Key Points
Diverse patient groups with GATA2 mutation develop mononuclear cytopenia and elevated Flt3 ligand.
Progressive cytopenias, rising Flt3 ligand, and terminal differentiation of lymphoid cells accompany clinical progression.
Abstract
Constitutive heterozygous GATA2 mutation is associated with deafness, lymphedema, mononuclear cytopenias, infection, myelodysplasia (MDS), and acute myeloid leukemia. In this study, we describe a cross-sectional analysis of 24 patients and 6 relatives with 14 different frameshift or substitution mutations of GATA2. A pattern of dendritic cell, monocyte, B, and natural killer (NK) lymphoid deficiency (DCML deficiency) with elevated Fms-like tyrosine kinase 3 ligand (Flt3L) was observed in all 20 patients phenotyped, including patients with Emberger syndrome, monocytopenia with Mycobacterium avium complex (MonoMAC), and MDS. Four unaffected relatives had a normal phenotype indicating that cellular deficiency may evolve over time or is incompletely penetrant, while 2 developed subclinical cytopenias or elevated Flt3L. Patients with GATA2 mutation maintained higher hemoglobin, neutrophils, and platelets and were younger than controls with acquired MDS and wild-type GATA2. Frameshift mutations were associated with earlier age of clinical presentation than substitution mutations. Elevated Flt3L, loss of bone marrow progenitors, and clonal myelopoiesis were early signs of disease evolution. Clinical progression was associated with increasingly elevated Flt3L, depletion of transitional B cells, CD56bright NK cells, naïve T cells, and accumulation of terminally differentiated NK and CD8+ memory T cells. These studies provide a framework for clinical and laboratory monitoring of patients with GATA2 mutation and may inform therapeutic decision-making.
Introduction
Constitutive heterozygous mutation of the GATA2 gene causes a complex disorder of hematopoiesis with variable extramedullary defects. We have previously characterized the loss of mononuclear cells that occurred in 4 patients with immunodeficiency as a syndrome of dendritic cell (DC), monocyte, B, and natural killer (NK) lymphoid (DCML) deficiency.1,2 Others have reported cytopenias in patients with various clinical syndromes of GATA2 mutation. These include monocytopenia with Mycobacterium avium complex (monoMAC)3-5; lymphedema, deafness, and myelodysplasia (MDS) (Emberger syndrome)6,7; and familial MDS/acute myeloid leukemia (AML).8-11 Recent work suggests that monoMAC, lymphedema, and familial MDS/AML are all facets of GATA2 mutation that may occur heterogeneously,9,12 even within a single pedigree.13 Some historical cases of familial AML are now also known to be due to GATA2 mutation.14-16 It is unknown whether failure of mononuclear cell development is a consequence of GATA2 mutation in all patient groups. In particular, hereditary AML may arise without a preceding “accessory” hematopoietic phenotype.8 Also, extramedullary complications such as lymphedema may or may not develop independently of hematopoietic failure.9,17,18
A wide range of genetic defects has been described in the GATA2 locus including deletions, regulatory mutations, frameshift mutations, and substitutions. Extramedullary developmental defects are associated with large deletions.9 Cohorts with lymphedema are enriched for frameshift mutations,7 while hereditary AML has been described particularly in families with substitutions clustering in the second zinc finger.8-10 These phenotypes are likely to be partially penetrant.9
Up to 50% of individuals with GATA2 mutation develop MDS associated with fibrosis and megakaryocyte dysplasia.4,5,10,11,19 However, many patients present with clinical problems prior to their meeting the standard criteria for MDS. Monocytopenia is a vital clue,1,3 but mild chronic neutropenia20 and NK deficiency21 are also associated with GATA2 mutation. A more precise understanding of the evolution of cellular deficiency and the progression of disease may further assist in the recognition and clinical management of this disorder.
It has previously been reported that Fms-like tyrosine kinase 3 ligand (Flt3L) is elevated in patients with DCML deficiency.1 Flt3L is an important factor in DC development, but elevated levels have also been reported in Fanconi and aplastic anemia suggesting that hematopoietic stress is a trigger.22,23 Further evaluation may indicate whether this is a useful marker for diagnosis and monitoring of GATA2 mutation.
The risk of infectious complications in GATA2 mutation is difficult to predict, but frequently remains low until the third or fourth decade. It appears from unremarkable childhood case histories, normal class-switched immunoglobulin, and a grossly intact T-cell compartment that the immune system is competent for sufficiently long to establish a level of immunologic memory. Nonetheless, the existence of a premorbid state without cytopenia has not been firmly established. Indeed, several patients have been characterized with long periods of cytopenia.1,3 Loss of CD56bright NK cells has been reported,21 but the B-cell compartment and remaining T cells have not been examined in detail.
It has been suggested that GATA2 mutation leads to poor risk AML.8,11,24 Although hematopoietic stem-cell transplantation is effective in treating MDS and in resolving life-threatening infectious complications,25 a better understanding of the trajectory of GATA2 disease is required to optimize the treatment strategy for patients.
In this study, we present an analysis of a European cohort of patients and their relatives with GATA2 mutation from a range of clinical backgrounds, describing in detail the evolution of cellular deficiency, the utility of Flt3L in diagnosing and monitoring disease progression, and the effects of failing mononuclear cell development upon peripheral lymphoid homeostasis.
Methods
Patients
Patients with GATA2 mutation were referred from a wide range of clinicians suspecting a GATA2-related disorder. There were no specific inclusion criteria and all patients found to have GATA2 mutation were reported. Patients with acquired MDS (World Health Organization classification: refractory cytopenia and multilineage dysplasia) were recruited from a local hematology ambulatory clinic. All MDS patients were symptomatic and some required transfusion support, but none had received high-dose cytoreductive therapy prior to testing. Direct sequencing confirmed wild-type (WT) GATA2 coding sequence in all cases. Patients with primary immunodeficiency disease (PID) were a heterogenous group with a history of suspected immunodeficiency and variable cellular deficiencies, were referred for investigation of possible GATA2 mutation but were found not to have a DCML deficiency phenotype or a GATA2 mutation. This population served as controls for further analyses performed. Blood, skin, and bone marrow (BM) aspirate surplus to diagnostic requirement was collected. Systematic collection of patient material, analysis, and collation of clinical details for publication was approved by the Newcastle and North Tyneside Research Ethics Committee 1 (Reference 08/H0906/72). Informed consent was obtained in accordance with the Declaration of Helsinki.
Flow cytometry
DCML profiling was performed as previously described.1 Antibodies are listed in supplemental Table 1 on the Blood Web site. Absolute cell counts were determined by Trucount analysis (BD Biosciences). Flow cytometry data were collected using an LSRII cytometer (BD Biosciences) and analyzed with FlowJo software (Tree Star, Ashland, OR).
