Abstract
Activation of prolactin (PRL)-dependent signaling occurs as the result of ligand-induced dimerization of receptor (PRLr). Although three PRLr isoforms (short, intermediate, and long) have been characterized and are variably coexpressed in PRL-responsive tissues, the functional effects of ligand-induced PRLr isoform heterodimerization have not been examined. To determine whether heterodimeric PRLr complexes were capable of ligand-induced signaling and cellular proliferation, chimeras consisting of the extracellular domain of either the alpha or beta subunit of human granulocyte-macrophage colony-stimulating factor receptor (GM-CSFr) and the intracellular domain of the rat intermediate or short PRLr isoforms (PRLr-I or PRLr-S) were synthesized. Because high affinity binding of GM-CSF is mediated by the extracellular domain of one alpha and beta GM-CSFr pair, use of GM-CSFr/PRLr chimera specifically directed the dimerization of the PRLr intracellular domains within ligand-receptor complexes. Stable transfection of these constructs into the Ba/F3 line was demonstrated by Northern blot and immunoprecipitation analyses. Flow cytometry revealed specific binding of a phycoerythrin-conjugated human GM-CSF to the transfectants, confirming cell surface expression of the chimeric receptors. When tested for their ability to proliferate in response to GM-CSF, only chimeric transfectants expressing GM-CSFr/PRLr-I homodimers demonstrated significant [3H]thymidine incorporation. GM-CSF stimulation of transfectants expressing either GM-CSFr/PRLr-S homodimers or GM-CSFr/PRLr-S+1 heterodimers failed to induce proliferation. Consistent with these data, the GM-CSF-induced activation of two phosphotyrosine kinases, Jak2 and Fyn, was observed only in homodimeric GM-CSFr/PRLr-I transfectants. These results show that the PRLr-S functions as a dominant negative isoform, down-regulating both signaling and proliferation mediated by the receptor complex. Thus, structural motifs necessary for Jak2 and Fyn activation within the carboxy terminus of the PRLr-I, absent in the PRLr-S, are required in each member of the dimeric PRLr complex.
Full text
PDF

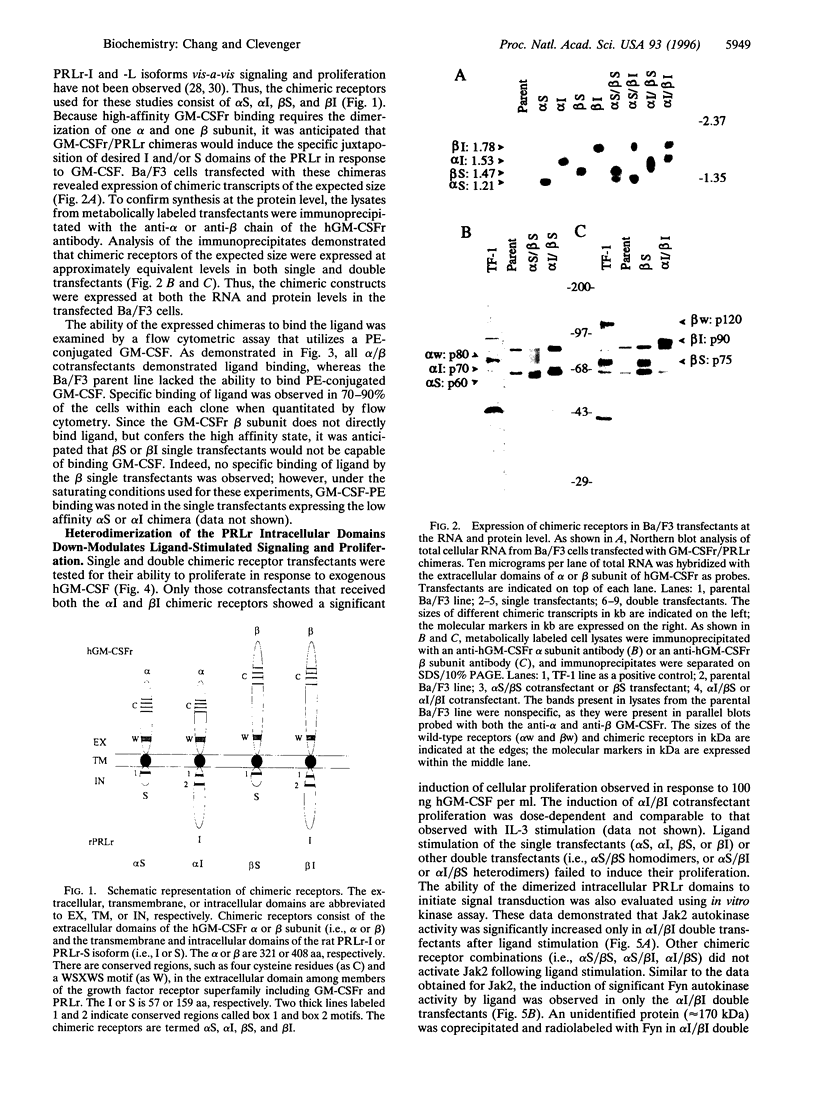
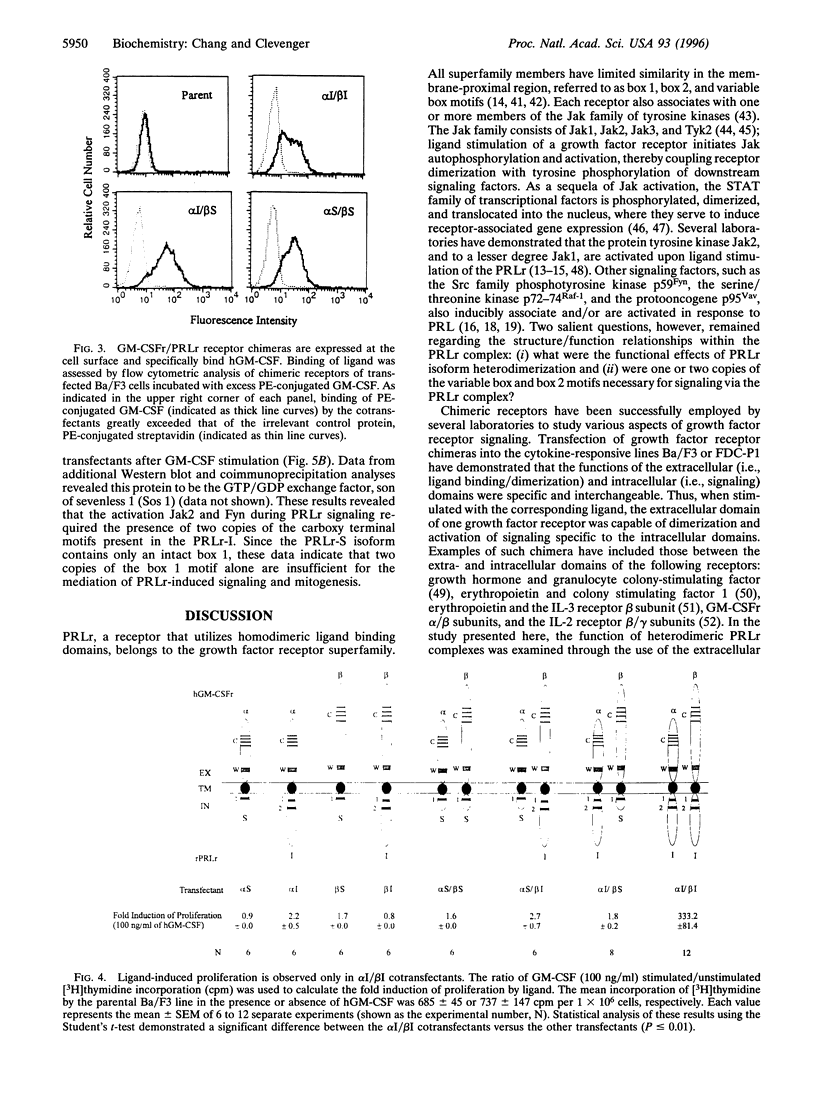


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S., Edery M., Pellegrini I., Lesueur L., Paly J., Djiane J., Kelly P. A. The Nb2 form of prolactin receptor is able to activate a milk protein gene promoter. Mol Endocrinol. 1992 Aug;6(8):1242–1248. doi: 10.1210/mend.6.8.1406702. [DOI] [PubMed] [Google Scholar]
- Ali S., Pellegrini I., Kelly P. A. A prolactin-dependent immune cell line (Nb2) expresses a mutant form of prolactin receptor. J Biol Chem. 1991 Oct 25;266(30):20110–20117. [PubMed] [Google Scholar]
- Baumbach W. R., Horner D. L., Logan J. S. The growth hormone-binding protein in rat serum is an alternatively spliced form of the rat growth hormone receptor. Genes Dev. 1989 Aug;3(8):1199–1205. doi: 10.1101/gad.3.8.1199. [DOI] [PubMed] [Google Scholar]
