Abstract
Background
Use of local therapy for prostate cancer may increase because of the perceived advantages of new technologies such as intensity-modulated radiotherapy (IMRT) and robotic prostatectomy.
Objective
To examine the association of market-level technological capacity with receipt of local therapy.
Design
Retrospective cohort.
Subjects
Patients with localized prostate cancer who were diagnosed between 2003 and 2007 (n=59,043) from the Surveillance Epidemiology and End Results (SEER) – Medicare database.
Measures
We measured the capacity for delivering treatment with new technology as the number of providers offering robotic prostatectomy or IMRT per population in a market (hospital referral region). The association of this measure with receipt of prostatectomy, radiotherapy, or observation was examined with multinomial logistic regression.
Results
For each 1,000 patients diagnosed with prostate cancer, 174 underwent prostatectomy, 490 radiotherapy, and 336 were observed. Markets with high robotic prostatectomy capacity had higher use of prostatectomy (146 vs. 118 per 1,000 men, p=0.008) but a trend towards decreased use of radiotherapy (574 vs. 601 per 1,000 men, p=0.068), resulting in a stable rate of local therapy. High versus low IMRT capacity did not significantly impact use of prostatectomy (129 vs. 129 per 1,000 men, p=0.947) and radiotherapy (594 vs. 585 per 1,000 men, p=0.579).
Conclusions
Although there was a small shift from radiotherapy to prostatectomy in markets with high robotic prostatectomy capacity, increased capacity for both robotic prostatectomy and IMRT did not change the overall rate of local therapy. Our findings temper concerns that new technology spurs additional therapy of prostate cancer.
Keywords: intensity-modulated radiotherapy, prostate cancer treatment, robotic prostatectomy, technology
Introduction
The development and dissemination of new technology is a major determinant of growth in Medicare spending,1,2 which is expected to double within the next 10 years.3 New biological agents, imaging tests, and medical devices are among the many sectors of new technology that play a disproportionate role in spending. However, equally important and under recognized is the dissemination of new non-pharmaceutical therapeutics, including surgical technology and new equipment to deliver radiotherapy. These non-pharmaceutical therapeutics vary in the extent to which they aim to substitute a new procedure for an existing one or provide a whole new way of treating patients. In the latter case, a new technology can expand the population eligible for treatment,4 in which case its incremental costs are spurred by greater overall rates of treatment in addition to any increase in per episode costs. For example, by decreasing morbidity by an order of magnitude, laparoscopic cholecystectomy was seen as a significant advance over the open procedure. The sharp contrast between the two morbidity profiles amplified the appeal of laparoscopy thereby reducing barriers to cholecystectomy for minimally symptomatic patients who otherwise would have gone untreated.5,6
Similar to laparoscopic cholecystectomy, new technologies for the treatment of prostate cancer, including robotic radical prostatectomy and intensity-modulated radiotherapy (IMRT), hold the promise of reduced morbidity profiles. Examining these new technologies provides a particularly rich context to gain insight into the effects of technology dissemination for several reasons. First, national prostate cancer spending accounts for almost ten percent of overall cancer spending and approaches $12 billion annually.7 Second, many men diagnosed with prostate cancer have a low risk of dying from the disease and thus are appropriate candidates for observation.8,9 Third, while the evidence is mixed, robotic prostatectomy and IMRT are thought to reduce side-effects affecting urinary, sexual, and gastrointestinal quality of life while also having the potential to improve cancer control when compared to their more traditional counterparts, i.e., open radical prostatectomy and three dimensional radiotherapy.10–12 However, both of these advanced treatments are associated with higher incremental costs compared to traditional treatments.13,14 In addition, the perception of lower morbidity associated with these new treatments, regardless of whether true or not, could shift decision making towards local therapy in lieu of observation and thus expand the population treated. This would further spur overall spending for prostate cancer care and, even more important, could lead to expansion of treatment among patients who have little to gain from local therapy (e.g., those with low-risk disease or advanced age) while being exposed to treatment related morbidity.8,9
For these reasons, we assessed the impact of new prostate cancer technology on utilization of treatment for prostate cancer. Including patients diagnosed with localized prostate cancer between 2003 and 2007, we examined the extent to which technology diffusion was associated with the substitution of one treatment for another and with expansion of the population treated.
Methods
Study population
We used linked Surveillance Epidemiology and End Results (SEER)-Medicare data to identify male beneficiaries with prostate cancer diagnosed between 2003 and 2007. We only included subjects 66 years of age or older to ensure accurate assessment of comorbidity based on Medicare claims for the 12 month period prior to diagnosis.15 Only men enrolled in the fee-for-service program and eligible for Parts A and B of Medicare for at least 12 months before and after prostate cancer diagnosis were included. We limited the cohort to men with prostate cancer as their only cancer (86.5% of the overall population) and excluded subjects with metastatic disease at diagnosis (6.0%). Using these criteria, our final study population consisted of 59,043 patients with localized prostate cancer managed with observation, surgery, or radiotherapy.