DNA sequencing
Peripheral blood was used as the source of DNA except for 3.II.6 (frozen muscle), 5.I.1, 7.I.1, 7.II.1, and 8.I.3 (dermal fibroblasts). Genomic DNA was extracted using the QIAamp DNA Mini Kit (QIAGEN), while polymerase chain reaction (PCR) amplification and Sanger sequencing was performed using primers and conditions described previously.2
Analysis of clonality
Genomic DNA was extracted with QIAamp DNA Mini Kit and genotyping of exonic single nucleotide polymorphisms from 3 X-chromosome genes (MPP1: G/T, FHL1: G/A, and IDS: C/T) was determined using TaqMan allele-discrimination assays on an Applied Biosystems 7500 Sequence Detection System. Total RNA was isolated from neutrophil pellets and peripheral blood mononuclear cells (PBMCs) using RNeasy Micro Kit (QIAGEN), and used for assessment of clonality. Quantitative allele-specific suppressive PCR was performed on a sequence detection system (7500 platform) and allele frequency of expressed exonic single nucleotide polymorphisms was calculated as previously described.26
Serum biomarker screening and ELISA
The quantities of 117 serum proteins from 16 patients with GATA2 mutation and 10 healthy adult controls were measured with MILLIPLEX Multiplex Assays (Millipore, Billerica, MA): Cytokine/Chemokine Panels I-III, Cancer Biomarker I-II, and Circulating Cytokine Receptor. Multiplex assays were processed on a MAGPIX Plate Reader (Luminex, Austin, TX), and analyzed with MILLIPLEX Analyst 5.1 software (Millipore). All samples were performed in duplicate. Significantly elevated markers were reanalyzed by enzyme-linked immunosorbent assay (ELISA) across the whole cohort. Serum ELISA was performed with quantikine human Flt3/Flk-2 ligand immunoassay, quantikine human epidermal growth factor (EGF) immunoassay, quantikine human soluble CD40 ligand (CD40L) immunoassay, quantikine human granulocyte-macrophage colony-stimulating factor (GM-CSF) immunoassay, and quantikine fibroblast growth factor (FGF) basic immunoassay (R&D Systems).
Real-time quantitative PCR
Total RNA was extracted using the RNeasy Micro Kit and treated with Dnase I. Complementary (cDNA) was synthesized using RevertAid H Minus First Strand cDNA Synthesis Kit (Thermo Scientific). Real-time PCR was performed with TaqMan Gene Expression Master Mix, and gene expression assays for FLT3LG (Hs00181740_m1) and glyceraldehyde-3-phosphate dehydrogenase (4352934E; Life Technologies) with Applied Biosystems 7900HT Fast Real-Time PCR System. Relative quantification of the messenger RNA (mRNA) levels was performed using glyceraldehyde-3-phosphate dehydrogenase as the reference.
Statistical analysis
Analysis of variance with Bonferroni Multiple Comparisons Test was used to compare groups in most analyses. Cell count data were obviously skewed in GATA2 deficiency, therefore, nonparametric tests were preferred (Mann-Whitney or Kruskal-Wallis with Dunn’s Multiple Comparison Test).
Results
Comparison of GATA2 mutation with acquired MDS
The cohort of 30 patients with GATA2 mutation comprised of 16 index cases and 14 relatives. These included 4 with Emberger syndrome, 6 with “monoMAC” (3 also with MDS), 8 with MDS, 6 with other clinical syndromes, and 6 asymptomatic patients. The 6 asymptomatic patients were all related to one or more persons who had developed MDS due to point mutation of the second zinc finger (Table 1). AML had been diagnosed historically in several families, but none of the patients reported here had developed leukemia.
Table 1.
Patient cohort
| Number | Kindred | Age* | cDNA | Protein | HPV = 1 | Myco = 1 | URTI = 1 | Lung = 1 | AI = 1 | Clinical Score | MDS | Lymph | Cancer |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.I.1† | 12 | c.599_600insG | G200fs | + | + | 2 | ||||||
| 2 | 2.I.1†§ | 26 | 1061 C>T | T354M | + | + | 2 | ||||||
| 3|| | 3.II.6§ | 34 | 1192 C>T | R398W | + | + | 2 | + | a | ||||
| 4 | 3.III.1† | 22 | 1192 C>T | R398W | + | + | 2 | b | |||||
| 5 | 3.III.3 | 26 | 1192 C>T | R398W | 0 | ||||||||
| 6 | 4.I.1† | 18 | c.1018-1 G>T | △340-381 | + | + | + | 3 | |||||
| 7 | 5.I.1§ | 40 | 1192 C>T | R398W | + | + | + | 3 | + | ||||
| 8 | 6.I.1 | 18 | c.594delG | G199fs | + | + | + | + | 4 | + | |||
| 9 | 6.II.1 | 17 | c.594delG | G199fs | + | 1 | + | ||||||
| 10 | 6.II.2 | 13 | c.594delG | G199fs | + | 1 | |||||||
| 11|| | 7.I.1§ | 10 | c.318_319insT | S106fs | + | + | 2 | ||||||
| 12|| | 7.II.1§ | 10 | c.318_319insT | S106fs | + | 1 | + | c | |||||
| 13 | 8.I.2 | 62 | 1193 G>A | R398Q | 0 | ||||||||
| 14|| | 8.I.3 | 25 | 1193 G>A | R398Q | + | 1 | mono 7 | ||||||
| 15 | 8.II.1 | 36 | 1193 G>A | R398Q | + | + | + | 3 | |||||
| 16 | 8.II.4 | 32 | 1193 G>A | R398Q | 0 | ||||||||
| 17 | 8.II.5 | 29 | 1193 G>A | R398Q | 0 | ||||||||
| 18 | 9.III.1‡ | 31 | 1061 C>T | T354M | + | + | + | 3 | + | ||||
| 19 | 9.III.2‡ | 29 | 1061 C>T | T354M | + | 1 | |||||||
| 20 | 9.III.3‡ | 22 | 1061 C>T | T354M | 0 | ||||||||
| 21 | 9.III.4‡ | 17 | 1061 C>T | T354M | 0 | ||||||||
| 22 | 9.III.5‡ | 17 | 1061 C>T | T354M | + | 1 | tri 8 | ||||||
| 23 | 10.I.1 | 22 | c.1114 G>A | A372T | + | + | 2 | ||||||
| 24 | 11.I.1 | 8 | c.257_258delGC | C85fs | + | 1 | mono 7 | ||||||
| 25 | 12.I.1§ | 22 | c.1018-1 G>A | △340-381 | + | + | + | + | 4 | + | d | ||
| 26 | 13.I.1 | 19 | c.735_736insC | P245fs | + | 1 | |||||||
| 27 | 14.I.1 | 60 | c.599_600insG | G200fs | + | + | 2 | tri 8 | |||||
| c.599_600insG | G200fs | ||||||||||||
| 28 | 14.II.2 | 30 | c.1168_1170del1AAG | 390delK | + | 1 | + | ||||||
| 29 | 15.I.1 | 4 | 1081 C>T | R361C | + | + | 2 | + | + | e | |||
| 30 | 16.I.1 | 9 | c.1081-3_1031del17 | A341fs | + | 1 | + | f |
A survey of the most common clinical features of the cohort is presented. HPV (persistent infection of hands, feet, or perineum with HPV); Myco (any history of mycobacterial infection); URTI (more than 3 episodes of recurrent bacterial sinusitis, otitis, or other URTI); Lung (loss of lung volume or transfer factor <80% predicted, history of bronchiectasis, chronic bronchitis, more than one episode of pneumonia, radiologically or pathologically confirmed pulmonary alveolar proteinosis); AI (autoimmunity: arthritis, panniculitis, or autoimmune cytopenia); MDS (WHO: refractory cytopenia with multilineage dysplasia); Lymph (lymphedema); and Cancer (nonhematopoietic malignancy). The clinical score was derived by giving 1 point for each of the categories: HPV, Myco, URTI, Lung, and AI. Cytogenetics are indicated in the MDS column: mono 7, monosomy 7, tri 8, and trisomy 8. Lowercase letters indicate solid malignancy, as follows: a, cervical intra-epithelial dysplasia 3; b, ano-genital dysplasia with vulval intra-epithelial neoplasia 3; c, cervical intra-epithelial dysplasia 3; d, schwannoma/neuroma; e, cervical intra-epithelial dysplasia 3 and squamous carcinoma of vulva; and f, ano-genital dysplasia. Blank spaces indicate the absence of a clinical feature.
