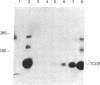
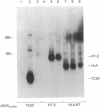
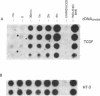
Abstract
Cyclosporin A (CsA) is a potent immunosuppressive agent, now gaining wide application in human organ transplantation. The immunosuppressive activity of CsA is at least in part due to inhibition of lymphokine production by activated T lymphocytes. Specifically, inhibition of T-cell growth factor (TCGF; also designated interleukin 2) production appears to be an important pathway by which CsA impairs T-cell function. To define further both the specificity of CsA and the level at which it interferes with lymphokine gene expression, we have studied its effects on TCGF mRNA accumulation as well as TCGF gene transcription. These studies were performed with a cloned human leukemic T-cell line (Jurkat, subclone 32), which can be induced with phytohemagglutinin and phorbol 12-myristate 13-acetate to produce large amounts of TCGF. In these cells, high levels of TCGF mRNA were present in induced but not in uninduced Jurkat cells as judged by hybridization to a cloned human TCGF cDNA probe. CsA completely inhibited induced TCGF mRNA accumulation at concentrations of 0.3-1.0 microgram/ml, whereas low levels of appropriately sized TCGF mRNA were present at 0.01 microgram/ml. In nuclear transcription experiments, CsA inhibited the synthesis of TCGF transcripts in a dose-dependent manner with complete inhibition at a concentration of 1 microgram/ml. In contrast, CsA did not inhibit the expression of two other inducible genes, TCGF receptor and HT-3. Further, HLA gene expression was also less affected than TCGF in CsA-treated cells. These data suggest a relatively selective action of CsA on TCGF gene transcription.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel J. F., Feurer C., Magnée C., Stähelin H. Effects of the new anti-lymphocytic peptide cyclosporin A in animals. Immunology. 1977 Jun;32(6):1017–1025. [PMC free article] [PubMed] [Google Scholar]
- Bunjes D., Hardt C., Röllinghoff M., Wagner H. Cyclosporin A mediates immunosuppression of primary cytotoxic T cell responses by impairing the release of interleukin 1 and interleukin 2. Eur J Immunol. 1981 Aug;11(8):657–661. doi: 10.1002/eji.1830110812. [DOI] [PubMed] [Google Scholar]
- Calne R. Y., Rolles K., White D. J., Thiru S., Evans D. B., McMaster P., Dunn D. C., Craddock G. N., Henderson R. G., Aziz S. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet. 1979 Nov 17;2(8151):1033–1036. doi: 10.1016/s0140-6736(79)92440-1. [DOI] [PubMed] [Google Scholar]
- Clark S. C., Arya S. K., Wong-Staal F., Matsumoto-Kobayashi M., Kay R. M., Kaufman R. J., Brown E. L., Shoemaker C., Copeland T., Oroszlan S. Human T-cell growth factor: partial amino acid sequence, cDNA cloning, and organization and expression in normal and leukemic cells. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2543–2547. doi: 10.1073/pnas.81.8.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dummer J. S., Hardy A., Poorsattar A., Ho M. Early infections in kidney, heart, and liver transplant recipients on cyclosporine. Transplantation. 1983 Sep;36(3):259–267. doi: 10.1097/00007890-198309000-00007. [DOI] [PubMed] [Google Scholar]
- Efrat S., Pilo S., Kaempfer R. Kinetics of induction and molecular size of mRNAs encoding human interleukin-2 and gamma-interferon. Nature. 1982 May 20;297(5863):236–239. doi: 10.1038/297236a0. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Horsburgh T., Wood P., Brent L. Suppression of in vitro lymphocyte reactivity by cyclosporin A: existence of a population of drug-resistant cytotoxic lymphocytes. Nature. 1980 Aug 7;286(5773):609–611. doi: 10.1038/286609a0. [DOI] [PubMed] [Google Scholar]