Characterizing market-level prostate cancer technology diffusion
As previously described,16 we assessed technology diffusion for prostate cancer treatment at the level of a health care market. Health care markets were defined by Hospital Referral Regions (HRRs), which represent geographical areas in which Medicare beneficiaries receive their tertiary medical care.17 We assigned Medicare beneficiaries to their respective HRRs (n=69) using their home ZIP codes.
Next, we used explicit Healthcare Common Procedure Coding System (HCPCS) and International Classification of Diseases, Ninth Revision, Clinical Modification (ICD9-CM) codes (see appendix) to categorize patients into mutually exclusive groups based on the primary treatment they received. We then assigned robotic prostatectomy patients to their treating surgeon, using Unique Physician Identifier and National Provider Identifier Numbers. For patients undergoing IMRT, we used the same identifiers to assign the provider who performed the clinical planning and simulation as the treating radiation oncologist.18 We then assigned each robotic prostatectomy provider and each IMRT provider to an HRR based on the provider's ZIP code.
Finally, we characterized the capacity for delivering treatment with new technology for each HRR and year by calculating robotic prostatectomy and IMRT provider densities. The numerator was the number of physicians providing robotic prostatectomy or IMRT treatments in each HRR in a given year. The denominator was the number of male Medicare beneficiaries residing within the HRR based on population estimates for the ZIP Code Tabulation Areas.19 We chose this denominator rather than the number of Medicare beneficiaries diagnosed with prostate cancer because we wanted to uncouple our exposure as much as possible from physician practice style. The number of beneficiaries diagnosed with prostate cancer in an HRR depends on physicians performing prostate biopsies. Thus, this number does depend on physician practice style whereas the number of beneficiaries residing in each HRR does not. Next, HRRs were categorized into those with low, medium, or high robotic prostatectomy or IMRT capacity based on tertiles of provider density. The claims-based empirical measure of technological capacity strongly correlated with the number of robotic prostatectomy providers in a given market as abstracted from historical websites (r=0.81,16 Supplemental Digital Content 1), suggesting that it represents a good proxy for technology penetration.
Outcome
Our primary outcome was the use of radical prostatectomy, radiotherapy, or observation. Using explicit HCPCS and ICD9-CM codes, we identified each patient's primary treatment as that occurring first within 12 months after diagnosis (see appendix). Notable exceptions to this rule were patients undergoing neoadjuvant androgen deprivation therapy, who were classified based on the first treatment that followed this therapy. Local therapy was defined as either radical prostatectomy or radiotherapy, while the remainder of the patients (i.e., those receiving primary androgen deprivation therapy or no prostate cancer directed treatment in the first 12 months after diagnosis) were categorized as undergoing observation.
Statistical analyses
We estimated bivariate associations of management approach with demographic characteristics (age, race, clinical stage, grade, D'Amico prostate cancer risk,20 comorbidity,15 education, income, rural versus urban residence), regional characteristics (number of urologists, number of radiation oncologists, and number of hospital beds per 100,000 population; Medicare managed care penetration; HRR-level provider volume), and technological capacity by calculating treatment rates (i.e., the number of men treated per 1,000 diagnosed) for each of the demographic or regional strata. The statistical significance of these bivariate associations was assessed with chi-square tests. Regional characteristics were obtained from the Health Resources and Services Administration's Area Resource File and categorized into tertiles. Because data on Gleason grade and PSA were not available in SEER prior to 2004, all analyses including D'Amico risk20 were restricted to patients diagnosed between 2004 and 2007. In order to describe variation in treatment rates across HRRs, we calculated treatment rates for each HRR and then described the median and range of these rates across all HRRs.
We fit multinomial logistic regression models with the patient as our unit of analysis, using Huber/White sandwich estimators of variance to account for heteroskedasticity.21 The dependent variable was the type of treatment received (prostatectomy, radiotherapy, or observation). We then examined the association of technological capacity with type of treatment received by adding to the models continuous measures of robotic prostatectomy and IMRT provider density. These measures were HRR and year specific, so technological capacity could change from year to year within a given HRR. We also included an interaction term between robotic prostatectomy and IMRT provider density, but removed it from the final models because it was not statistically significant. The multivariable models were adjusted for the patient and regional characteristics described above, and for year of diagnosis. From these models, we calculated adjusted treatment rates for HRRs with low versus high robotic prostatectomy capacity (i.e., robotic prostatectomy capacity in the lowest [0 providers per 100,000 in 2007] versus highest tertile [9.6 providers per 100,000 in 2007]) and low versus high IMRT capacity (i.e., IMRT capacity in the lowest [9.2 providers per 100,000 in 2007] versus highest tertile [18.9 providers per 100,000 in 2007]). We estimated these treatment rates for white patients with no comorbidity diagnosed in 2007 with all other covariates set at the mean. We also explored modeling regional technological capacity as a lagged independent variable (with a one year lag), but this did not substantially affect the results (data not shown). To assess the contribution of specific covariates (i.e., robotic prostatectomy capacity, IMRT capacity, age, race, and comorbidity) to the models, we compared Akaike's Information Criterion of the full model to that of a model excluding that covariate.22 To provide more detailed information on the association between technological capacity and type of treatment received, we fit similar multinomial logistic regression models, but with the dependent variable being the specific treatment received (see appendix).