Age at clinical presentation or detection of mutation, if asymptomatic.
Patients published.2
Pedigree previously described.14
Deceased.
Genotype and clinical phenotype only.
The most common early complications were documented from a review of the case notes, and a simple clinical score was derived (Table 1). We focused on early complications because management is less certain at this stage than when patients have already developed MDS. MDS, lymphedema, and solid malignancy were recorded, but were excluded from the clinical score.
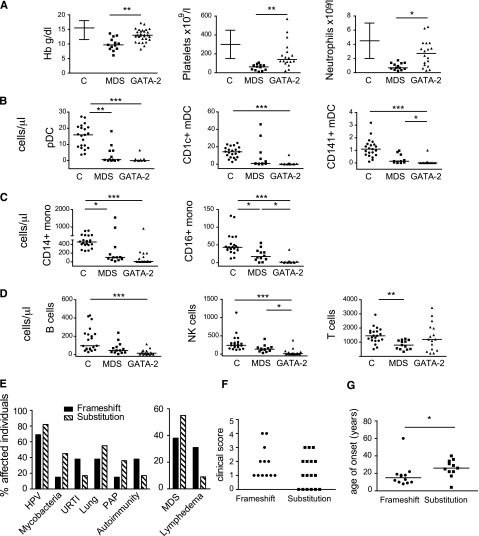
Mononuclear cytopenia, in particular the loss of DCs, has not been systematically investigated in all presentations of GATA2 mutation. Furthermore, it is not known how distinct the phenotype of DCML deficiency is from cytopenias that occur in acquired MDS with WT GATA2. To explore these issues, we performed an extended mononuclear profile on 26 of 30 patients with GATA2 mutation, including 20 symptomatic patients with monoMAC, Emberger syndrome, or MDS, and 6 asymptomatic relatives. DCML-deficiency was evident in all 20 symptomatic cases, including representative patients with monoMAC, Emberger syndrome, and MDS (Figure 1). We then compared 18 patients (with at least one clinical manifestation) with acquired MDS patients receiving ambulatory care who were known to be GATA2 WT (n = 12). Patients with MDS were significantly older (median age = 65 years, range 42 to 83 years vs median age = 20 years, range 4 to 60 years) and had reduced hemoglobin (Hb), neutrophils, and platelets compared with those with GATA2 mutation (Figure 2A).
Figure 1.
Mononuclear cell profiles of patients with Emberger syndrome, monoMAC, and familial MDS associated with GATA2 mutation. Examples of mononuclear profiling in familial MDS (#18; T354M), monoMAC (#25; del340-381), and Emberger syndrome (#30; A341fs) showing that a DCML-deficiency phenotype may be associated with diverse clinical manifestations and different GATA2 mutations. Populations: (1) CD14+ monocyte; (2) CD16+ monocyte; (3) pDC; (4) CD34+ progenitors; (5) CD141+ mDC; (6) CD1c+ mDC; (7) B cells; and (8) NK cells. Note expansion of CD34+ progenitors.
Figure 2.
Comparison of GATA2-mutated patients with MDS patients and genotype-phenotype correlations. (A-D) Comparison of controls (n = 21), patients with MDS (n = 12), and patients with symptomatic GATA2 mutation (n = 18). (A) Automated blood counts. (B-D) DCs, monocytes, and lymphocyte subsets by Trucount analysis. Analysis was performed as previously described.1 (E) Summary of clinical features among symptomatic carriers of GATA2 mutation by genotype (11 frameshift and 13 substitutions). (F) Profile of clinical score as defined in Table 1 according to genotype. (G) Age at presentation by genotype. *P < .05; **P < .01; ***P < .001. C, controls (or reference range); HPV, human papilloma virus; mono, monocyte; PAP, pulmonary alveolar proteinosis; URTI, upper respiratory tract infection.
Patients with GATA2 mutation often had normal hematologic parameters: 9 of 18 (50%), 11 of 18 (61%), and 9 of 18 (50%) had Hb, neutrophils, and platelets, respectively, within the reference range. A total of 7 of 18 (39%) were normal for all three parameters. DC, monocyte, B cell, and NK cell counts were significantly reduced in all symptomatic carriers of GATA2 mutation (Figure 2B-D).
Interestingly, patients with acquired MDS also had mild mononuclear cytopenias of plasmacytoid DCs (pDCs), and both classical and nonclassical monocytes. There were trends for lower myeloid DCs (mDCs), B cells, and NK cells in MDS, but this did not achieve significance after adjustment for multiple comparisons.
Genotype-phenotype correlations
Although GATA2 mutations are diverse and we screened all the promoters, exons, intron 5 enhancer, and 3′ untranslated regions in this cohort, we detected only frameshift mutations in the coding region 5′ to the second zinc finger, or substitutions (plus one inframe deletion) in the second zinc finger of GATA2 (supplemental Figure 1). We did not detect larger gene deletions as have been described in patients with congenital defects, which might have been under-represented in our cohort. Common clinical manifestations showed little difference between the 2 genotype groups (Figure 2E). Among symptomatic patients (n = 24; 11 frameshift and 13 substitution mutations), a higher proportion of frameshift mutations occurred in lymphedema (3 of 13 vs 1 of 11), while substitution mutations were more prevalent in the MDS group (7 of 11 vs 5 of 13). Neither trait achieved significance in Fisher’s exact test with this relatively small number of patients. The clinical score derived from Table 1 showed a slightly higher but nonsignificant weighting in the frameshift group (Figure 2F). The age of presentation was younger in the frameshift group compared with the substitution group (median age = 18 vs 26 years; P < .05) (Figure 2G).
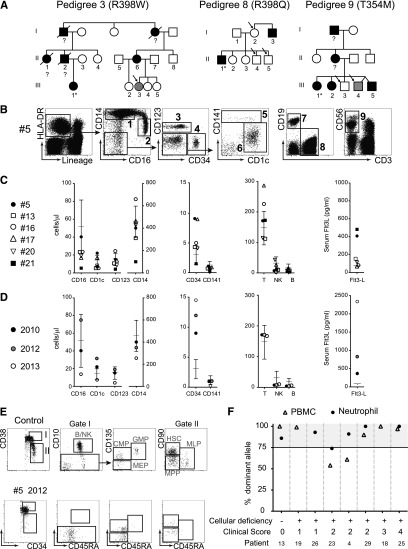
Evolution of mononuclear cytopenia in asymptomatic relatives with GATA2 mutation
We identified 3 pedigrees with mutations: R398W, R398Q, and T354M containing 6 asymptomatic relatives (Figure 3A). Two developed cytopenia and elevation of Flt3L, even though they were unaffected clinically (clinical score = 0), but 4 cases (#13, #16, #17, and #20) remained phenotypically normal (Figure 3B-C). Sequential monitoring of #5 showed declining cell counts over 3 years with progressive elevation of Flt3L and circulating CD34+ progenitors (Figure 3D). Despite normal peripheral cell counts and unremarkable BM histology (not shown), flow cytometry revealed that the progenitor compartment was already depleted of B and NK cells, multilymphoid progenitor (MLP), and granulocyte-macrophage progenitor (GMP) fractions (Figure 3E).
