Abstract
Many plant species have mating systems characterized by a mixture of self-fertilization and outcrossing. Statistical estimation of the outcrossing rate has relied on a model of the mating process that assumes that successive outcross events within a family arise from independent draws of pollen from the total population of male plants. Although this assumption is likely to be most appropriate for wind-pollinated plants, it is not appropriate in certain insect-pollinated plants. An alternative model is developed that assumes that successive outcross events within a family involve pollen drawn from a single male parent. The estimation of the parameters that index this model is outlined and a procedure for calculating the variances of the parameter estimates is presented. Monte Carlo simulations of the sampling processes assumed by each model are also presented. The simulations show that application of the incorrect estimation model to data can lead to a large bias in parameter estimates.
Keywords: mixed-mating model, mating type, outcrossing rate, allozyme, multiple paternity
Full text
PDF

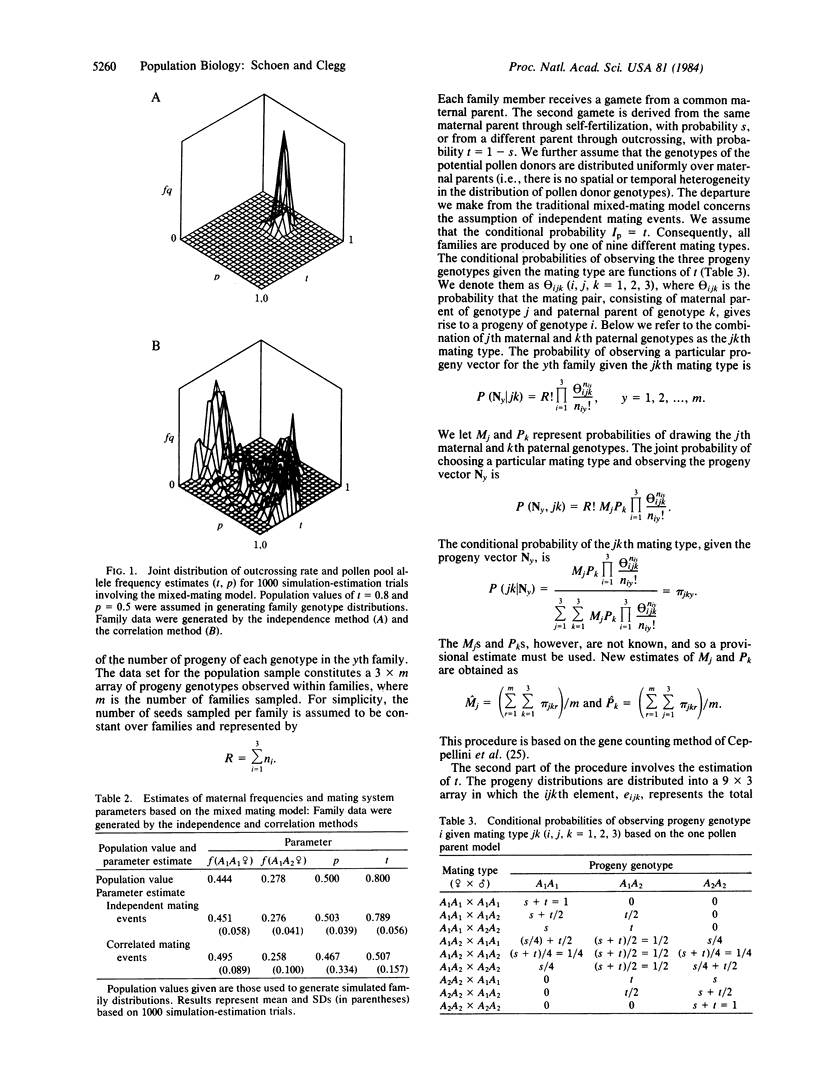
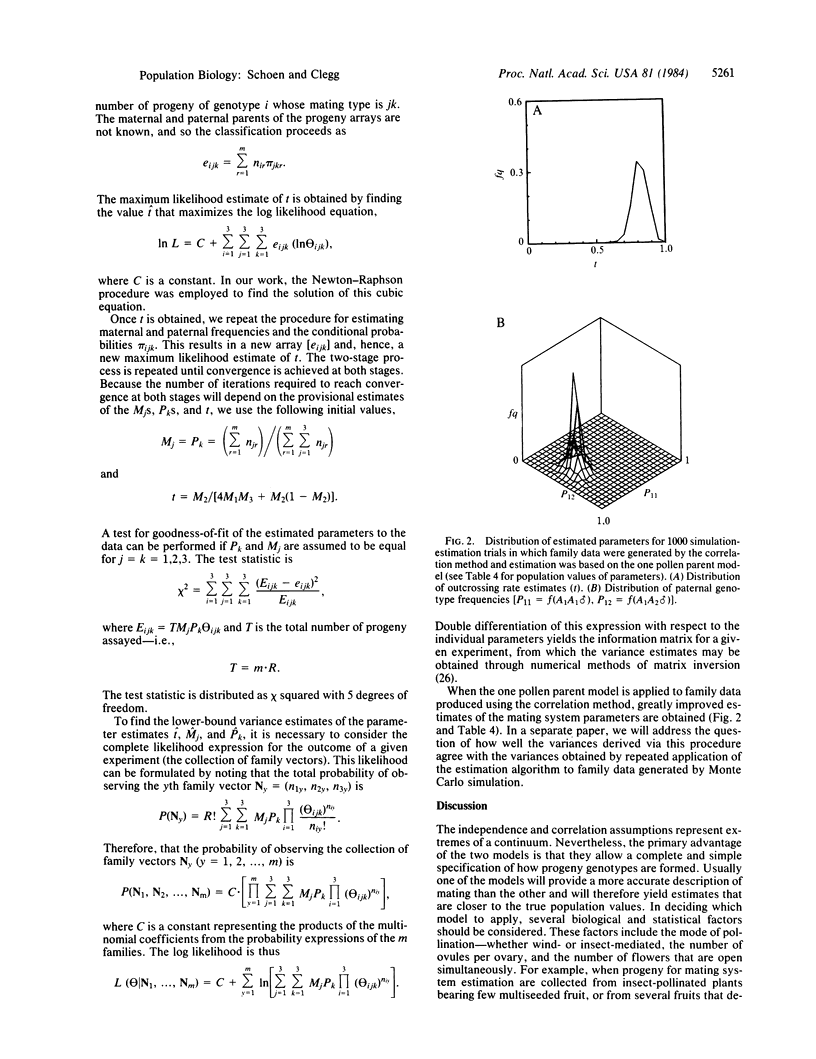