Because new technology, particularly a new surgical technology, may have a more substantive effect on treatment choice among younger patients, we evaluated the association of technological capacity with type of treatment in patients aged 66 to 69. Given that expansion of local therapy would be particularly worrisome among patients who are least likely to benefit from it (i.e., those aged 70 and older with low-risk prostate cancer20,23 and those aged 85 and older24,25), we also investigated the effect of technological capacity on treatment in these subgroups.
Because of concern that there may be other factors influencing the choice of procedure in each of the 69 HRRs, we additionally fit fixed effects models to help isolate the role of technology more closely. Thus, we examined how treatment decisions changed within each HRR while new technology was disseminating. Moreover, we performed sensitivity analyses excluding HRRs that crossed SEER boundaries into areas from which SEER data were not available (21 of 69 HRRs). We also estimated models not adjusting for market-level provider volume, because adjusting for this covariate could have masked the effect of technological capacity. Results from these sensitivity analyses were not materially different in direction or effect size compared with those of our main analyses, so only the latter are presented.
We performed all analyses using Stata version 12SE and SAS version 9.3. All tests were 2-tailed; and we considered p<0.05 as statistically significant. The University of Michigan Medical School Institutional Review Board exempted this study from review.
Results
On average, for every 1,000 patients diagnosed with prostate cancer, 174 underwent prostatectomy, 490 radiotherapy, and 336 were observed. Treatment rates varied widely across HRRs, with a median surgery rate of 168 (range 71 to 419), a median radiotherapy rate of 483 (range 176 to 663), and a median rate of observation of 336 (range 241 to 558) per 1,000 men diagnosed. Men who were African American and those with higher stage and more comorbid disease were less likely to undergo surgery (Table 1). Patients with advanced age, less education, and lower income were more likely to be observed (Table 1). In bivariate analyses, increased regional technological capacity for robotic prostatectomy was associated with higher rates of surgery (p<0.001, Table 1). Similarly, increased technological capacity for IMRT was associated with higher rates of radiotherapy (p<0.001, Table 1).
Table 1.
Distribution of treatment with surgery or radiation versus observation by patient and regional characteristics. p<0.001 for all bivariate comparisons.
| Characteristics | N | Surgery (number treated per 1,000 diagnosed) | Radiation (number treated per 1,000 diagnosed) | Observation (number managed per 1,000 diagnosed) |
|---|---|---|---|---|
| Age, years | ||||
| 66-69 | 16,218 | 365 | 450 | 185 |
| 70-74 | 18,361 | 193 | 578 | 228 |
| 75-79 | 13,811 | 52 | 572 | 375 |
| 80-84 | 7,404 | 09 | 362 | 629 |
| 85+ | 3,249 | 04 | 134 | 862 |
| Race/ethnicity | ||||
| White | 48,318 | 182 | 494 | 324 |
| Black | 6,245 | 114 | 469 | 417 |
| Hispanic | 1,204 | 153 | 453 | 395 |
| Asian | 1,688 | 126 | 508 | 366 |
| Other/unknown | 1,588 | 238 | 470 | 292 |
| Clinical Stage | ||||
| T1 | 30,462 | 170 | 507 | 323 |
| T2 | 27,036 | 180 | 468 | 352 |
| T3 | 1,309 | 152 | 580 | 268 |
| T4 | 236 | 59 | 386 | 555 |
| D'Amico prostate cancer risk# | ||||
| Low | 12,767 | 149 | 562 | 289 |
| Intermediate | 14,202 | 244 | 519 | 237 |
| High | 12,513 | 157 | 526 | 317 |
| Grade | ||||
| Well or moderately differentiated | 29,987 | 141 | 497 | 362 |
| Poorly or undifferentiated | 29,056 | 208 | 483 | 309 |
| Comorbidity | ||||
| 0 | 38,635 | 202 | 485 | 313 |
| 1 | 13,039 | 144 | 519 | 337 |
| 2 | 4,386 | 94 | 486 | 421 |
| 3+ | 2,983 | 55 | 442 | 504 |
| Lived in Census Tract in which 25% or more of adults had a college education | ||||
| No | 31,047 | 156 | 478 | 366 |
| Yes | 27,996 | 194 | 503 | 302 |
| Median annual household income in Census Tract | ||||
| Low (≤ $39,926) | 19,651 | 148 | 462 | 391 |
| Intermediate | 19,697 | 178 | 489 | 333 |
| High (≥ $58,412) | 19,695 | 196 | 520 | 284 |
| Residing in urban area | ||||
| No | 4,834 | 165 | 444 | 391 |
| Yes | 54,209 | 175 | 494 | 331 |