Figure 3.
Asymptomatic carriers of GATA2 mutation may develop cellular deficiency, elevated Flt3L, loss of BM progenitors, and clonal myelopoeisis. (A) Three pedigrees identified (mutation indicated) containing asymptomatic relatives (clinical core = 0), carrying GATA2 mutation (open symbols, arrowed). Gray symbols identify 2 patients with either elevated Flt3L (>200 pg/ml) or cytopenia. Filled symbols indicate affected patients with mutation (clinical score = 1 to 4). (B) DC, monocyte, and lymphocyte profiles of patient #5, 1 of 3 healthy carriers of GATA2 mutation showing a normal cellular phenotype at the first point of analysis in 2010. Populations: (1) CD14+ monocyte; (2) CD16+ monocyte; (3) pDC; (4) CD34+ progenitors; (5) CD141+ mDC; (6) CD1c+ mDC; (7) B cell; (8) T cell; and (9) NK cell. (C) Summary of DC and monocyte counts relative to reference ranges for the asymptomatic carriers. Case #5 (filled circle) is shown at first analysis in 2010. Case #21 (filled square) already has cytopenia. (D) Detailed analysis of case #5 showing the loss of cells and rising Flt3L over a 3-year period. (E) BM analysis of case #5 showing loss of B, NK, MLP, and GMP progenitors at midpoint when no cytopenia was evident. CMP, common myeloid progenitor; MEP, megakaryocyte-erythroid progenitor; MPP, multi-potent progenitor. (F) Pattern of X inactivation in females with GATA2 mutation at different stages of clinical evolution. Dominance of >75% is considered evidence of clonal hematopoiesis.
The early elevation of Flt3L and the loss of specific progenitors suggested that hematopoiesis was already under stress. To corroborate this, clonality testing was performed by looking for nonrandom X inactivation in female patients, as previously described.26 Surprisingly, this revealed a >75% bias toward one allele, consistent with clonal hematopoiesis, in all neutrophil samples and most PBMCs tested (Figure 3F). PBMC clonality was always less marked than that of neutrophils, and in 2 patients with the lowest neutrophil clonal bias, PBMCs remained evenly balanced. T cells are a major component of PBMCs, especially in these patients, and the lag in PBMC clonality presumably reflects the slow turnover of peripheral T cells from BM-derived precursors.
Although hematopoiesis appears clonal, this does not explain the selective loss of MLP, GMP, and mononuclear cells associated with GATA2 mutation. Seeking evidence that GATA2 had a direct role in specifying the development of MLP, GMP, or their progeny, we attempted to knock-down GATA2 expression in hematopoietic stem cells using short hairpin RNA-expressing lentiviral vectors. However, this failed to alter the generation of hematopoietic progenitors or balance of lymphoid/myeloid output using a xenotransplant readout (supplemental Figure 2).
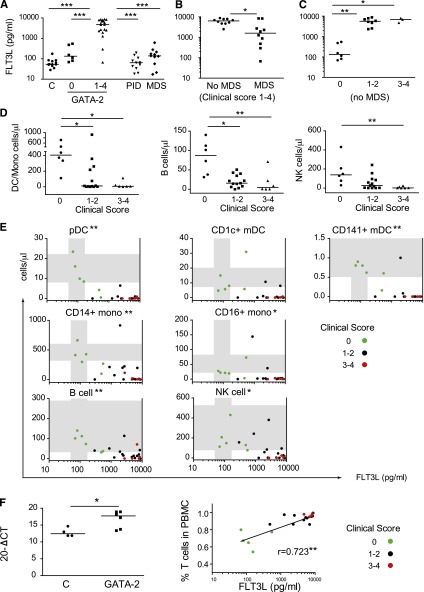
Elevated Flt3L is a marker of GATA2 mutation
It was previously reported that GATA2 mutation is associated with elevated serum Flt3L.1 Comparison of patients with GATA2 mutation at different clinical stages (n = 24) with relatives who did not carry GATA2 mutation (n = 13), patients with acquired MDS and WT GATA2 (n = 11) or PID (n = 11) indicated significantly elevated Flt3L for symptomatic GATA2 mutation (Figure 4A). Compared with GATA2 WT controls, 2 of the 6 relatives with a clinical score of 0 had supranormal Flt3L (>200 pg/mL). Within the symptomatic GATA2 cohort (clinical score = 1 to 4), the development of MDS was associated with lower Flt3L, but still above the level seen in acquired MDS patients (Figure 4B). When patients with MDS were removed, advancing clinical stage was clearly associated with progressively increasing FLt3L (Figure 4C). Together, these results suggest a biphasic relationship, where Flt3L becomes progressively elevated but then declines as MDS develops.
Figure 4.
Flt3L is a specific marker of GATA2 mutation. (A) Flt3L was measured by ELISA in the serum of unaffected relatives with WT GATA2 (n = 13), individuals with GATA2 mutation (n = 24), patients with other PID (n = 11), and MDS patients (n = 11). For patients with GATA2 mutation, the clinical score (0 or 1 to 4) is indicated. (B) The relationship between Flt3L and the development of MDS (n = 24). (C) Relationship between Flt3L and clinical score, excluding patients with MDS (n = 18). (D) Decline in DCs/monocytes, B cells, and NK cells with increasing clinical score (n = 24). (E) Relationship between cell counts, Flt3L, and clinical stage (n = 24; shaded regions indicate normal ranges and asterisks indicate P values for Spearman correlation coefficients). (F) Elevation of Flt3L mRNA detected by Q-PCR in GATA2 patients compared with controls, and relationship between serum Flt-3L and percentage of CD3 (ie, T cells in PBMCs). *P < .05; **P < .01; ***P < .001. Q-PCR, quantitative polymerase chain reaction.
In all patients, progressive mononuclear cytopenias correlated with clinical stage (Figure 4D). The relationships between cytopenia, advancing clinical stage, and Flt3L were evident in all mononuclear fractions (Figure 4E). Flt3L mRNA was increased in the PBMCs of patients with GATA2 mutation and correlated with the percentage of T cells in PBMCs, consistent with T cells being one source of Flt3L (Figure 4F). From these data, we conclude that serial measurements of Flt3L may be useful in identifying and assessing the prognosis of patients with GATA2 mutation.
A proteomic screen of 117 cytokines, chemokines, growth factors, and other immune mediators was initiated to seek other correlative biomarkers. Confirmatory testing of a second larger cohort by ELISA revealed trends for increased FGF-2, EGF, GM-CSF, and CD40L in patients compared with healthy controls (supplemental Figure 3).
