213.Zelenitsky SA, Harding GK, Sun S, Ubhi K, Ariano RE. Treatment and outcome of Pseudomonas aeruginosa bacteraemia: an antibiotic pharmacodynamic analysis. J Antimicrob Chemother. 2003;52:668–74. doi: 10.1093/jac/dkg403. [DOI] [PubMed] [Google Scholar]
- 214.Ambrose PG, Bhavnani SM, Owens RC., Jr. Clinical pharmacodynamics of quinolones. Infect Dis Clin North Am. 2003;17:529–43. doi: 10.1016/S0891-5520(03)00061-8. [DOI] [PubMed] [Google Scholar]
- 215.Forrest A, Nix DE, Ballow CH, Goss TF, Birmingham MC, Schentag JJ. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother. 1993;37:1073–81. doi: 10.1128/AAC.37.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zelenitsky SA, Ariano RE. Support for higher ciprofloxacin AUC 24/MIC targets in treating Enterobacteriaceae bloodstream infection. J Antimicrob Chemother. 2010;65:1725–32. doi: 10.1093/jac/dkq211. [DOI] [PubMed] [Google Scholar]
- 217.Preston SL, Drusano GL, Berman AL, Fowler CL, Chow AT, Dornseif B, Reichl V, Natarajan J, Corrado M. Pharmacodynamics of levofloxacin: a new paradigm for early clinical trials. JAMA. 1998;279:125–9. doi: 10.1001/jama.279.2.125. [DOI] [PubMed] [Google Scholar]
- 218.Drusano GL, Preston SL, Fowler C, Corrado M, Weisinger B, Kahn J. Relationship between fluoroquinolone area under the curve: minimum inhibitory concentration ratio and the probability of eradication of the infecting pathogen, in patients with nosocomial pneumonia. J Infect Dis. 2004;189:1590–7. doi: 10.1086/383320. [DOI] [PubMed] [Google Scholar]
- 219.Jumbe NL, Lovie A, Liv M, et al. Pharmacodynamics of levofloxacin with different organisms: one size does not fit all (abstract 29). Program and Abstracts of the 40th Interscience Conference of Anti-Microbial Agents and Chemotherapy (Toronto)Washington, DC: American Society for Microbiology 2000. [Google Scholar]
- 220.Kullar R, Davis SL, Levine DP, Rybak MJ. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis. 2011;52:975–81. doi: 10.1093/cid/cir124. [DOI] [PubMed] [Google Scholar]
- 221.Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43:925–42. doi: 10.2165/00003088-200443130-00005. [DOI] [PubMed] [Google Scholar]
- 222.Zelenitsky S, Rubinstein E, Ariano R, Iacovides H, Dodek P, Mirzanejad Y, Kumar A, Cooperative Antimicrobial Therapy of Septic Shock-CATSS Database Research Group Vancomycin pharmacodynamics and survival in patients with methicillin-resistant Staphylococcus aureus-associated septic shock. Int J Antimicrob Agents. 2013;41:255–60. doi: 10.1016/j.ijantimicag.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 223.De Jongh CA, Joshi JH, Thompson BW, Newman KA, Finley RS, Moody MR, Salvatore PC, Tenney JH, Drusano GL, Schimpff SC. A double beta-lactam combination versus an aminoglycoside-containing regimen as empiric antibiotic therapy for febrile granulocytopenic cancer patients. Am J Med. 1986;80(5C):101–11. [PubMed] [Google Scholar]
- 224.Klastersky J, Zinner SH. Synergistic combinations of antibiotics in gram-negative bacillary infections. Rev Infect Dis. 1982;4:294–301. doi: 10.1093/clinids/4.2.294. [DOI] [PubMed] [Google Scholar]
- 225.Anderson ET, Young LS, Hewitt WL. Antimicrobial synergism in the therapy of gram-negative rod bacteremia. Chemotherapy. 1978;24:45–54. doi: 10.1159/000237759. [DOI] [PubMed] [Google Scholar]
- 226.Giamarellou H. Aminoglycosides plus beta-lactams against gram-negative organisms. Evaluation of in vitro synergy and chemical interactions. Am J Med. 1986;80(6B):126–37. doi: 10.1016/0002-9343(86)90490-0. [DOI] [PubMed] [Google Scholar]