| Number of urologists per 100,000 men 65 and over | ||||
| Low (≤ 55) | 19,704 | 158 | 495 | 347 |
| Intermediate | 21,663 | 207 | 455 | 338 |
| High (≥88) | 17,676 | 151 | 528 | 321 |
| Number of radiation oncologists per 100,000 men 65 and over | ||||
| Low (≤ 23) | 20,327 | 159 | 499 | 342 |
| Intermediate | 22,061 | 197 | 464 | 339 |
| High (≥ 38) | 16,655 | 162 | 513 | 325 |
| Number of hospital beds per 100,000 men 65 and over | ||||
| Low(≤ 4,797) | 20,077 | 181 | 494 | 326 |
| Intermediate | 22,811 | 164 | 491 | 345 |
| High (≥ 6,861) | 16,155 | 180 | 485 | 335 |
| Medicare managed care penetration | ||||
| Low (≤ 5.2%) | 20,083 | 156 | 487 | 357 |
| Intermediate | 19,072 | 149 | 543 | 308 |
| High (≥ 19.4%) | 19,888 | 216 | 443 | 342 |
| Average market-level yearly prostatectomy volume per provider | ||||
| Low (< 2.8 prostatectomies per year) | 20,047 | 127 | 530 | 343 |
| Intermediate | 19,578 | 169 | 498 | 333 |
| High (≥ 5.1 prostatectomies per year) | 19,418 | 228 | 440 | 332 |
| Average market-level yearly radiotherapy volume per provider | ||||
| Low (≤ 12.6 patients treated per year) | 19,762 | 211 | 427 | 362 |
| Intermediate | 19,444 | 163 | 502 | 335 |
| High (> 20.4 patients treated per year) | 19,837 | 149 | 541 | 311 |
| Surgical technology | ||||
| Low (0 providers per 100,000 in 2007) | 10,704 | 166 | 486 | 348 |
| Intermediate | 29,685 | 166 | 493 | 341 |
| High (9.6 providers per 100,000 in 2007) | 18,654 | 192 | 487 | 321 |
| IMRT technology | ||||
| Low (9.2 providers per 100,000 in 2007) | 16,556 | 190 | 480 | 330 |
| Intermediate | 28,107 | 167 | 495 | 338 |
| High (18.9 providers per 100,000 in 2007) | 14,380 | 170 | 492 | 338 |
prostate cancer risk according to D'Amico et al.20 Because G eason grade and PSA were not available in SEER-Medicare until 2004, only patients diagnosed in 2004 and later are included.
In multivariable analyses, the use of radical prostatectomy was significantly higher in markets with high robotic prostatectomy capacity (146 vs. 118 per 1,000 men diagnosed, p=0.008, detailed model output shown in Supplemental Digital Content 2). However, these markets had a trend towards lower rates of radiotherapy (574 vs. 601 per 1,000 men diagnosed, p=0.068), such that rates of observation remained stable (280 vs. 281, p=0.910, Figure 1A). In these markets, the increased use of radical prostatectomy was primarily driven by an increase in use of robotic prostatectomy (85 vs. 42 per 1,000 men diagnosed, p<0.001, Supplemental Digital Content 3). Similar effects were observed among the patients in the youngest age group (n=16,218): 447 vs. 389 per 1,000 men were treated with prostatectomy comparing high to low surgical technology HRRs (p=0.022, Figure 1B).
Figure 1.
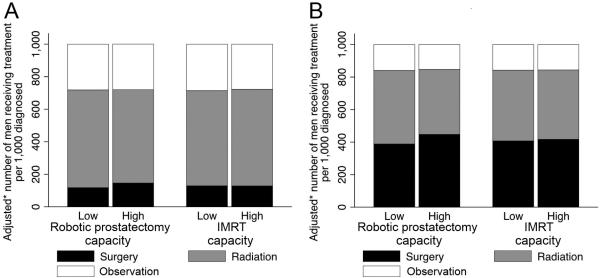
Rates of surgical treatment, radiotherapy, and observation according to market-level technological capacity. The use of radical prostatectomy was significantly higher in markets with high robotic prostatectomy capacity, both among all patients (p=0.008, panel A) and among men 66 to 69 years old (p=0.022, panel B). However, rates of observation remained stable. * Models were adjusted for year, patient characteristics (age, comorbidity, stage, grade, socioeconomic status), and market characteristics (number of urologists, of radiation oncologists, and of hospital beds; managed care penetration; average provider volume in market).
High versus low IMRT capacity did not significantly impact use of prostatectomy (129 vs. 129 per 1,000 men, p=0.947), radiotherapy (594 vs. 585 per 1,000 men, p=0.579), or observation (277 vs. 286 per 1,000 men, p=0.384, Figure 1A). Nevertheless, high IMRT capacity was associated with a significant increase in use of IMRT (352 vs. 220 per 1,000 men, p<0.001), but this was offset by decreases in use of external beam radiotherapy and brachytherapy (Supplemental Digital Content 3). Removing patient-level covariates such as age, race, or comorbidity from the full model led to much more substantial increases in Akaike's Information Criterion (15008, 524, and 509, respectively) than removing technological capacity (56 for robotic prostatectomy capacity and 12 for IMRT capacity). This implies that the effect of these patient-level covariates on treatment was much larger than the impact of technological capacity (Figure 2).
Figure 2.
