Abstract
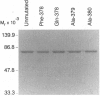
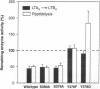
Leukotriene A4 (LTA4) hydrolase [(7E,9E,11Z,14Z)-(5S,6S)-5,6-epoxyicosa-7, 9,11,14-tetraenoate hydrolase; EC 3.3.2.6] is a bifunctional zinc metalloenzyme that catalyzes the final step in the biosynthesis of the potent chemotactic agent leukotriene B4 (LTB4). LTA4 hydrolase/aminopeptidase is suicide inactivated during catalysis via an apparently mechanism-based irreversible binding of LTA4 to the protein in a 1:1 stoichiometry. Previously, we have identified a henicosapeptide, encompassing residues Leu-365 to Lys-385 in human LTA4 hydrolase, which contains a site involved in the covalent binding of LTA4 to the native enzyme. To investigate the role of Tyr-378, a potential candidate for this binding site, we exchanged Tyr for Phe or Gln in two separate mutants. In addition, each of two adjacent and potentially reactive residues, Ser-379 and Ser-380, were exchanged for Ala. The mutated enzymes were expressed as (His)6-tagged fusion proteins in Escherichia coli, purified to apparent homogeneity, and characterized. Enzyme activity determinations and differential peptide mapping, before and after repeated exposure to LTA4, revealed that wild-type enzyme and the mutants [S379A] and [S380A]LTA4hydrolase were equally susceptible to suicide inactivation whereas the mutants in position 378 were no longer inactivated or covalently modified by LTA4. Furthermore, in [Y378F]LTA4 hydrolase, the value of kcat for epoxide hydrolysis was increased 2.5-fold over that of the wild-type enzyme. Thus, by a single-point mutation in LTA4 hydrolase, catalysis and covalent modification/inactivation have been dissociated, yielding an enzyme with increased turnover and resistance to mechanism-based inactivation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blomster M., Wetterholm A., Mueller M. J., Haeggström J. Z. Evidence for a catalytic role of tyrosine 383 in the peptidase reaction of leukotriene A4 hydrolase. Eur J Biochem. 1995 Aug 1;231(3):528–534. doi: 10.1111/j.1432-1033.1995.0528d.x. [DOI] [PubMed] [Google Scholar]
- Evans J. F., Kargman S. Bestatin inhibits covalent coupling of [3H]LTA4 to human leukocyte LTA4 hydrolase. FEBS Lett. 1992 Feb 3;297(1-2):139–142. doi: 10.1016/0014-5793(92)80345-h. [DOI] [PubMed] [Google Scholar]
- Evans J. F., Nathaniel D. J., Zamboni R. J., Ford-Hutchinson A. W. Leukotriene A3. A poor substrate but a potent inhibitor of rat and human neutrophil leukotriene A4 hydrolase. J Biol Chem. 1985 Sep 15;260(20):10966–10970. [PubMed] [Google Scholar]
- Haeggström J. Z., Wetterholm A., Medina J. F., Samuelsson B. Leukotriene A4 hydrolase: structural and functional properties of the active center. J Lipid Mediat. 1993 Mar-Apr;6(1-3):1–13. [PubMed] [Google Scholar]
- Haeggström J. Z., Wetterholm A., Vallee B. L., Samuelsson B. Leukotriene A4 hydrolase: an epoxide hydrolase with peptidase activity. Biochem Biophys Res Commun. 1990 Nov 30;173(1):431–437. doi: 10.1016/s0006-291x(05)81076-9. [DOI] [PubMed] [Google Scholar]
- McGee J., Fitzpatrick F. Enzymatic hydration of leukotriene A4. Purification and characterization of a novel epoxide hydrolase from human erythrocytes. J Biol Chem. 1985 Oct 15;260(23):12832–12837. [PubMed] [Google Scholar]
- Medina J. F., Wetterholm A., Rådmark O., Shapiro R., Haeggström J. Z., Vallee B. L., Samuelsson B. Leukotriene A4 hydrolase: determination of the three zinc-binding ligands by site-directed mutagenesis and zinc analysis. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7620–7624. doi: 10.1073/pnas.88.17.7620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami M., Ohishi N., Mutoh H., Izumi T., Bito H., Wada H., Seyama Y., Toh H., Shimizu T. Leukotriene A4 hydrolase is a zinc-containing aminopeptidase. Biochem Biophys Res Commun. 1990 Dec 14;173(2):620–626. doi: 10.1016/s0006-291x(05)80080-4. [DOI] [PubMed] [Google Scholar]
- Mueller M. J., Samuelsson B., Haeggström J. Z. Chemical modification of leukotriene A4 hydrolase. Indications for essential tyrosyl and arginyl residues at the active site. Biochemistry. 1995 Mar 21;34(11):3536–3543. doi: 10.1021/bi00011a007. [DOI] [PubMed] [Google Scholar]
- Mueller M. J., Wetterholm A., Blomster M., Jörnvall H., Samuelsson B., Haeggström J. Z. Leukotriene A4 hydrolase: mapping of a henicosapeptide involved in mechanism-based inactivation. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8383–8387. doi: 10.1073/pnas.92.18.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orning L., Gierse J. K., Fitzpatrick F. A. The bifunctional enzyme leukotriene-A4 hydrolase is an arginine aminopeptidase of high efficiency and specificity. J Biol Chem. 1994 Apr 15;269(15):11269–11273. [PubMed] [Google Scholar]
- Orning L., Gierse J., Duffin K., Bild G., Krivi G., Fitzpatrick F. A. Mechanism-based inactivation of leukotriene A4 hydrolase/aminopeptidase by leukotriene A4. Mass spectrometric and kinetic characterization. J Biol Chem. 1992 Nov 15;267(32):22733–22739. [PubMed] [Google Scholar]
- Orning L., Jones D. A., Fitzpatrick F. A. Mechanism-based inactivation of leukotriene A4 hydrolase during leukotriene B4 formation by human erythrocytes. J Biol Chem. 1990 Sep 5;265(25):14911–14916. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar G., Sommer S. S. The "megaprimer" method of site-directed mutagenesis. Biotechniques. 1990 Apr;8(4):404–407. [PubMed] [Google Scholar]
- Sun F. F., McGuire J. C. Metabolism of arachidonic acid by human neutrophils. Characterization of the enzymatic reactions that lead to the synthesis of leukotriene B4. Biochim Biophys Acta. 1984 Jun 6;794(1):56–64. doi: 10.1016/0005-2760(84)90297-2. [DOI] [PubMed] [Google Scholar]
- Wetterholm A., Medina J. F., Rådmark O., Shapiro R., Haeggström J. Z., Vallee B. L., Samuelsson B. Leukotriene A4 hydrolase: abrogation of the peptidase activity by mutation of glutamic acid-296. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9141–9145. doi: 10.1073/pnas.89.19.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetterholm A., Medina J. F., Rådmark O., Shapiro R., Haeggström J. Z., Vallee B. L., Samuelsson B. Recombinant mouse leukotriene A4 hydrolase: a zinc metalloenzyme with dual enzymatic activities. Biochim Biophys Acta. 1991 Oct 25;1080(2):96–102. doi: 10.1016/0167-4838(91)90134-l. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Y., Rådmark O., Samuelsson B. Mutagenesis of some conserved residues in human 5-lipoxygenase: effects on enzyme activity. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):485–489. doi: 10.1073/pnas.89.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]