Abstract
Background
Techniques for achieving hemispheric disconnection in patients with epilepsy continue to evolve.
Objective
To review the outcomes of the first 50 hemispherectomy surgeries performed by a single surgeon with an emphasis on outcomes, complications, and how these results led to changes in practice.
Methods
The first 50 hemispherectomy cases performed by the lead author were identified from a prospectively maintained database. Patient demographics, surgical details, clinical outcomes, and complications were critically reviewed.
Results
From 2004 to 2012, 50 patients underwent hemispherectomy surgery (mean follow-up time 3.5 years). Modified lateral hemispherotomy (MLH) became the preferred technique and was performed on 44 patients. Forty patients (80%) achieved complete seizure freedom (Engel I). Pre- and post-surgical neuropsychological evaluations demonstrated cognitive stability. Two cases were performed for palliation only. Previous hemispherectomy surgery was associated with worsened seizure outcome (2 of 6 seizure-free, P = .005). The use of Avitene was associated with a higher incidence of postoperative hydrocephalus (56% v. 18%, P = .03). In MLH patients without the use of Avitene, the incidence of hydrocephalus was 13%. Complications included: infection (3), incomplete disconnection requiring reoperation (1), reversible ischemic neurologic deficit (1), and craniosynostosis (1). There were no (unanticipated) permanent neurological deficits or deaths. Minor technique modifications were made in response to specific complications.
Conclusion
The modified lateral hemispherotomy is effective and safe for both initial and revision hemispherectomy surgery. Avitene use appears to result in a greater incidence of postoperative hydrocephalus.
Keywords: Hemispherectomy, modified lateral hemispherotomy, epilepsy, hydrocephalus, outcomes, complications
INTRODUCTION
In 1938, the Canadian neurosurgeon KG McKenzie reported results from the first hemispherectomy performed for epilepsy1. The first series of patients was reported by Krynauw in 1950, who described 12 children with infantile hemiplegia who underwent anatomic hemispherectomies for epilepsy and/or “mental changes”2. In this series, all surviving patients with epilepsy demonstrated seizure freedom (there was one unexplained immediate postoperative death). Though effective for treating seizures, early hemispherectomy surgery was associated with significant morbidity and mortality2–8. Over time it became clear that a substantial number of patients were suffering long-term problems related to repeated hemorrhages into the large resection cavities and subsequent superficial cerebral hemosiderosis3, 4, 6, 9. By 1968, Rasmussen began to leave up to one-third of the epileptogenic hemisphere intact which effectively eliminated superficial hemosiderosis at the cost of decreased seizure efficacy6, 7. This evolved into Rasmussen’s initial description of functional hemispherectomy involving a combination of resection and disconnection of the diseased hemisphere in 19837.
Since that time, the evolutionary line for hemispherectomy surgery has branched considerably. Experienced epilepsy centers now perform a wide variety of techniques, including: traditional anatomic hemispherectomies, Rasmussen-style functional hemispherectomies, peri-Sylvian hemispherotomies, and hemicorticectomies, each with their own variations, twists, pros and cons. There is no clear optimal technique and comparing outcomes between techniques is fraught with difficulties due to variations in patient selection, surgical experience, and outcome metrics. Nevertheless, every published series adds to the common knowledge and moves the field forward. The purpose of this study is to share the results from the first 50 consecutive hemispherectomies from a single surgeon with an emphasis on outcomes, predictors of seizure control, postoperative hydrocephalus, complications, and how these results led to practice modifications.
METHODS
Study design
An institutional review board-approved review was undertaken of the first 50 consecutive pediatric hemispherectomy surgeries performed by the lead author (SML) between September 2004 and May 2012. For the first year of this time period, surgeries were performed with the senior author (WMM) as a co-surgeon. A prospectively maintained database was utilized, as were individual chart reviews for additional data collection. Preoperative data included general demographics, seizure history, etiology of epilepsy, prior surgical history, magnetic resonance imaging (MRI) findings, electroencephalography (EEG) findings, neuropsychological evaluations, and goals of surgery (palliative versus seizure freedom). Data was collected regarding surgical technique, time, and blood loss for each procedure. Follow-up data were available for all patients and included seizure outcome, neuropsychological evaluations, histology results, and complications. The follow-up time period and seizure outcome were determined from the last clinical encounter.