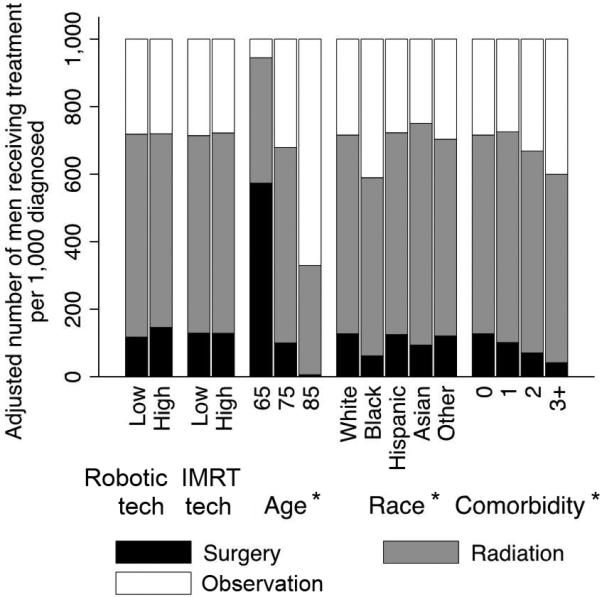
Effect of technological capacity on rates of prostatectomy, radiotherapy, or observation compared with the effects of other covariates. Models were adjusted for year, patient characteristics (age, comorbidity, stage, grade, socioeconomic status), and market characteristics (number of urologists, of radiation oncologists, and of hospital beds; managed care penetration; average provider volume in market). * Denotes p<0.001; tech = technology.
Next, we assessed whether there was expansion of treatment among men who are least likely to benefit. Rates of observation were not significantly different comparing markets with high technological capacity to those with low technological capacity, both among men age 70 and older who were diagnosed with low-risk cancer (n=8,497, p≥0.494, Figure 3A) and among men 85 years and older (n=3,249, p≥0.226, Figure 3B).
Figure 3.
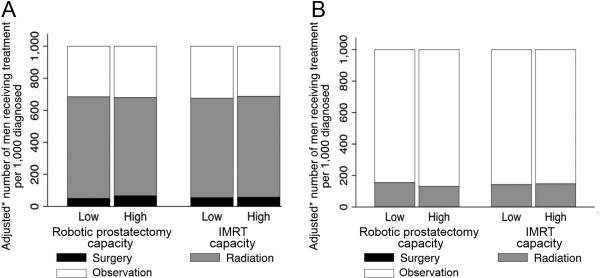
Rates of surgical treatment, radiotherapy, and observation according to market-level technological capacity among men who are least likely to benefit from active treatment (those 70 years and older with D'Amico low-risk disease20 [n=8,497, panel A] and those 85 years and older [n=3,249, panel B]). Robotic prostatectomy and IMRT capacity did not significantly impact rates of observation (p≥0.226). * Models were adjusted for year, patient characteristics (age, comorbidity, stage, grade, socioeconomic status), and market characteristics (number of urologists, of radiation oncologists, and of hospital beds; managed care penetration; average provider volume in market).
Finally, we examined the effect of increasing technological capacity within each HRR by fitting fixed effects models. Based on these models, a change from low to high robotic prostatectomy capacity was associated with an increase in use of prostatectomy (from 117 to 137 per 1,000 men diagnosed, p=0.031) and a trend towards decreased use of radiotherapy (from 602 to 580 per 1,000 men diagnosed, p=0.097), with rates of observation again remaining stable (281 versus 282 per 1,000 men diagnosed, p=0.915, Supplemental Digital Content 4).
Discussion
We found that treatment rates varied widely across HRRs. While the capacity for delivering IMRT was not associated with type of treatment, increased capacity for robotic prostatectomy was associated with greater use of prostatectomy and less use of radiotherapy. However, a greater capacity for delivering either new technology did not affect the rate of observation. The impact of technological capacity on prostate cancer treatment was much smaller than the effect of patient-level factors. Our results reflect some redistribution of treatment from radiotherapy to surgery after dissemination of robotic technology, rather than expansion of treatment among those who were managed with observation. There was also no evidence of expansion of treatment in patients who are least likely to benefit from aggressive treatment, including those 70 years of age and older with low-risk cancer and those aged 85 years and up.
While previous studies have taken a monolithic approach and evaluated either use of prostatectomy or radiotherapy, this study represents the first comprehensive assessment of technology dissemination and its effect on prostate cancer treatments. Regarding use of prostatectomy, it has been shown that adoption of robotic prostatectomy by a hospital or within a region was associated with increased rates of radical prostatectomy.26–28 Similarly, dissemination of IMRT was associated with a small increase in radiotherapy rates.29 However, none of these studies was focused on the important issue of treatment expansion and therefore did not include a population-level assessment of treatment among all patients with localized prostate cancer. We measured technological capacity for both robotic prostatectomy and IMRT and evaluated the distinct effects of these technologies on treatment selection. Thus, we were able to examine whether technology dissemination was associated with expansion of treatments (i.e., increased use of local therapy in lieu of observation) or with redistribution of treatments from one treatment to another. Based on our findings, a greater capacity for delivering treatment with new technology was not associated with increased overall treatment rates. Rather, increasing robotic prostatectomy capacity was associated with a small proportion of treatments being redistributed from radiotherapy to radical prostatectomy. However, patient-level covariates were more important for treatment selection, which appears appropriate. For example, older patients are significantly more likely to experience side-effects from prostatectomy and are increasingly less likely to die of prostate cancer, thus justifying less aggressive management.30 Similarly, men with more comorbid disease tend to have a higher risk of perioperative complications, which would discourage the use of radical prostatectomy.31
Robotic prostatectomy and IMRT promise decreased morbidity and increased cancer control, which may increase the palatability of treatment. Many worry that this would lead to expansion of treatments among those at low risk of dying from prostate cancer, who would be appropriate candidates for observation.32 However, our findings seem to temper these concerns. We evaluated the effect of technological capacity on the distribution of treatments among those 70 years of age and older with low-risk cancer or those age 85 and up. Low-risk prostate cancer is rarely lethal;23 some have even argued to remove the “cancer” label from this entity to decrease the urge for overtreatment.33 Men aged 85 and older are much more likely to die from causes other than localized prostate cancer, given their average life expectancy.24,25 Our results imply that technology dissemination did not affect treatment rates in these men. Nevertheless, a substantial number of these men were treated, which is in line with previous studies describing potential overuse of curative treatments in men with non-lethal prostate cancer.34–36 While the use of new technologies is rising both among all men with prostate cancer29,37 and among men unlikely to benefit from local therapy,36,38 new technology appears to substitute for the prior standard as opposed to increasing the population treated.