- Bazan J. F. A novel family of growth factor receptors: a common binding domain in the growth hormone, prolactin, erythropoietin and IL-6 receptors, and the p75 IL-2 receptor beta-chain. Biochem Biophys Res Commun. 1989 Oct 31;164(2):788–795. doi: 10.1016/0006-291x(89)91528-3. [DOI] [PubMed] [Google Scholar]
- Berczi I., Nagy E., Kovacs K., Horvath E. Regulation of humoral immunity in rats by pituitary hormones. Acta Endocrinol (Copenh) 1981 Dec;98(4):506–513. doi: 10.1530/acta.0.0980506. [DOI] [PubMed] [Google Scholar]
- Bernton E. W., Meltzer M. S., Holaday J. W. Suppression of macrophage activation and T-lymphocyte function in hypoprolactinemic mice. Science. 1988 Jan 22;239(4838):401–404. doi: 10.1126/science.3122324. [DOI] [PubMed] [Google Scholar]
- Boutin J. M., Jolicoeur C., Okamura H., Gagnon J., Edery M., Shirota M., Banville D., Dusanter-Fourt I., Djiane J., Kelly P. A. Cloning and expression of the rat prolactin receptor, a member of the growth hormone/prolactin receptor gene family. Cell. 1988 Apr 8;53(1):69–77. doi: 10.1016/0092-8674(88)90488-6. [DOI] [PubMed] [Google Scholar]
- Campbell G. S., Argetsinger L. S., Ihle J. N., Kelly P. A., Rillema J. A., Carter-Su C. Activation of JAK2 tyrosine kinase by prolactin receptors in Nb2 cells and mouse mammary gland explants. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5232–5236. doi: 10.1073/pnas.91.12.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevenger C. V., Medaglia M. V. The protein tyrosine kinase P59fyn is associated with prolactin (PRL) receptor and is activated by PRL stimulation of T-lymphocytes. Mol Endocrinol. 1994 Jun;8(6):674–681. doi: 10.1210/mend.8.6.7935483. [DOI] [PubMed] [Google Scholar]
- Clevenger C. V., Ngo W., Sokol D. L., Luger S. M., Gewirtz A. M. Vav is necessary for prolactin-stimulated proliferation and is translocated into the nucleus of a T-cell line. J Biol Chem. 1995 Jun 2;270(22):13246–13253. doi: 10.1074/jbc.270.22.13246. [DOI] [PubMed] [Google Scholar]
- Clevenger C. V., Sillman A. L., Hanley-Hyde J., Prystowsky M. B. Requirement for prolactin during cell cycle regulated gene expression in cloned T-lymphocytes. Endocrinology. 1992 Jun;130(6):3216–3222. doi: 10.1210/endo.130.6.1534539. [DOI] [PubMed] [Google Scholar]
- Clevenger C. V., Torigoe T., Reed J. C. Prolactin induces rapid phosphorylation and activation of prolactin receptor-associated RAF-1 kinase in a T-cell line. J Biol Chem. 1994 Feb 25;269(8):5559–5565. [PubMed] [Google Scholar]
- Colosi P., Wong K., Leong S. R., Wood W. I. Mutational analysis of the intracellular domain of the human growth hormone receptor. J Biol Chem. 1993 Jun 15;268(17):12617–12623. [PubMed] [Google Scholar]
- Cunningham B. C., Ultsch M., De Vos A. M., Mulkerrin M. G., Clauser K. R., Wells J. A. Dimerization of the extracellular domain of the human growth hormone receptor by a single hormone molecule. Science. 1991 Nov 8;254(5033):821–825. doi: 10.1126/science.1948064. [DOI] [PubMed] [Google Scholar]