- 227.Comber KR, Basker MJ, Osborne CD, Sutherland R. Synergy between ticarcillin and tobramycin against Pseudomonas aeruginosa and Enterobacteriaceae in vitro and in vivo. Antimicrob Agents Chemother. 1977;11:956–64. doi: 10.1128/AAC.11.6.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Archer G, Fekety FR., Jr. Experimental endocarditis due to Pseudomonas aeruginosa. II. Therapy with carbenicillin and gentamicin. J Infect Dis. 1977;136:327–35. doi: 10.1093/infdis/136.3.327. [DOI] [PubMed] [Google Scholar]
- 229.Yoshikawa TT, Shibata SA. In vitro antibacterial activity of amikacin and ticarcillin, alone and in combination, against Pseudomonas aerurginosa. Antimicrob Agents Chemother. 1978;13:997–9. doi: 10.1128/AAC.13.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kluge RM, Standiford HC, Tatem B, Young VM, Greene WH, Schimpff SC, Calia FM, Hornick RB. Comparative activity of tobramycin, amikacin, and gentamicin alone and with carbenicillin against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1974;6:442–6. doi: 10.1128/AAC.6.4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Gimeno C, Borja J, Navarro D, Valdés L, García-Barbal J, García-de-Lomas J. In vitro interaction between ofloxacin and cefotaxime against gram-positive and gram-negative bacteria involved in serious infections. Chemotherapy. 1998;44:94–8. doi: 10.1159/000007098. [DOI] [PubMed] [Google Scholar]
- 232.Gradelski E, Kolek B, Bonner DP, Valera L, Minassian B, Fung-Tomc J. Activity of gatifloxacin and ciprofloxacin in combination with other antimicrobial agents. Int J Antimicrob Agents. 2001;17:103–7. doi: 10.1016/S0924-8579(00)00317-4. [DOI] [PubMed] [Google Scholar]
- 233.Zembower TR, Noskin GA, Postelnick MJ, Nguyen C, Peterson LR. The utility of aminoglycosides in an era of emerging drug resistance. Int J Antimicrob Agents. 1998;10:95–105. doi: 10.1016/S0924-8579(98)00033-8. [DOI] [PubMed] [Google Scholar]
- 234.Neu HC. Synergy and antagonism of fluoroquinolones with other classes of antimicrobial agents. Drugs. 1993;45(Suppl 3):54–8. doi: 10.2165/00003495-199300453-00011. [DOI] [PubMed] [Google Scholar]
- 235.Pohlman JK, Knapp CC, Ludwig MD, Washington JA. Timed killing kinetic studies of the interaction between ciprofloxacin and beta-lactams against gram-negative bacilli. Diagn Microbiol Infect Dis. 1996;26:29–33. doi: 10.1016/S0732-8893(96)00174-5. [DOI] [PubMed] [Google Scholar]
- 236.Milatovic D, Wallrauch C. In vitro activity of trovafloxacin in combination with ceftazidime, meropenem, and amikacin. Eur J Clin Microbiol Infect Dis. 1996;15:688–93. doi: 10.1007/BF01691162. [DOI] [PubMed] [Google Scholar]
- 237.Pankuch GA, Lin G, Seifert H, Appelbaum PC. Activity of meropenem with and without ciprofloxacin and colistin against Pseudomonas aeruginosa and Acinetobacter baumannii. Antimicrob Agents Chemother. 2008;52:333–6. doi: 10.1128/AAC.00689-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Lin E, Stanek RJ, Mufson MA. Lack of synergy of erythromycin combined with penicillin or cefotaxime against Streptococcus pneumoniae in vitro. Antimicrob Agents Chemother. 2003;47:1151–3. doi: 10.1128/AAC.47.3.1151-1153.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.MacGowan AR, Bowker K, Bedford KA, Holt HA, Reeves DS. Synergy testing of macrolide combinations using the chequerboard technique. J Antimicrob Chemother. 1993;32:913–5. doi: 10.1093/jac/32.6.913. [DOI] [PubMed] [Google Scholar]
- 240.Furuya R, Nakayama H, Kanayama A, Saika T, Iyoda T, Tatewaki M, Matsuzaki K, Kobayashi I, Tanaka M. In vitro synergistic effects of double combinations of beta-lactams and azithromycin against clinical isolates of Neisseria gonorrhoeae. J Infect Chemother. 2006;12:172–6. doi: 10.1007/s10156-006-0445-z. [DOI] [PubMed] [Google Scholar]