Peripheral lymphoid homeostasis in GATA2 mutation
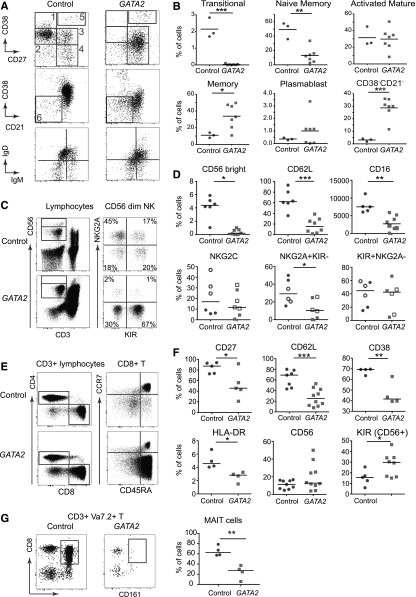
GATA2 mutation is associated with a complete loss of B- and NK-progenitors within the CD34+ compartment of the BM.1 This implies that these compartments become depleted by a shortage of immature or naïve cells, although it is also possible that GATA2 mutation compromises the differentiation or survival of mature cells. For more precise definition, we examined the B and NK compartments in intermediate stages of evolution of DCML deficiency. Transitional B cells were absent and memory B cells were skewed toward a mature phenotype (Figure 5A-B).27 In keeping with normal immunoglobulin levels, IgD and IgM class-switched–B cells were detectable. In addition, there was expansion of a small subset of CD38−CD21− B cells associated with autoimmunity.28 As previously reported, the most juvenile population of CD56bright NK cells was absent in patients with GATA2 mutation,21 supporting the model of NK differentiation from CD56bright to CD56dim populations (Figure 5C-D).29 Within the CD56dim compartment, there was further evidence of skewing toward a more highly differentiated phenotype characterized by the loss of NKG2A and CD62L, and expression of killer-cell immunoglobulin-like receptors (KIR).30
Figure 5.
Highly differentiated phenotype of the peripheral lymphoid compartment of patients with GATA2 mutation. (A) Example of B-cell profile of patient with GATA2 mutation compared with control according to published descriptions.23,27 Populations: (1) transitional; (2) naïve mature; (3) mature activated; (4) resting memory; (5) plasmablast; and (6) CD38−CD21− (autoimmune-associated). (B) Quantification of GATA2-mutated patients vs controls showing depletion of transitional B cells and naïve memory B cells, and accumulation of memory B cells and CD38−CD21− B cells. (C) Example of NK-cell profile of patient with GATA2 mutation compared with control showing the distribution of CD56bright NK cells, and NKG2A+ and KIR+ cells within the CD56dim population. (D) Quantification of GATA2-mutated patients vs controls showing CD56bright NK cells and the expression of differentiation-associated antigens within the CD56dim population. Cytomegalovirus seropositivity is indicated by open symbols. (E) Example of CD3+ T-cell profile of a patient with GATA2 mutation compared with control showing CD4:CD8 profile and differentiation according to expression of CCR7 and CD45RA. (F) Quantification of antigen expression by CD8+ T cells of GATA2-mutated patients vs controls showing the acquisition of a terminally differentiated phenotype and increased expression of KIR on the CD56+ subset. (G) CD8+ CD161+ Va7.2+ MAIT cells are decreased in patients relative to controls. *P < .05; **P < .01; ***P < .001.
More detailed phenotyping of the CD8+ T-cell compartment disclosed a reduction of naïve and central memory cells but an accumulation of CCR7− CD45RA+ effector memory and CCR7− CD45RA+ terminal effector populations (Figure 5E).31,32 In keeping with this, CD8+ T cells of patients expressed lower CD27, CD62L, CD38, and HLA-DR than controls (Figure 5F). The expansion of CD56+CD3+ T cells observed in many patients was consistent with the accumulation of terminal effector CD8+ T cells which was also found to express higher levels of KIR. There was no expansion of γδ T cells, invariant NK T cells, or CD161+ mucosal-associated invariant T cells (MAIT cells) within the CD56+ population. Unexpectedly, there was a significant depletion of MAIT cells in GATA2 patients (Figure 5G).
Discussion
This study describes a cross-sectional analysis of 30 patients that reveals new information about the evolution of mononuclear cytopenia or DCML deficiency in GATA2 mutation (Figure 6). We demonstrated DCML deficiency in 20 symptomatic members of the cohort, and variable cytopenias in 2 of 6 of the asymptomatic individuals who were phenotyped. Our findings concur with the descriptions of cellular deficiency in Emberger syndrome7 and other cases of lymphedema.9 Mononuclear cytopenia is also a key feature of monoMAC3 and has been reported in patients with MDS and GATA2 mutation.4,9-12,20
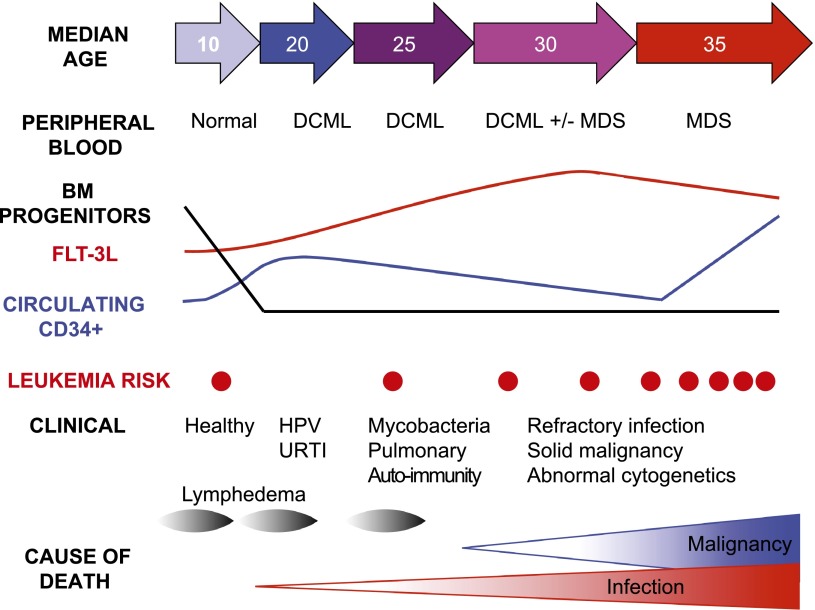
Figure 6.
Schematic diagram summarizing the evolution of cellular deficiency in GATA2 mutation. BM MLPs are rapidly lost even in healthy carriers (see Figure 2). Peripheral blood CD34 counts are elevated in many patients (see examples in Figures 1 and 2) and tend to decline with advancing disease (not shown). Flt3L is progressively elevated, but declines as patients develop MDS (see Figure 4). A rapid rise in CD34+ cells and decline in Flt3L may signify the onset of MDS or AML, although AML may occur sporadically without prior cytopenia.
Patients were recruited from diverse clinical backgrounds, but cytopenia often triggered referral, therefore the frequent observation of DCML deficiency is perhaps not surprising. Notably, the pattern of DCML deficiency was reproducible across variable clinical phenotypes and the degree of cytopenia correlated with the elevation of Flt3L and clinical severity.