- DaSilva L., Howard O. M., Rui H., Kirken R. A., Farrar W. L. Growth signaling and JAK2 association mediated by membrane-proximal cytoplasmic regions of prolactin receptors. J Biol Chem. 1994 Jul 15;269(28):18267–18270. [PubMed] [Google Scholar]
- Darnell J. E., Jr, Kerr I. M., Stark G. R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994 Jun 3;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Das R., Vonderhaar B. K. Transduction of prolactin's (PRL) growth signal through both long and short forms of the PRL receptor. Mol Endocrinol. 1995 Dec;9(12):1750–1759. doi: 10.1210/mend.9.12.8614411. [DOI] [PubMed] [Google Scholar]
- Dinerstein H., Lago F., Goujon L., Ferrag F., Esposito N., Finidori J., Kelly P. A., Postel-Vinay M. C. The proline-rich region of the GH receptor is essential for JAK2 phosphorylation, activation of cell proliferation, and gene transcription. Mol Endocrinol. 1995 Dec;9(12):1701–1707. doi: 10.1210/mend.9.12.8614406. [DOI] [PubMed] [Google Scholar]
- Dusanter-Fourt I., Gaye P., Belair L., Pétridou B., Kelly P. A., Djiane J. Prolactin receptor gene expression in the rabbit: identification, characterization and tissue distribution of several prolactin receptor messenger RNAs encoding a unique precursor. Mol Cell Endocrinol. 1991 May;77(1-3):181–192. doi: 10.1016/0303-7207(91)90073-2. [DOI] [PubMed] [Google Scholar]
- Dusanter-Fourt I., Muller O., Ziemiecki A., Mayeux P., Drucker B., Djiane J., Wilks A., Harpur A. G., Fischer S., Gisselbrecht S. Identification of JAK protein tyrosine kinases as signaling molecules for prolactin. Functional analysis of prolactin receptor and prolactin-erythropoietin receptor chimera expressed in lymphoid cells. EMBO J. 1994 Jun 1;13(11):2583–2591. doi: 10.1002/j.1460-2075.1994.tb06548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edens A., Southard J. N., Talamantes F. Mouse growth hormone-binding protein and growth hormone receptor transcripts are produced from a single gene by alternative splicing. Endocrinology. 1994 Dec;135(6):2802–2805. doi: 10.1210/endo.135.6.7988474. [DOI] [PubMed] [Google Scholar]
- Erwin R. A., Kirken R. A., Malabarba M. G., Farrar W. L., Rui H. Prolactin activates Ras via signaling proteins SHC, growth factor receptor bound 2, and son of sevenless. Endocrinology. 1995 Aug;136(8):3512–3518. doi: 10.1210/endo.136.8.7628388. [DOI] [PubMed] [Google Scholar]
- Goldsmith M. A., Lai S. Y., Xu W., Amaral M. C., Kuczek E. S., Parent L. J., Mills G. B., Tarr K. L., Longmore G. D., Greene W. C. Growth signal transduction by the human interleukin-2 receptor requires cytoplasmic tyrosines of the beta chain and non-tyrosine residues of the gamma c chain. J Biol Chem. 1995 Sep 15;270(37):21729–21737. doi: 10.1074/jbc.270.37.21729. [DOI] [PubMed] [Google Scholar]