- 241.Maioli E, Debbia EA, Gualco L, Marchese A. In vitro interaction between mecillinam and piperacillin-tazobactam in the presence of azithromycin against members of the Enterobacteriaceae family and Pseudomonas aeruginosa. New Microbiol. 2008;31:37–46. [PubMed] [Google Scholar]
- 242.Allen NE, Epp JK. Mechanism of penicillin-erythromycin synergy on antibiotic-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1978;13:849–53. doi: 10.1128/AAC.13.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kumar A, Mensing J, Zelenitsky S, Ariano R, Parrillo JE. Effect of antibiotic sequence on blood bacterial counts in a rat model of E. coli peritonitis/septic shock. ICAAC Proceedings 2004:page 26; A-1296. [Google Scholar]
- 244.Darras-Joly C, Bédos JP, Sauve C, Moine P, Vallée E, Carbon C, Azoulay-Dupuis E. Synergy between amoxicillin and gentamicin in combination against a highly penicillin-resistant and -tolerant strain of Streptococcus pneumoniae in a mouse pneumonia model. Antimicrob Agents Chemother. 1996;40:2147–51. doi: 10.1128/aac.40.9.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Bennett JE, Dismukes WE, Duma RJ, Medoff G, Sande MA, Gallis H, Leonard J, Fields BT, Bradshaw M, Haywood H, et al. A comparison of amphotericin B alone and combined with flucytosine in the treatment of cryptoccal meningitis. N Engl J Med. 1979;301:126–31. doi: 10.1056/NEJM197907193010303. [DOI] [PubMed] [Google Scholar]
- 246.De Jongh CA, Joshi JH, Newman KA, Moody MR, Wharton R, Standiford HC, Schimpff SC. Antibiotic synergism and response in gram-negative bacteremia in granulocytopenic cancer patients. Am J Med. 1986;80(5C):96–100. [PubMed] [Google Scholar]
- 247.Bouza E, Muñoz P. Monotherapy versus combination therapy for bacterial infections. Med Clin North Am. 2000;84:1357–89, v. doi: 10.1016/S0025-7125(05)70293-5. [DOI] [PubMed] [Google Scholar]
- 248.Marcus R, Paul M, Elphick H, Leibovici L. Clinical implications of β-lactam-aminoglycoside synergism: systematic review of randomised trials. Int J Antimicrob Agents. 2011;37:491–503. doi: 10.1016/j.ijantimicag.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 249.Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis. 2004;4:519–27. doi: 10.1016/S1473-3099(04)01108-9. [DOI] [PubMed] [Google Scholar]
- 250.Paul M, Benuri-Silbiger I, Soares-Weiser K, Leibovici L. Beta lactam monotherapy versus beta lactam-aminoglycoside combination therapy for sepsis in immunocompetent patients: systematic review and meta-analysis of randomised trials. BMJ. 2004;328:668–79. doi: 10.1136/bmj.38028.520995.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Paul M, Soares-Weiser K, Leibovici L. β lactam monotherapy versus β lactam-aminoglycoside combination therapy for fever with neutropenia: systematic review and meta-analysis. BMJ. 2003;326:1111–8. doi: 10.1136/bmj.326.7399.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Waterer GW, Somes GW, Wunderink RG. Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia. Arch Intern Med. 2001;161:1837–42. doi: 10.1001/archinte.161.15.1837. [DOI] [PubMed] [Google Scholar]
- 253.Baddour LM, Yu VL, Klugman KP, Feldman C, Ortqvist A, Rello J, Morris AJ, Luna CM, Snydman DR, Ko WC, et al. International Pneumococcal Study Group Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170:440–4. doi: 10.1164/rccm.200311-1578OC. [DOI] [PubMed] [Google Scholar]
- 254.Rodríguez A, Mendia A, Sirvent JM, Barcenilla F, de la Torre-Prados MV, Solé-Violán J, Rello J, CAPUCI Study Group Combination antibiotic therapy improves survival in patients with community-acquired pneumonia and shock. Crit Care Med. 2007;35:1493–8. doi: 10.1097/01.CCM.0000266755.75844.05. [DOI] [PubMed] [Google Scholar]