Ultimately, whether more use of new technology is beneficial depends, in part, on whether the benefits of new technology exceed the costs. While recent analyses have shown similar cost-effectiveness of open and robotic prostatectomy, IMRT was significantly more expensive than traditional three-dimensional radiotherapy without a meaningful gain in quality adjusted life years.39 In addition, surgical treatment tended to be more cost-effective than radiotherapy.39 As such, substitution of radiotherapy with surgery after dissemination of robotic technology may be associated with lower costs for payers, but substitution of traditional three-dimensional radiotherapy with IMRT is associated with higher costs. It is thus difficult to judge the extent to which these substitutions affect overall costs of treatment. However, our findings alleviate concerns that technology dissemination leads to expansion of prostate cancer treatments, which would clearly be associated with additional overall costs.
Our study has several limitations. First, it is possible that we did not capture all providers who use robotic prostatectomy and IMRT to treat men with prostate cancer in HRRs with sections that fall outside the SEER catchment areas. Therefore, we performed sensitivity analyses only including HRRs that were completely contained within SEER, with results not materially differing from those reported here. Second, our findings are only generalizable to men older than 65 given the limitations inherent to using Medicare data. However, older men are less likely to benefit from prostate cancer treatment and therefore are at higher risk for overtreatment.40 Thus, we are primarily concerned with this age group when examining the hypothesis that dissemination of new technology may lead to expansion of treatments among men with localized prostate cancer. Third, given the observational nature of our data, we cannot exclude the possibility of confounding secondary to unmeasured differences in patients across markets. To mitigate this problem as much as possible, we included many patient- and market-level covariates in our multivariable analyses and conducted additional analyses fitting fixed effects models. Finally, some may argue that the data from the time period examined (2003 to 2007) are rather old. However, we believe that this time period is highly relevant for several reasons. First, most of the dissemination of both robotic prostatectomy and IMRT had already happened by 2007.38 Thus, our data are representative of the effects of technology dissemination on treatment during the main period of dissemination. Second, we calculated treatment rates for typical HRRs with low or high technological capacity. While the absolute provider density may have increased in later years, it is unlikely that the relative categorization into low or high technological capacity would change significantly by including more recent data.
These limitations notwithstanding, our study suggests that new technology for the treatment of prostate cancer is not expanding the population treated, which has important implications for patients, payers, and policy makers. For patients, increased technological capacity is not driving additional treatment of disease and is thus not expanding the pool of patients at risk for side-effects from treatment. For payers and policymakers, who are considering coverage for other emerging treatments of prostate cancer, our findings alleviate concerns that technology dissemination may spur more prostate cancer treatment, which would be associated with higher costs.
Supplementary Material
Acknowledgments
Funding: FRS: T32 DK07782 from the NIH/NIDDK and Postdoctoral Fellowship PF-12-118-01-CPPB from the American Cancer Society. BLJ: Postdoctoral Fellowship 121805-PF-12-008-01-CPHPS from the American Cancer Society. SAS: Washington University Career Development Awards Program KL2 TR000450. BKH: RSGI-13-323-01-CPHPS from the American Cancer Society.
Appendix
Appendix.
Healthcare Common Procedure Coding System (HCPCS) and International Classification of Diseases, Ninth Revision, Clinical Modification (ICD9-CM) codes used in the analyses.