- Kunkl A., Klaus G. G. Selective effects of cyclosporin A on functional B cell subsets in the mouse. J Immunol. 1980 Dec;125(6):2526–2531. [PubMed] [Google Scholar]
- Larsson E. L. Cyclosporin A and dexamethasone suppress T cell responses by selectively acting at distinct sites of the triggering process. J Immunol. 1980 Jun;124(6):2828–2833. [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Uchiyama T., Smith K. A., Waldmann T. A., Greene W. C. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characterization of the receptor. Nature. 1982 Nov 18;300(5889):267–269. doi: 10.1038/300267a0. [DOI] [PubMed] [Google Scholar]
- Manzari V., Gallo R. C., Franchini G., Westin E., Ceccherini-Nelli L., Popovic M., Wong-Staal F. Abundant transcription of a cellular gene in T cells infected with human T-cell leukemia-lymphoma virus. Proc Natl Acad Sci U S A. 1983 Jan;80(1):11–15. doi: 10.1073/pnas.80.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh L. C., Thompson A. W. Activity of the mononuclear phagocyte system in cyclosporin A-treated mice. Transplantation. 1980 Nov;30(5):384–386. doi: 10.1097/00007890-198011000-00017. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- Miyawaki T., Yachie A., Ohzeki S., Nagaoki T., Taniguchi N. Cyclosporin A does not prevent expression of Tac antigen, a probable TCGF receptor molecule, on mitogen-stimulated human T cells. J Immunol. 1983 Jun;130(6):2737–2742. [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Neckers L. M., Cossman J. Transferrin receptor induction in mitogen-stimulated human T lymphocytes is required for DNA synthesis and cell division and is regulated by interleukin 2. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3494–3498. doi: 10.1073/pnas.80.11.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orosz C. G., Roopenian D. C., Widmer M. B., Bach F. H. Analysis of cloned T cell function. II. Differential blockade of various cloned T cell functions by cyclosporine. Transplantation. 1983 Dec;36(6):706–711. doi: 10.1097/00007890-198336060-00024. [DOI] [PubMed] [Google Scholar]
- Palacios R., Möller G. Cyclosporin A blocks receptors for HLA-DR antigens on T cells. Nature. 1981 Apr 30;290(5809):792–794. doi: 10.1038/290792a0. [DOI] [PubMed] [Google Scholar]
- Reem G. H., Cook L. A., Vilcek J. Gamma interferon synthesis by human thymocytes and T lymphocytes inhibited by cyclosporin A. Science. 1983 Jul 1;221(4605):63–65. doi: 10.1126/science.6407112. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Greene W. C. Direct demonstration of the identity of T cell growth factor binding protein and the Tac antigen. J Exp Med. 1983 Oct 1;158(4):1332–1337. doi: 10.1084/jem.158.4.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood A. K., Pereira D., Weissman S. M. Isolation and partial nucleotide sequence of a cDNA clone for human histocompatibility antigen HLA-B by use of an oligodeoxynucleotide primer. Proc Natl Acad Sci U S A. 1981 Jan;78(1):616–620. doi: 10.1073/pnas.78.1.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzl T. E., Klintmalm G. B., Porter K. A., Iwatsuki S., Schröter G. P. Liver transplantation with use of cyclosporin a and prednisone. N Engl J Med. 1981 Jul 30;305(5):266–269. doi: 10.1056/NEJM198107303050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D., Fellous M., Revel M. Preferential effect of gamma interferon on the synthesis of HLA antigens and their mRNAs in human cells. Nature. 1982 Oct 28;299(5886):833–836. doi: 10.1038/299833a0. [DOI] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]
- Yoshie O., Schmidt H., Reddy E. S., Weissman S., Lengyel P. Mouse interferons enhance the accumulation of a human HLA RNA and protein in transfected mouse and hamster cells. J Biol Chem. 1982 Nov 25;257(22):13169–13172. [PubMed] [Google Scholar]