Patient selection
The pre-operative workups were tailored to each patient. All patients underwent video-EEG monitoring and MRI. Non-infant patients had pre-operative neuropsychological evaluations. Other diagnostic studies performed in some but not all patients included: single-photon emission computed tomography (SPECT), positron emission tomography (PET), magnetoencephalography (MEG), functional MRI (fMRI), and Wada tests. All cases were formally reviewed at a multidisciplinary epilepsy conference prior to offering hemispherectomy surgery. Two patients were identified pre-operatively as palliative cases; for the remaining 48 patients the goal of surgery was seizure freedom. One patient with cortical dysplasia underwent placement of intracranial electrodes prior to her resection while the remainder of the patients had single stage procedures. One other patient with cortical dysplasia underwent a posterior quadrantectomy followed by intraoperative electrocorticography (ECoG) which prompted conversion to a hemispherectomy (all done as a single surgical procedure). This was the only case which utilized intraoperative ECoG. Of note, one patient (who had not had prior epilepsy surgery) underwent a modified lateral hemispherotomy (MLH) that was deemed incomplete on her postoperative imaging (see Case Illustration #2) and during the same hospitalization returned to surgery to complete the procedure. This patient’s procedure was categorized as not having prior epilepsy surgery for purposes of outcome analysis despite the two procedures.
Neuropsychological evaluations
In order to characterize cognitive outcomes related to hemispherectomy, presurgical and postsurgical neuropsychological evaluations were planned for each patient. When a patient’s level of functioning allowed, a full battery of neuropsychological measures assessing general intellectual abilities, fine motor skills, attention, executive functioning, language skills, visual-spatial skills, memory, academic achievement and adaptive functioning were administered. A measure of general intellectual abilities was administered to all patients within this sample. Due to the variability of functioning and age within the patient population, a number of different measures were used to assess each patient’s overall level of cognitive functioning, Measures used included a version of the Wechsler Intelligence Scale for Children (WISC-III or WISC-IV)10, 11, the Wechsler Adult Intelligence Scale (WAIS-III)12, a version of the Differential Abilities Scale (DAS or DAS-II)13, Mullen Early Learning Scales14, or the Stanford-Binet15. Each of these measures provides an overall score consistent with the Full Scale Intelligence Quotient (FSIQ) calculated on Wechsler measures. Adaptive behavioral functioning was measured using the structured interview format of the Vineland Adaptive Behavior Scales (VABS)16, which also provides an overall composite score. The resulting composite scores for both the FSIQ and adaptive behavior measures is a standard score with a mean of 100 and a standard deviation of 15.
Surgical Techniques
The first five surgeries in this series were either Rasmussen-style hemispherectomies7, 17 or anatomic hemispherectomies2, 8. Due to a general dissatisfaction with these techniques, the modified lateral hemispherotomy was adopted as the technique of choice. Aside from a single additional anatomic procedure performed for a revision hemispherectomy, all subsequent hemispherectomies, including 3 revision hemispherectomies, were done with the MLH technique. This technique was initially described by Cook et al in 200418 and is derivative of the peri-insular hemispherotomy techniques described by Schramm19, 20 and Villemeure21 in the 1990s. The features that distinguish this technique from peri-insular hemispherotomy techniques are: a) sacrifice of the middle cerebral artery (MCA) to facilitate hemostasis, thus limiting blood loss, b) resection of a central opercular block of tissue to provide generous exposure of the ventricular system and removal of the insula with portions of the basal ganglia and thalamus, and c) resection of the anterior temporal lobe. At our institution, minor modifications have been made to the technique initially described by Cook et al18. The osteoplastic craniotomy is considerably smaller, with the superior extent at the superior temporal line rather than the mid-pupillary line. We have found that we do not require visualization of anything outside of the ventricular system. We forgo the orbitofrontal cortex resection that was performed as part of the deep frontal disconnection. The frontal disconnection is now made using the proximal anterior cerebral artery (visible through the arachnoid at the base of the genu of the corpus callosum) as a landmark to create a disconnection sufficiently posterior to avoid leaving connected mesial basal posterior frontal tissue (Figure 1). A complete detailed description of the procedure is included in Supplemental Content 1. The same principles of this technique were applied to patients with previous epilepsy surgery, including 3 patients with previous hemispherectomies performed elsewhere. In these cases, preoperative imaging was scrutinized for evidence of retained connected ipsilateral brain which was then targeted for disconnection. Frameless stereotactic navigation is not routinely used, but is reserved for patients with a history of prior surgery affecting the normal anatomical landmarks or distorted baseline ventricular anatomy.