- Gouilleux F., Wakao H., Mundt M., Groner B. Prolactin induces phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. EMBO J. 1994 Sep 15;13(18):4361–4369. doi: 10.1002/j.1460-2075.1994.tb06756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goujon L., Allevato G., Simonin G., Paquereau L., Le Cam A., Clark J., Nielsen J. H., Djiane J., Postel-Vinay M. C., Edery M. Cytoplasmic sequences of the growth hormone receptor necessary for signal transduction. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):957–961. doi: 10.1073/pnas.91.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett R. H., Wang Y. D., Larner A. C. Mapping of the cytoplasmic domain of the human growth hormone receptor required for the activation of Jak2 and Stat proteins. J Biol Chem. 1995 Sep 8;270(36):21326–21330. doi: 10.1074/jbc.270.36.21326. [DOI] [PubMed] [Google Scholar]
- Hartmann D. P., Holaday J. W., Bernton E. W. Inhibition of lymphocyte proliferation by antibodies to prolactin. FASEB J. 1989 Aug;3(10):2194–2202. doi: 10.1096/fasebj.3.10.2787766. [DOI] [PubMed] [Google Scholar]
- He T. C., Jiang N., Zhuang H., Quelle D. E., Wojchowski D. M. The extended box 2 subdomain of erythropoietin receptor is nonessential for Jak2 activation yet critical for efficient mitogenesis in FDC-ER cells. J Biol Chem. 1994 Jul 15;269(28):18291–18294. [PubMed] [Google Scholar]
- Ihle J. N. Cytokine receptor signalling. Nature. 1995 Oct 19;377(6550):591–594. doi: 10.1038/377591a0. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Kerr I. M. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 1995 Feb;11(2):69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- Ishizaka-Ikeda E., Fukunaga R., Wood W. I., Goeddel D. V., Nagata S. Signal transduction mediated by growth hormone receptor and its chimeric molecules with the granulocyte colony-stimulating factor receptor. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):123–127. doi: 10.1073/pnas.90.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe B. D., Sabath D. E., Johnson G. D., Moscinski L. C., Johnson K. R., Rovera G., Nauseef W. M., Prystowsky M. B. Myeloperoxidase and oncogene expression in GM-CSF induced bone marrow differentiation. Oncogene. 1988 Feb;2(2):167–174. [PubMed] [Google Scholar]
- Kitamura T., Hayashida K., Sakamaki K., Yokota T., Arai K., Miyajima A. Reconstitution of functional receptors for human granulocyte/macrophage colony-stimulating factor (GM-CSF): evidence that the protein encoded by the AIC2B cDNA is a subunit of the murine GM-CSF receptor. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5082–5086. doi: 10.1073/pnas.88.12.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T., Tange T., Terasawa T., Chiba S., Kuwaki T., Miyagawa K., Piao Y. F., Miyazono K., Urabe A., Takaku F. Establishment and characterization of a unique human cell line that proliferates dependently on GM-CSF, IL-3, or erythropoietin. J Cell Physiol. 1989 Aug;140(2):323–334. doi: 10.1002/jcp.1041400219. [DOI] [PubMed] [Google Scholar]
- Lebrun J. J., Ali S., Goffin V., Ullrich A., Kelly P. A. A single phosphotyrosine residue of the prolactin receptor is responsible for activation of gene transcription. Proc Natl Acad Sci U S A. 1995 Apr 25;92(9):4031–4035. doi: 10.1073/pnas.92.9.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun J. J., Ali S., Sofer L., Ullrich A., Kelly P. A. Prolactin-induced proliferation of Nb2 cells involves tyrosine phosphorylation of the prolactin receptor and its associated tyrosine kinase JAK2. J Biol Chem. 1994 May 13;269(19):14021–14026. [PubMed] [Google Scholar]