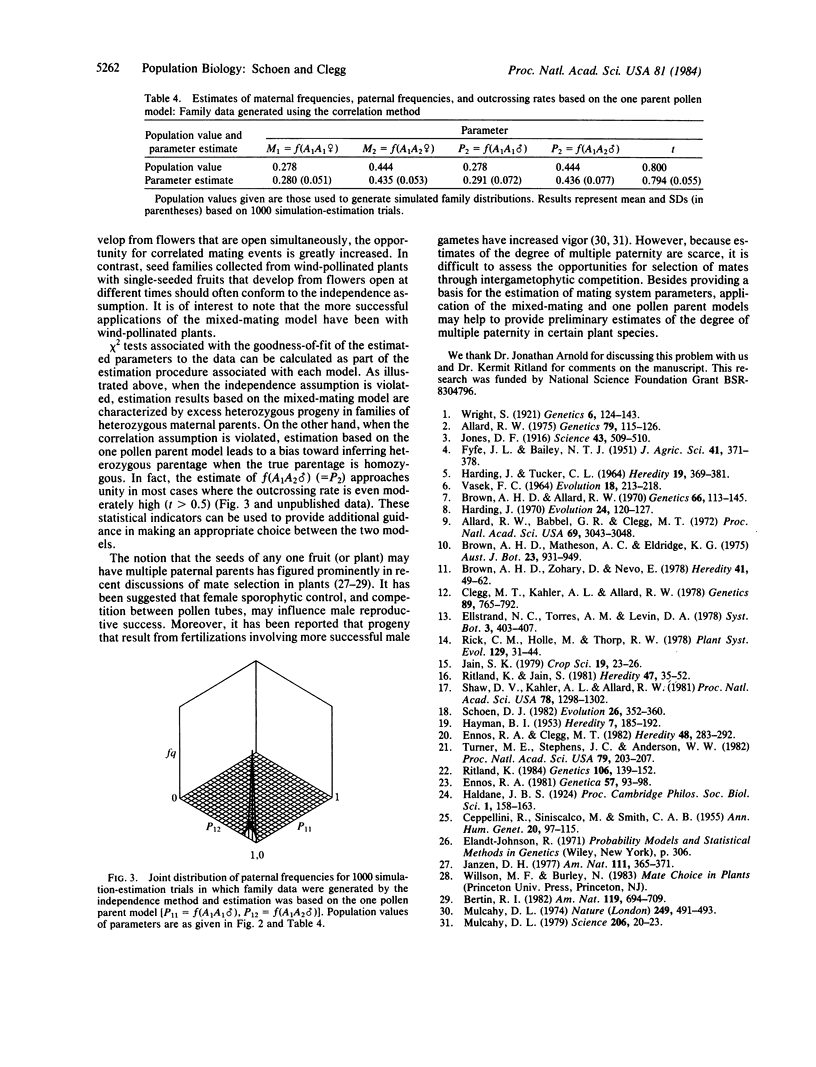
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allard R. W., Babbel G. R., Clegg M. T., Kahler A. L. Evidence for coadaptation in Avena barbata. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3043–3048. doi: 10.1073/pnas.69.10.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard R. W. The mating system and microevolution. Genetics. 1975 Jun;79 (Suppl):115–126. [PubMed] [Google Scholar]
- Brown A. H., Allard R. W. Estimation of the mating system in open-pollinated maize populations using isozyme polymorphisms. Genetics. 1970 Sep;66(1):133–145. doi: 10.1093/genetics/66.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CEPPELLINI R., SINISCALCO M., SMITH C. A. The estimation of gene frequencies in a random-mating population. Ann Hum Genet. 1955 Oct;20(2):97–115. doi: 10.1111/j.1469-1809.1955.tb01360.x. [DOI] [PubMed] [Google Scholar]
- Clegg M. T., Kahler A. L., Allard R. W. Estimation of life cycle components of selection in an experimental plant population. Genetics. 1978 Aug;89(4):765–792. doi: 10.1093/genetics/89.4.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. F. NATURAL CROSS-POLLINATION IN THE TOMATO. Science. 1916 Apr 7;43(1110):509–510. doi: 10.1126/science.43.1110.509. [DOI] [PubMed] [Google Scholar]
- Mulcahy D. L. The rise of the angiosperms: a genecological factor. Science. 1979 Oct 5;206(4414):20–23. doi: 10.1126/science.206.4414.20. [DOI] [PubMed] [Google Scholar]
- Ritland K. The effective proportion of self-fertilization with consanguineous matings in inbred populations. Genetics. 1984 Jan;106(1):139–152. doi: 10.1093/genetics/106.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D. V., Kahler A. L., Allard R. W. A multilocus estimator of mating system parameters in plant populations. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1298–1302. doi: 10.1073/pnas.78.2.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M. E., Stephens J. C., Anderson W. W. Homozygosity and patch structure in plant populations as a result of nearest-neighbor pollination. Proc Natl Acad Sci U S A. 1982 Jan;79(1):203–207. doi: 10.1073/pnas.79.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. Systems of Mating. II. the Effects of Inbreeding on the Genetic Composition of a Population. Genetics. 1921 Mar;6(2):124–143. doi: 10.1093/genetics/6.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]