Figure 1.
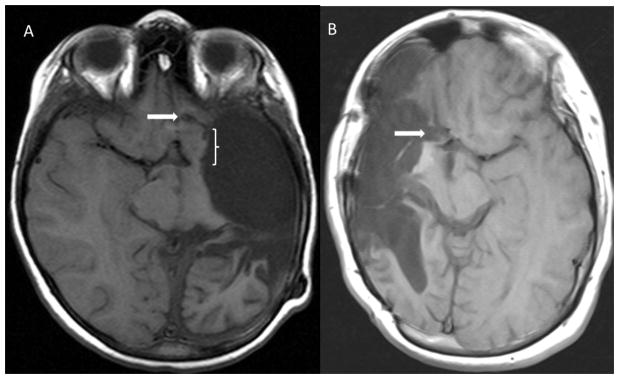
Postoperative axial T1-weighted MR images demonstrating frontal disconnections (white arrows) from the first MLH procedure performed (A) and one performed later in the series (B) utilizing a trough extending to the pia-arachnoid overlying the ipsilateral ACA. Note the significant residual basal frontal tissue (bracketed) left connected in (A).
Statistical analysis
Associations between categorical variables were made using Fisher’s test, exact Pearson chi-square test, or exact Mantel-Hanszel chi-square tests, and the Jonchheere-Terpstra test for ordinal-by-continuous comparisons (Engel score and age/time). Comparisons of continuous variables were made using Wilcoxon Rank-sum test (some exact), Spearman correlation, or paired t-tests for change-in FSIQ scores. Multivariate logistic regression was performed to examine the simultaneous effect of variables on seizure outcome. For this analysis on dichotomized Engel score (I vs. II, III, or IV), two palliative cases were excluded, (as these surgeries were already expected to have poor seizure outcome). For the examination of risk factors related to the development of hydrocephalus, the 7 patients with pre-existing hydrocephalus were excluded. A P < .05 was prospectively determined to indicate a significant difference. Standard deviations were presented with means as “mean ± SD” unless otherwise specified. The analysis was performed using SAS version 9.3 (The SAS Institute, Cary, NC).
RESULTS
General
Etiologies of epilepsy, additional diagnoses, and previous epilepsy surgeries are listed in Table 1. The etiology of epilepsy of the one patient listed as “Tumor/radiation therapy” requires some explanation. This patient underwent resection of a large left hemisphere atypical teratoid/rhabdoid tumor (ATRT) at the age of 13 months. He underwent a second resection coupled with intracavitary radiation shortly thereafter. At the age of 2 years he developed medically refractory epilepsy and he underwent hemispherectomy surgery at the age of 3 years.
Table 1.
Demographics (n=50)
| Mean ± SD (range) | |
|---|---|
| Age at seizure onset | 2.1 ± 2.7 yrs (3 days – 13.8 yrs) |
| Age at surgery | 9.1 ± 5.3 yrs (2.5 mos – 21.2 yrs) |
| Duration of seizures prior to surgery | 7.0 ± 5.0 yrs (1 mo – 20.2 yrs) |
| Length of follow-up | 3.5 ± 2.1 yrs (3 mos – 7.8 yrs) |
| # (%) of patients | |
| Side of hemispherectomy | |
| Left | 32 (64%) |
| Right | 18 (36%) |
| Etiologies of Epilepsy | |
| Infarct | 25 (50%) |
| Malformations of cortical development | 13 (26%) |
| Rasmussen’s encephalitis | 7 (14%) |
| Sturge-Weber | 2 (4%) |
| Trauma | 1 (2%) |
| Hemiconvulsion-hemiplegia-epilepsy syndrome | 1 (2%) |
| Tumor/radiation therapy | 1 (2%) |
| Additional diagnoses | |
| Pre-existing hemiplegia | 28 (56%) |
| Pre-existing hydrocephalus | 7 (14%) |
| Prior resective surgery | |
| None | 37 (74%) |
| Non-hemispherectomy resection | 7 (14%) |
| Previous hemispherectomy | 6 (12%) |
| Goal of surgery | |
| Seizure freedom | 48 (96%) |
| Palliation | 2 (4%) |
The mean age at time of surgery was 9.1 years (2.5 months – 16.9 years). The mean age at onset of seizures was 2.1 years (3 days – 21.2 years), with a mean duration of seizures prior to surgery of 7.0 years (7 months – 20.2 years). The mean operative time was 260 min (range 126–498 min) with a mean estimated blood loss (EBL) of 340 mL (range 25–1000 mL). There was a trend for decreased blood loss and operative time with experience. For the last 10 procedures (all MLH technique), the mean operative time was 208 min (166 – 272 min) with a mean EBL of 218 mL (50–650 mL). The mean follow-up period is 3.5 years (3 months – 7.5 years).