Six symptomatic relatives were recruited from 3 different pedigrees with substitutions of the second zinc finger (R398W, R398Q, and T354M) and a history of MDS or AML. Cytopenia and elevated Flt3L was seen in 2 cases but 4 were phenotypically normal, including a relative aged 62 years, suggesting that partial penetrance may occur. Case #5 clearly developed a subclinical cellular phenotype with loss of BM progenitors, progressive elevation of Flt3L, and evolution cytopenia over a 3-year period of observation. Together with other cases already described,9 this is consistent with the notion that DCML deficiency may evolve over several decades but remain undetected. Whether cytopenias and elevated Flt3L always precede MDS or AML remains unclear. Firstly, we have not yet prospectively documented a case of DCML deficiency evolving into MDS or AML; and secondly, it is entirely possible that unaffected carriers may undergo spontaneous transformation without any sign of DCML deficiency. Further prospective studies will be required to ascertain whether DCML deficiency can be considered a true “accessory” hematologic phenotype to GATA2-related MDS/AML, in the manner of thrombocytopenia in RUNX1 and eosinophilia in CEBPA mutations.33,34 In particular, we note that cytopenia was not reported in association with the T354M and T355del mutations of GATA2, originally described in familial MDS/AML.8 From a pragmatic stance, however, our data suggests that it may be informative to monitor the development of cytopenia and elevation of Flt3L in asymptomatic family members at risk for MDS/AML.
Although GATA2 mutation is a constitutive genetic risk for developing MDS,4,8-10,20 patients with GATA2 mutation may be distinguished from those with acquired MDS and WT GATA2 on several grounds. GATA2 mutation is associated with a much younger age of presentation, better preserved Hb, neutrophils, and platelets, and much more severe defects of DCs, monocytes, and lymphoid cells than patients with MDS. Flt3L may be useful as a diagnostic test in this setting; a level in excess of 1000 pg would identify GATA2 mutation with 89% sensitivity and 100% specificity compared with MDS patients. The observation that control MDS patients did not have GATA2 mutations is consistent with a recent large cohort study showing an incidence of mutation in only 4 of 603 MDS patients.35
We did not encounter large deletions or regulatory mutations of GATA2 in this cohort despite sequencing the promoters, intron 5 enhancer, and untranslated regions of the gene. As in other studies, frameshifts 5′ to the second zinc finger and substitution mutations in the second zinc finger predominate here.4,9,20 We found a younger age of presentation and higher clinical score in the frameshift group. An association between lymphedema and frameshift mutation is suggested by a survey of published cases.7,9,12 Although our data did not reach significance, 3 of 4 patients with lymphedema had frameshift mutations. This is a similar proportion to a previously reported study (6 of 8 pedigrees).7 Significance was not reached because of cohort size, as well as the low penetrance of this trait (8 of 11 symptomatic frameshift patients did not develop lymphedema). In a similar fashion, MDS was more often associated with substitution mutations, but not all patients with substitution (including 4 of 6 with T354M), developed MDS. Preterm labor has been recognized in women carrying GATA2 mutations.3 In this cohort, there was 1 case in each genotype group with an overall incidence of 14% of live births in the cohort. This compares with the European average of 7%.36
A simple clinical score aimed at the early complications suggested that clinical progression was associated with evolving mononuclear cytopenia and progressively elevated FLt3L. Flt3L is a trophic factor for human progenitors and DCs.37-39 GATA2 mutation presumably elicits a combined response to stem-cell attrition, DC deficiency, and systemic infection.40,41 Flt3L is produced by activated T cells and stromal cells,41-43 and serum Flt3L and Flt3L mRNA in PBMCs increased in parallel with peripheral T-cell enrichment. A longitudinal study of patient #5 indicated that the loss of progenitors and the elevation of Flt3L preceded the development of cellular deficiency, suggesting that the major driver relates to the attrition of hematopoietic progenitors rather than peripheral DC homeostasis. In more advanced disease, it appeared that the development of MDS in GATA2-mutated individuals was associated with a secondary decline in Flt3L, possibly due to an expansion of marrow cellularity and consumption of Flt3L. Further longitudinal study is required, but this may be a useful indicator of hematologic progression.
The factors that promote the evolution of cytopenias remain uncertain. Extrinsic infection has been mooted and may be consistent with the high numbers of terminally differentiated peripheral T cells seen in many patients. Alternatively, progressive cytopenia and clonal hematopoiesis occurring in asymptomatic individuals, favors cell intrinsic mechanisms. GATA2 mutation is known to compromise stem-cell longevity in animal models,44-46 but the mechanism is poorly understood.
Flt3L was the only serum marker of 118 markers screened, to be markedly elevated in GATA2 mutation. Mild increases of FGF, EGF, and M-CSF were seen, together with CD40L and GM-CSF. Stromal growth factors (FGF and EGF), exerted a similar effect to Flt3L in protecting animal models against hematopoietic stress.47 CD40L and GM-CSF indicated immune activation. Of note, EGF and CD40L were also elevated in mycobacterial infection and HIV infection.48,49 The modest induction of these mediators is of interest, but unlikely to be useful in clinical monitoring.
GATA2 mutation and DCML deficiency provide new insights into the maintenance of long-term immunocompetence in adult humans. It is surprising that an almost complete absence of DCs, monocytes, NK cells, and B cells is compatible with long-term survival. Normal IgG and memory T-cell development appears to sustain host resistance to many pathogens and is probably established before cytopenias develop. Patients with evolving DCML deficiency lose transitional B cells, CD56bright NK cells, and naïve T lymphocytes. The immunophenotype that emerges is strongly reminiscent of the pattern of terminal differentiation seen in aged individuals and chronic viral infections such as cytomegalovirus, hepatitis C, and HIV.50 NK cells of GATA2-deficient patients lose CD16, NKG2A, and acquire KIR expression. Concomitantly, CD8 T cells express CD45RA (TEMRA phenotype), lose CD27, CD62L, and activation markers HLA-DR and CD38, but acquire CD56 and KIR.51 The function of terminally differentiated cells has been described as defective in many studies, but more recent data indicate that viral infections leave specific adaptive signatures on NK- and T-cell phenotype.52,53 The absence of professional antigen-presenting cells led us to speculate that invariant T cells including invariant NK T cells, γδ T cells, or MAIT cells might be relatively expanded. The converse was observed, particularly a reduction in MAIT cells, which is another finding consistent with persistent infection and susceptibility to mycobacteria.54
In summary, DCML deficiency or mononuclear cytopenia evolves in diverse clinical groups of GATA2 mutation including Emberger syndrome, monoMAC, and hereditary MDS, but may not be completely penetrant or is an invariant precursor of malignant transformation. GATA2 mutation appears to cause a complex process of progenitor cell loss, associated with clonal myelopoiesis and elevated Flt3L. Preservation of hematopoiesis in early life allows most individuals to establish a degree of protective immunity. The results presented in this study define the pathogenesis of GATA2 disease in more detail and will assist in the development of individualized care for patients. However, significant questions remain concerning the molecular mechanisms of hematopoietic failure and malignant transformation, caused by heterozygous GATA2 mutation in humans.
Supplementary Material
Acknowledgments
The authors thank Lisa Strain, David Bourn, and the Molecular Genetics Laboratory of The Northern Regional Genetics Service, Newcastle upon Tyne, for sequencing of MDS patients.
This work was supported by Lymphoma and Leukaemia Research, The British Society of Hematology, Bright Red, The George Walker Trust, and The Wellcome Trust.