- Lesueur L., Edery M., Ali S., Paly J., Kelly P. A., Djiane J. Comparison of long and short forms of the prolactin receptor on prolactin-induced milk protein gene transcription. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):824–828. doi: 10.1073/pnas.88.3.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Subleski M., Fusaki N., Yamamoto T., Copeland T., Princler G. L., Kung H., Kamata T. Catalytic activity of the mouse guanine nucleotide exchanger mSOS is activated by Fyn tyrosine protein kinase and the T-cell antigen receptor in T cells. Proc Natl Acad Sci U S A. 1996 Feb 6;93(3):1001–1005. doi: 10.1073/pnas.93.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura O., Cleveland J. L., Ihle J. N. Inactivation of erythropoietin receptor function by point mutations in a region having homology with other cytokine receptors. Mol Cell Biol. 1993 Mar;13(3):1788–1795. doi: 10.1128/mcb.13.3.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura O., D'Andrea A., Kabat D., Ihle J. N. Induction of tyrosine phosphorylation by the erythropoietin receptor correlates with mitogenesis. Mol Cell Biol. 1991 Oct;11(10):4895–4902. doi: 10.1128/mcb.11.10.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M., Narazaki M., Hibi M., Yawata H., Yasukawa K., Hamaguchi M., Taga T., Kishimoto T. Critical cytoplasmic region of the interleukin 6 signal transducer gp130 is conserved in the cytokine receptor family. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11349–11353. doi: 10.1073/pnas.88.24.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Nakauchi H. A truncated erythropoietin receptor and cell death: a reanalysis. Science. 1994 Apr 22;264(5158):588–589. doi: 10.1126/science.8160019. [DOI] [PubMed] [Google Scholar]
- Nelson B. H., Lord J. D., Greenberg P. D. Cytoplasmic domains of the interleukin-2 receptor beta and gamma chains mediate the signal for T-cell proliferation. Nature. 1994 May 26;369(6478):333–336. doi: 10.1038/369333a0. [DOI] [PubMed] [Google Scholar]
- O'Neal K. D., Schwarz L. A., Yu-Lee L. Y. Prolactin receptor gene expression in lymphoid cells. Mol Cell Endocrinol. 1991 Dec;82(2-3):127–135. doi: 10.1016/0303-7207(91)90023-l. [DOI] [PubMed] [Google Scholar]
- O'Neal K. D., Yu-Lee L. Y. Differential signal transduction of the short, Nb2, and long prolactin receptors. Activation of interferon regulatory factor-1 and cell proliferation. J Biol Chem. 1994 Oct 21;269(42):26076–26082. [PubMed] [Google Scholar]