| Treatment received | HCPCS code | ICD9-CM code |
|---|---|---|
| Prostatectomy | ||
| Open prostatectomy | 55840, 55842, 55845 | 60.4 |
| Robotic prostatectomy | 55866 | not applicable |
| Other prostatectomy | 55810, 55812, 55815 | 60.5, 60.62 |
| Radiotherapy | ||
| External beam radiotherapy | 77373, 77401, 77402, 77403, 77404, 77406, 77407, 77408, 77409, 77411, 77412, 77413, 77414, 77416, 77417, 77419, 77420, 77421, 77425, 77427, 77430, 77431, 77435, 77470, 77499 | 92.21, 92.22, 92.24, V58.0 |
| IMRT | G0174, 77418, 0073T | not applicable |
| Brachytherapy | 55859, 55860, 55862, 55865, 55875, 76873, 76965, 77750, 77761, 77762, 77763, 77776, 77777, 77778, 77781, 77782, 77783, 77784, 77785, 77786, 77787, 77789, 77790, 77799, C1164, C1174, C1325, C1350, C1700, C1701, C1702, C1703 C1704, C1705, C1706, C1707, C1708, C1709, C1710, C1711, C1712, C1715, C1716, C1717, C1718, C1719, C1720, C1728, C1790, C1791, C1792, C1793, C1794, C1795, C1796, C1797 C1798, C1799, C1800, C1801, C1802, C1803, C1804, C1805, C1806, C2632, C2633, C2634, C2635, C2636, C2638, C2639, C2640, C2641, C2642, C2643, G0256, G0261, Q3001 | 92.20, 92.27 |
| Proton beam radiotherapy | 77380, 77381, 77520, 77522, 77523, 77525 | 92.33 |
| Observation | ||
| Androgen-deprivation therapy | C9430, J1675, J1950, J3315, J9202, J9217, J9218, J9219, J9225, J9226, Q2020, S0133, S9560, 11980, 11981, 11982, 11983, 96400, 96402, G0356, C9216, J0128, S0165, J0970, J1000, J1056, J1380, J1390, J1410, J9165, J9155, 54520, 54522, 54530, 54535 | 62.3, 62.4, 62.41, 62.42, 99.24 |
| No cancer directed therapy | not applicable | not applicable |
References
- 1.Newhouse JP. Medical care costs: how much welfare loss? J Econ Perspect. 1992;6:3–21. doi: 10.1257/jep.6.3.3. [DOI] [PubMed] [Google Scholar]
- 2.Maxwell S, Zuckerman S, Berenson RA. Use of Physicians’ Services under Medicare's Resource-Based Payments. N. Engl. J. Med. 2007;356:1853–1861. doi: 10.1056/NEJMsa063258. [DOI] [PubMed] [Google Scholar]
- 3.Medicare Payment Advisory Commission [March 12, 2013];March 2012 Report to the Congress: Medicare Payment Policy. 2012 Available at: http://medpac.gov/documents/Mar12_EntireReport.pdf.
- 4.Cutler DM, McClellan M. Is technological change in medicine worth it? Health Affairs. 2001;20:11–29. doi: 10.1377/hlthaff.20.5.11. [DOI] [PubMed] [Google Scholar]
- 5.Legorreta AP, Silber JH, Costantino GN, et al. Increased cholecystectomy rate after the introduction of laparoscopic cholecystectomy. JAMA. 1993;270:1429–1432. [PubMed] [Google Scholar]
- 6.Escarce JJ, Chen W, Schwartz JS. Falling cholecystectomy thresholds since the introduction of laparoscopic cholecystectomy. JAMA. 1995;273:1581–1585. [PubMed] [Google Scholar]
- 7.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J. Natl. Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stamatiou K, Alevizos A, Mariolis A, et al. Do clinically insignificant tumors of the prostate exist? Urol. Int. 2008;81:379–382. doi: 10.1159/000167832. [DOI] [PubMed] [Google Scholar]
- 9.Klotz L, Zhang L, Lam A, et al. Clinical Results of Long-Term Follow-Up of a Large, Active Surveillance Cohort With Localized Prostate Cancer. J. Clin. Oncol. 2010;28:126–131. doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 10.Staffurth J. A review of the clinical evidence for intensity-modulated radiotherapy. Clin Oncol (R Coll Radiol) 2010;22:643–657. doi: 10.1016/j.clon.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Ficarra V, Cavalleri S, Novara G, et al. Evidence from robot-assisted laparoscopic radical prostatectomy: a systematic review. Eur. Urol. 2007;51:45–56. doi: 10.1016/j.eururo.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs BL, Zhang Y, Skolarus TA, et al. Comparative Effectiveness of External-Beam Radiation Approaches for Prostate Cancer. [September 25, 2012];Eur. Urol. 2012 doi: 10.1016/j.eururo.2012.06.055. epub ahead of print. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22790288. [DOI] [PMC free article] [PubMed]
- 13.Scales CD, Jr, Jones PJ, Eisenstein EL, et al. Local cost structures and the economics of robot assisted radical prostatectomy. J. Urol. 2005;174:2323–2329. doi: 10.1097/01.ju.0000181830.43340.e7. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen PL, Gu X, Lipsitz SR, et al. Cost Implications of the Rapid Adoption of Newer Technologies for Treating Prostate Cancer. J. Clin. Oncol. 2011;29:1517–1524. doi: 10.1200/JCO.2010.31.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. Journal of Clinical Epidemiology. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 16.Schroeck FR, Kaufman SR, Jacobs BL, et al. Technology diffusion and diagnostic testing for prostate cancer. J. Urol. 2013 doi: 10.1016/j.juro.2013.05.007. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Dartmouth Institute for Health Policy & Clinical Practice. [April 7, 2012];Glossary - Dartmouth Atlas of Health Care. 2012 Available at: http://www.dartmouthatlas.org/tools/glossary.aspx.