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.E.D. performed experiments, analyzed data, and wrote the manuscript; P.M. and L.J. performed experiments and analyzed data; S.Z. and S.I.S. performed experiments, analyzed data, and wrote the manuscript; N.M. performed experiments and analyzed data; S.C. performed experiments; Z.F. and A.L. performed experiments and analyzed data; S.P. performed experiments; A.G., T.H.K., S. Hämäläinen, M.S., M. Helbert., E.T., E.G., S.R., M.G., J.E.T., E.M., G.H., A.G.R., S.J., and C.M.B. provided clinical material; S. Hambleton provided clinical material and analyzed data; M. Haniffa analyzed data; Y.B. and C.A. performed experiments and analyzed data; J.T.P. and J.E.D. analyzed data and wrote the manuscript; and V.B. and M.C. designed the study, performed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Matthew Collin, Institute for Cellular Medicine, Newcastle University, Framlington Place, Newcastle upon Tyne, NE2 4HH, United Kingdom; e-mail: matthew.collin@ncl.ac.uk.
References
- 1.Bigley V, Haniffa M, Doulatov S, et al. The human syndrome of dendritic cell, monocyte, B and NK lymphoid deficiency. J Exp Med. 2011;208(2):227–234. doi: 10.1084/jem.20101459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dickinson RE, Griffin H, Bigley V, et al. Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood. 2011;118(10):2656–2658. doi: 10.1182/blood-2011-06-360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vinh DC, Patel SY, Uzel G, et al. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood. 2010;115(8):1519–1529. doi: 10.1182/blood-2009-03-208629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu AP, Sampaio EP, Khan J, et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood. 2011;118(10):2653–2655. doi: 10.1182/blood-2011-05-356352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu AP, Johnson KD, Falcone EL, et al. GATA2 haploinsufficiency caused by mutations in a conserved intronic element leads to MonoMAC syndrome. Blood. 2013;121(19):3830–3837, S1-S7. doi: 10.1182/blood-2012-08-452763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mansour S, Connell F, Steward C, et al. Lymphoedema Research Consortium. Emberger syndrome-primary lymphedema with myelodysplasia: report of seven new cases. Am J Med Genet A. 2010;152A(9):2287–2296. doi: 10.1002/ajmg.a.33445. [DOI] [PubMed] [Google Scholar]
- 7.Ostergaard P, Simpson MA, Connell FC, et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nat Genet. 2011;43(10):929–931. doi: 10.1038/ng.923. [DOI] [PubMed] [Google Scholar]
- 8.Hahn CN, Chong CE, Carmichael CL, et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat Genet. 2011;43(10):1012–1017. doi: 10.1038/ng.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kazenwadel J, Secker GA, Liu YJ, et al. Loss-of-function germline GATA2 mutations in patients with MDS/AML or MonoMAC syndrome and primary lymphedema reveal a key role for GATA2 in the lymphatic vasculature. Blood. 2012;119(5):1283–1291. doi: 10.1182/blood-2011-08-374363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holme H, Hossain U, Kirwan M, Walne A, Vulliamy T, Dokal I. Marked genetic heterogeneity in familial myelodysplasia/acute myeloid leukaemia. Br J Haematol. 2012;158(2):242–248. doi: 10.1111/j.1365-2141.2012.09136.x. [DOI] [PubMed] [Google Scholar]
- 11.Bödör C, Renneville A, Smith M, et al. Germ-line GATA2 p.THR354MET mutation in familial myelodysplastic syndrome with acquired monosomy 7 and ASXL1 mutation demonstrating rapid onset and poor survival. Haematologica. 2012;97(6):890–894. doi: 10.3324/haematol.2011.054361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishida H, Imai K, Honma K, et al. GATA-2 anomaly and clinical phenotype of a sporadic case of lymphedema, dendritic cell, monocyte, B- and NK-cell (DCML) deficiency, and myelodysplasia. Eur J Pediatr. 2012;171(8):1273–1276. doi: 10.1007/s00431-012-1715-7. [DOI] [PubMed] [Google Scholar]
- 13.Mutsaers PG, van de Loosdrecht AA, Tawana K, Bödör C, Fitzgibbon J, Menko FH. Highly variable clinical manifestations in a large family with a novel GATA2 mutation. Leukemia. 2013;27(11):2247–2248. doi: 10.1038/leu.2013.105. [DOI] [PubMed] [Google Scholar]
- 14.Kaur J, Catovsky D, Valdimarsson H, Jensson O, Spiers AS. Familial acute myeloid leukaemia with acquired Pelger-Huet anomaly and aneuploidy of C group. BMJ. 1972;4(5836):327–331. doi: 10.1136/bmj.4.5836.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson CW, Norman JE, Cleland LG, Ford JH. Dermal necrosis and chromosome Iq abnormality in a man with a familial myeloproliferative disorder. Aust N Z J Med. 1983;13(2):141–145. doi: 10.1111/j.1445-5994.1983.tb02669.x. [DOI] [PubMed] [Google Scholar]
- 16.Horwitz M, Sabath DE, Smithson WA, Radich J. A family inheriting different subtypes of acute myelogenous leukemia. Am J Hematol. 1996;52(4):295–304. doi: 10.1002/(SICI)1096-8652(199608)52:4<295::AID-AJH9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 17.Lim KC, Hosoya T, Brandt W, et al. Conditional Gata2 inactivation results in HSC loss and lymphatic mispatterning. J Clin Invest. 2012;122(10):3705–3717. doi: 10.1172/JCI61619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson KD, Hsu AP, Ryu MJ, et al. Cis-element mutated in GATA2-dependent immunodeficiency governs hematopoiesis and vascular integrity. J Clin Invest. 2012;122(10):3692–3704. doi: 10.1172/JCI61623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calvo KR, Vinh DC, Maric I, et al. Myelodysplasia in autosomal dominant and sporadic monocytopenia immunodeficiency syndrome: diagnostic features and clinical implications. Haematologica. 2011;96(8):1221–1225. doi: 10.3324/haematol.2011.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasquet M, Bellanné-Chantelot C, Tavitian S, et al. High frequency of GATA2 mutations in patients with mild chronic neutropenia evolving to MonoMac syndrome, myelodysplasia, and acute myeloid leukemia. Blood. 2013;121(5):822–829. doi: 10.1182/blood-2012-08-447367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mace EM, Hsu AP, Monaco-Shawver L, et al. Mutations in GATA2 cause human NK cell deficiency with specific loss of the CD56(bright) subset. Blood. 2013;121(14):2669–2677. doi: 10.1182/blood-2012-09-453969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lyman SD, Seaberg M, Hanna R, et al. Plasma/serum levels of flt3 ligand are low in normal individuals and highly elevated in patients with Fanconi anemia and acquired aplastic anemia. Blood. 1995;86(11):4091–4096. [PubMed] [Google Scholar]
- 23.Haidar JH, Bazarbachi A, Mahfouz R, Haidar HA, Jaafar H, Daher R. Serum Flt3 ligand variation as a predictive indicator of hematopoietic stem cell mobilization. J Hematother Stem Cell Res. 2002;11(3):533–538. doi: 10.1089/15258160260090997. [DOI] [PubMed] [Google Scholar]