- 255.Martin-Loeches I, Lisboa T, Rodriguez A, Putensen C, Annane D, Garnacho-Montero J, Restrepo MI, Rello J. Combination antibiotic therapy with macrolides improves survival in intubated patients with community-acquired pneumonia. Intensive Care Med. 2010;36:612–20. doi: 10.1007/s00134-009-1730-y. [DOI] [PubMed] [Google Scholar]
- 256.Chow JW, Fine MJ, Shlaes DM, Quinn JP, Hooper DC, Johnson MP, Ramphal R, Wagener MM, Miyashiro DK, Yu VL. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med. 1991;115:585–90. doi: 10.7326/0003-4819-115-8-585. [DOI] [PubMed] [Google Scholar]
- 257.Hilf M, Yu VL, Sharp J, Zuravleff JJ, Korvick JA, Muder RR. Antibiotic therapy for Pseudomonas aeruginosa bacteremia: outcome correlations in a prospective study of 200 patients. Am J Med. 1989;87:540–6. doi: 10.1016/S0002-9343(89)80611-4. [DOI] [PubMed] [Google Scholar]
- 258.Korvick JA, Bryan CS, Farber B, Beam TR, Jr., Schenfeld L, Muder RR, Weinbaum D, Lumish R, Gerding DN, Wagener MM, et al. Prospective observational study of Klebsiella bacteremia in 230 patients: outcome for antibiotic combinations versus monotherapy. Antimicrob Agents Chemother. 1992;36:2639–44. doi: 10.1128/AAC.36.12.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kumar A, Safdar N, Kethireddy S, Chateau D. A survival benefit of combination antibiotic therapy for serious infections associated with sepsis and septic shock is contingent only on the risk of death: a meta-analytic/meta-regression study. Crit Care Med. 2010;38:1651–64. doi: 10.1097/CCM.0b013e3181e96b91. [DOI] [PubMed] [Google Scholar]
- 260.Kumar A, Zarychanski R, Light B, Parrillo JE, Maki D, Simon D, Laporta D, Lapinsky S, Ellis P, Mirzanejad Y, et al. Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group Early combination antibiotic therapy yields improved survival compared with monotherapy in septic shock: a propensity-matched analysis. Crit Care Med. 2010;38:1773–85. doi: 10.1097/CCM.0b013e3181eb3ccd. [DOI] [PubMed] [Google Scholar]
- 261.Brunkhorst FM, Oppert M, Marx G, Bloos F, Ludewig K, Putensen C, Nierhaus A, Jaschinski U, Meier-Hellmann A, Weyland A, et al. German Study Group Competence Network Sepsis (SepNet) Effect of empirical treatment with moxifloxacin and meropenem vs meropenem on sepsis-related organ dysfunction in patients with severe sepsis: a randomized trial. JAMA. 2012;307:2390–9. doi: 10.1001/jama.2012.5833. [DOI] [PubMed] [Google Scholar]
- 262.Al-Hasan MN, Wilson JW, Lahr BD, Thomsen KM, Eckel-Passow JE, Vetter EA, Tleyjeh IM, Baddour LM. Beta-lactam and fluoroquinolone combination antibiotic therapy for bacteremia caused by gram-negative bacilli. Antimicrob Agents Chemother. 2009;53:1386–94. doi: 10.1128/AAC.01231-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Delannoy PY, Boussekey N, Devos P, Alfandari S, Turbelin C, Chiche A, Meybeck A, Georges H, Leroy O. Impact of combination therapy with aminoglycosides on the outcome of ICU-acquired bacteraemias. Eur J Clin Microbiol Infect Dis. 2012;31:2293–9. doi: 10.1007/s10096-012-1568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Díaz-Martín A, Martínez-González ML, Ferrer R, Ortiz-Leyba C, Piacentini E, Lopez-Pueyo MJ, Martín-Loeches I, Levy MM, Artigas A, Garnacho-Montero J, for the Edusepsis Study Group Antibiotic prescription patterns in the empiric therapy of severe sepsis: combination of antimicrobials with different mechanisms of action reduces mortality. Crit Care. 2012;16:R223. doi: 10.1186/cc11869. [DOI] [PMC free article] [PubMed] [Google Scholar]