- 18.Pollack CE, Weissman G, Bekelman J, et al. Physician social networks and variation in prostate cancer treatment in three cities. Health Serv Res. 2012;47:380–403. doi: 10.1111/j.1475-6773.2011.01331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.US Census Bureau [March 27, 2013];ZIP Code Tabulation Areas. Available at: http://www.census.gov/geo/ZCTA/zcta.html.
- 20.D'Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 21.Williams RL. A Note on Robust Variance Estimation for Cluster-Correlated Data. Biometrics. 2000;56:645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 22.Vittinghoff E, Glidden DW, Shiboski SC, et al. Regression Methods in Biostatistics. 2nd ed. Springer US; New York: 2012. Chapter 10: Predictor Selection. pp. 395–429. [Google Scholar]
- 23.Lu-Yao GL, Albertsen PC, Moore DF, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009;302:1202–1209. doi: 10.1001/jama.2009.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walter LC, Bertenthal D, Lindquist K, et al. PSA screening among elderly men with limited life expectancies. JAMA. 2006;296:2336–2342. doi: 10.1001/jama.296.19.2336. [DOI] [PubMed] [Google Scholar]
- 25.So C, Kirby KA, Mehta K, et al. Medical center characteristics associated with PSA screening in elderly veterans with limited life expectancy. J Gen Intern Med. 2012;27:653–660. doi: 10.1007/s11606-011-1945-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makarov DV, Yu JB, Desai RA, et al. The Association Between Diffusion of the Surgical Robot and Radical Prostatectomy Rates. Med Care. 2011;49:333–339. doi: 10.1097/MLR.0b013e318202adb9. [DOI] [PubMed] [Google Scholar]
- 27.Neuner JM, See WA, Pezzin LE, et al. The association of robotic surgical technology and hospital prostatectomy volumes. Cancer. 2012;118:371–377. doi: 10.1002/cncr.26271. [DOI] [PubMed] [Google Scholar]
- 28.Stitzenberg KB, Wong Y, Nielsen ME, et al. Trends in radical prostatectomy: centralization, robotics, and access to urologic cancer care. Cancer. 2012;118:54–62. doi: 10.1002/cncr.26274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobs BL, Zhang Y, Skolarus TA, et al. Growth Of High-Cost Intensity-Modulated Radiotherapy For Prostate Cancer Raises Concerns About Overuse. Health Affairs. 2012;31:750–759. doi: 10.1377/hlthaff.2011.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N. Engl. J. Med. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 31.Brooks MJ, Sutton R, Sarin S. Comparison of Surgical Risk Score, POSSUM and p-POSSUM in higher-risk surgical patients. Br. J. Surg. 2005;92:1288–1292. doi: 10.1002/bjs.5058. [DOI] [PubMed] [Google Scholar]
- 32.Zietman A, Goitein M, Tepper JE. Technology evolution: is it survival of the fittest? J. Clin. Oncol. 2010;28:4275–4279. doi: 10.1200/JCO.2010.29.4645. [DOI] [PubMed] [Google Scholar]
- 33.Carter HB, Partin AW, Walsh PC, et al. Gleason Score 6 Adenocarcinoma: Should It Be Labeled As Cancer? J. Clin. Oncol. 2012;30:4294–4296. doi: 10.1200/JCO.2012.44.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller DC, Gruber SB, Hollenbeck BK, et al. Incidence of initial local therapy among men with lower-risk prostate cancer in the United States. J. Natl. Cancer Inst. 2006;98:1134–1141. doi: 10.1093/jnci/djj308. [DOI] [PubMed] [Google Scholar]
- 35.Etzioni R, Mucci LA, Chen S, et al. Increasing use of radical prostatectomy for non-lethal prostate cancer in Sweden. Clin. Cancer Res. 2012;18:6742–6747. doi: 10.1158/1078-0432.CCR-12-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carreyrou J, Tamman M. A Device to Kill Cancer, Lift Revenue. [January 4, 2013];Wall Street Journal. 2010 Available at: http://online.wsj.com/article/SB10001424052748703904804575631222900534954.html.
- 37.Barbash GI, Glied SA. New Technology and Health Care Costs–The Case of Robot-Assisted Surgery. N Engl J Med. 2010;363:701–704. doi: 10.1056/NEJMp1006602. [DOI] [PubMed] [Google Scholar]
- 38.Jacobs BL, Zhang Y, Schroeck FR, et al. Use of Advanced Treatment Technologies among Men at Low Risk of Dying from Prostate Cancer. JAMA. 2013;309:2587–2595. doi: 10.1001/jama.2013.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooperberg MR, Ramakrishna NR, Duff SB, et al. Primary treatments for clinically localised prostate cancer: a comprehensive lifetime cost-utility analysis. BJU International. 2013;111:437–450. doi: 10.1111/j.1464-410X.2012.11597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooperberg MR, Lubeck DP, Meng MV, et al. The Changing Face of Low-Risk Prostate Cancer: Trends in Clinical Presentation and Primary Management. J. Clin. Oncol. 2004;22:2141–2149. doi: 10.1200/JCO.2004.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