- Ohashi H., Maruyama K., Liu Y. C., Yoshimura A. Ligand-induced activation of chimeric receptors between the erythropoietin receptor and receptor tyrosine kinases. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):158–162. doi: 10.1073/pnas.91.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Steinmetz M. Il-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985 Jul;41(3):727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- Pellegrini I., Lebrun J. J., Ali S., Kelly P. A. Expression of prolactin and its receptor in human lymphoid cells. Mol Endocrinol. 1992 Jul;6(7):1023–1031. doi: 10.1210/mend.6.7.1508218. [DOI] [PubMed] [Google Scholar]
- Prystowsky M. B., Clevenger C. V. Prolactin as a second messenger for interleukin 2. Immunomethods. 1994 Aug;5(1):49–55. doi: 10.1006/immu.1994.1037. [DOI] [PubMed] [Google Scholar]
- Sato N., Sakamaki K., Terada N., Arai K., Miyajima A. Signal transduction by the high-affinity GM-CSF receptor: two distinct cytoplasmic regions of the common beta subunit responsible for different signaling. EMBO J. 1993 Nov;12(11):4181–4189. doi: 10.1002/j.1460-2075.1993.tb06102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipman P. M., Sabath D. E., Fischer A. H., Comber P. G., Sullivan K., Tan E. M., Prystowsky M. B. Cyclin mRNA and protein expression in recombinant interleukin 2-stimulated cloned murine T lymphocytes. J Cell Biochem. 1988 Nov;38(3):189–198. doi: 10.1002/jcb.240380306. [DOI] [PubMed] [Google Scholar]
- Shirota M., Banville D., Ali S., Jolicoeur C., Boutin J. M., Edery M., Djiane J., Kelly P. A. Expression of two forms of prolactin receptor in rat ovary and liver. Mol Endocrinol. 1990 Aug;4(8):1136–1143. doi: 10.1210/mend-4-8-1136. [DOI] [PubMed] [Google Scholar]
- VanderKuur J. A., Wang X., Zhang L., Campbell G. S., Allevato G., Billestrup N., Norstedt G., Carter-Su C. Domains of the growth hormone receptor required for association and activation of JAK2 tyrosine kinase. J Biol Chem. 1994 Aug 26;269(34):21709–21717. [PubMed] [Google Scholar]
- Venkitaraman A. R., Cowling R. J. Interleukin 7 receptor functions by recruiting the tyrosine kinase p59fyn through a segment of its cytoplasmic tail. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12083–12087. doi: 10.1073/pnas.89.24.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams N. G., Roberts T. M., Li P. Both p21ras and pp60v-src are required, but neither alone is sufficient, to activate the Raf-1 kinase. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2922–2926. doi: 10.1073/pnas.89.7.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witthuhn B. A., Quelle F. W., Silvennoinen O., Yi T., Tang B., Miura O., Ihle J. N. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993 Jul 30;74(2):227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- Ziemiecki A., Harpur A. G., Wilks A. F. JAK protein tyrosine kinases: their role in cytokine signalling. Trends Cell Biol. 1994 Jun;4(6):207–212. doi: 10.1016/0962-8924(94)90143-0. [DOI] [PubMed] [Google Scholar]
- Zon L. I., Moreau J. F., Koo J. W., Mathey-Prevot B., D'Andrea A. D. The erythropoietin receptor transmembrane region is necessary for activation by the Friend spleen focus-forming virus gp55 glycoprotein. Mol Cell Biol. 1992 Jul;12(7):2949–2957. doi: 10.1128/mcb.12.7.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]