- 24.West RR, Hsu AP, Holland SM, Cuellar-Rodriguez J, Hickstein DD. Acquired ASXL1 mutations are common in patients with inherited GATA2 mutations and correlate with myeloid transformation [published online ahead of print September 27, 2013]. Haematologica. doi: 10.3324/haematol.2013.090217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuellar-Rodriguez J, Gea-Banacloche J, Freeman AF, et al. Successful allogeneic hematopoietic stem cell transplantation for GATA2 deficiency. Blood. 2011;118(13):3715–3720. doi: 10.1182/blood-2011-06-365049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swierczek SI, Agarwal N, Nussenzveig RH, et al. Hematopoiesis is not clonal in healthy elderly women. Blood. 2008;112(8):3186–3193. doi: 10.1182/blood-2008-03-143925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luning Prak ET, Ross J, Sutter J, Sullivan KE. Age-related trends in pediatric B-cell subsets. Pediatr Dev Pathol. 2011;14(1):45–52. doi: 10.2350/10-01-0785-OA.1. [DOI] [PubMed] [Google Scholar]
- 28.Wehr C, Eibel H, Masilamani M, et al. A new CD21low B cell population in the peripheral blood of patients with SLE. Clin Immunol. 2004;113(2):161–171. doi: 10.1016/j.clim.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Béziat V, Duffy D, Quoc SN, et al. CD56brightCD16+ NK cells: a functional intermediate stage of NK cell differentiation. J Immunol. 2011;186(12):6753–6761. doi: 10.4049/jimmunol.1100330. [DOI] [PubMed] [Google Scholar]
- 30.Béziat V, Descours B, Parizot C, Debré P, Vieillard V. NK cell terminal differentiation: correlated stepwise decrease of NKG2A and acquisition of KIRs. PLoS ONE. 2010;5(8):e11966. doi: 10.1371/journal.pone.0011966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamann D, Baars PA, Rep MH, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186(9):1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 33.Owen CJ, Toze CL, Koochin A, et al. Five new pedigrees with inherited RUNX1 mutations causing familial platelet disorder with propensity to myeloid malignancy. Blood. 2008;112(12):4639–4645. doi: 10.1182/blood-2008-05-156745. [DOI] [PubMed] [Google Scholar]
- 34.Carmichael CL, Wilkins EJ, Bengtsson H, et al. Poor prognosis in familial acute myeloid leukaemia with combined biallelic CEBPA mutations and downstream events affecting the ATM, FLT3 and CDX2 genes. Br J Haematol. 2010;150(3):382–385. doi: 10.1111/j.1365-2141.2010.08204.x. [DOI] [PubMed] [Google Scholar]
- 35.Papaemmanuil E, Gerstung M, Malcovati L, et al. Chronic Myeloid Disorders working group of the International Cancer Genome Consortium. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122(22):3616–3627. doi: 10.1182/blood-2013-08-518886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang HH, Larson J, Blencowe H, et al. Born Too Soon preterm prevention analysis group. Preventing preterm births: analysis of trends and potential reductions with interventions in 39 countries with very high human development index. Lancet. 2013;381(9862):223–234. doi: 10.1016/S0140-6736(12)61856-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dao MA, Hannum CH, Kohn DB, Nolta JA. FLT3 ligand preserves the ability of human CD34+ progenitors to sustain long-term hematopoiesis in immune-deficient mice after ex vivo retroviral-mediated transduction. Blood. 1997;89(2):446–456. [PubMed] [Google Scholar]
- 38.McKenna HJ, Stocking KL, Miller RE, et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95(11):3489–3497. [PubMed] [Google Scholar]
- 39.Pulendran B, Banchereau J, Burkeholder S, et al. Flt3-ligand and granulocyte colony-stimulating factor mobilize distinct human dendritic cell subsets in vivo. J Immunol. 2000;165(1):566–572. doi: 10.4049/jimmunol.165.1.566. [DOI] [PubMed] [Google Scholar]
- 40.Darrasse-Jèze G, Deroubaix S, Mouquet H, et al. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med. 2009;206(9):1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saito Y, Boddupalli CS, Borsotti C, Manz MG. Dendritic cell homeostasis is maintained by nonhematopoietic and T-cell-produced Flt3-ligand in steady state and during immune responses. Eur J Immunol. 2013;43(6):1651–1658. doi: 10.1002/eji.201243163. [DOI] [PubMed] [Google Scholar]
- 42.McClanahan T, Culpepper J, Campbell D, et al. Biochemical and genetic characterization of multiple splice variants of the Flt3 ligand. Blood. 1996;88(9):3371–3382. [PubMed] [Google Scholar]
- 43.Hannum C, Culpepper J, Campbell D, et al. Ligand for FLT3/FLK2 receptor tyrosine kinase regulates growth of haematopoietic stem cells and is encoded by variant RNAs. Nature. 1994;368(6472):643–648. doi: 10.1038/368643a0. [DOI] [PubMed] [Google Scholar]
- 44.Tsai FY, Orkin SH. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood. 1997;89(10):3636–3643. [PubMed] [Google Scholar]
- 45.Ling KW, Ottersbach K, van Hamburg JP, et al. GATA-2 plays two functionally distinct roles during the ontogeny of hematopoietic stem cells. J Exp Med. 2004;200(7):871–882. doi: 10.1084/jem.20031556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodrigues NP, Janzen V, Forkert R, et al. Haploinsufficiency of GATA-2 perturbs adult hematopoietic stem-cell homeostasis. Blood. 2005;106(2):477–484. doi: 10.1182/blood-2004-08-2989. [DOI] [PubMed] [Google Scholar]
- 47.Gratwohl A, John L, Baldomero H, et al. FLT-3 ligand provides hematopoietic protection from total body irradiation in rabbits. Blood. 1998;92(3):765–769. [PubMed] [Google Scholar]
- 48.Djoba Siawaya JF, Chegou NN, van den Heuvel MM, et al. Differential cytokine/chemokines and KL-6 profiles in patients with different forms of tuberculosis. Cytokine. 2009;47(2):132–136. doi: 10.1016/j.cyto.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 49.Sipsas NV, Sfikakis PP, Kontos A, Kordossis T. Levels of soluble CD40 ligand (CD154) in serum are increased in human immunodeficiency virus type 1-infected patients and correlate with CD4(+) T-cell counts. Clin Diagn Lab Immunol. 2002;9(3):558–561. doi: 10.1128/CDLI.9.3.558-561.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strindhall J, Skog M, Ernerudh J, et al. The inverted CD4/CD8 ratio and associated parameters in 66-year-old individuals: the Swedish HEXA immune study. Age (Dordr) 2013;35(3):985–991. doi: 10.1007/s11357-012-9400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Björkström NK, Béziat V, Cichocki F, et al. CD8 T cells express randomly selected KIRs with distinct specificities compared with NK cells. Blood. 2012;120(17):3455–3465. doi: 10.1182/blood-2012-03-416867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Béziat V, Liu LL, Malmberg JA, et al. NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood. 2013;121(14):2678–2688. doi: 10.1182/blood-2012-10-459545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Newell EW, Sigal N, Bendall SC, Nolan GP, Davis MM. Cytometry by time-of-flight shows combinatorial cytokine expression and virus-specific cell niches within a continuum of CD8+ T cell phenotypes. Immunity. 2012;36(1):142–152. doi: 10.1016/j.immuni.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Le Bourhis L, Martin E, Péguillet I, et al. Antimicrobial activity of mucosal-associated invariant T cells [published correction appears in Nat Immunol. 2010;11(10):969]. Nat Immunol. 2010;11(8):701–708. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.