Palliative cases
Forty (80%) of the 50 patients achieved seizure freedom (Engel I). Two patients in this series were identified preoperatively as candidates for hemispherectomy for palliation only, without a chance of complete seizure freedom. Their families were counseled as such. Both patients had pre-existing motor deficits, catastrophic epilepsy, and seizures predominantly (but not solely) arising from a single hemisphere. The first patient was a 10-year-old male with a history of infantile spasms, Lennox-Gaustaut syndrome, microcephaly, cortical visual loss, spastic quadraparesis, status post vagal nerve stimulator placement at the age of 22 months, and a complete corpus callosotomy at 8 years of age. At the time of surgery he was on felbamate, valproic acid, and clorazepate, with over 25 tonic seizures per day. Semiology for most of his seizures post-callosotomy involved tonic extension of the right upper extremity first. His workup demonstrated generalized ictal discharges, with >90% of interictal discharges from the left hemisphere. A left MLH was offered and performed. Histology revealed widespread diffuse dysplasia consistent with Palmini IIA and IIB changes but not focal. Hippocampal sclerosis was also present. At last follow-up (9 months post-op), he was having 1–3 seizures/day (Engel IIIA).
The second palliative case involved a 5-year-old male born premature at 33 weeks, with a left MCA perinatal stroke. Long-term video-EEG monitoring revealed predominantly left-sided seizures but clear independent right-onset seizures as well. He also demonstrated severe developmental delays (non-verbal and non-ambulatory) that suggested right hemispheric dysfunction. He was averaging 5 seizures/day on valproic acid, levetiracetam, clonazepam, lacosamide, and felbamate. A left MLH was performed for palliation. He had several generalized seizures in the first month after surgery. At his most recent follow-up, 12 months after surgery, he was having only rare simple partial seizures (Engel IIC), on 3 anti-epileptic medications.
Non-palliative cases
Of the remaining 48 non-palliative cases, 40 (83%) resulted in seizure freedom (Engel I). An additional 4 (8%) patients achieved an Engel II outcome, while 4 (8%) patients continue to have a significant seizure burden (3 Engel III, 1 Engel IV). Multiple variables were tested as possible determinants of seizure outcome. Age and duration of seizures did not correlate with seizure outcome (Table 2). Preoperative MRI findings can be found in Table 3. All reported findings are for MRIs that preceded any cranial surgeries. Presurgical imaging was not available for one patient who underwent a revision MRI after prior surgery elsewhere. The majority of patients had diffuse MRI abnormalities involving the affected side. However, none of the MRI findings were found to correlate with seizure outcome (Table 3). Pre-operative EEG findings, seizure semiology and their relation to seizure outcome are presented in Table 4. While there were no correlations that reached statistical significance, the presence of bilateral synchrony trended towards significance as a predictor of worsened seizure outcome (P = .07).
Table 2.
Seizure outcome relative to age and duration of epilepsy in non-palliative cases (n=48).
| All | Engel | P* | ||||
|---|---|---|---|---|---|---|
| I (n=40) | II (n=4) | III (n=3) | IV (n=1) | |||
| Age at seizure onset (yrs, mean ± SD) | 2.1 ± 2.8 | 2.1 ± 2.9 | 2.7 ± 2.3 | 1.6 ± 2.5 | 2.0 | .71 |
| Age at hemispherectomy (yrs, mean ± SD) | 9.1 ± 5.3 | 8.8 ± 5.2 | 10.4 ± 6.6 | 9.1 ± 6.8 | 15.5 | .26 |
| Duration of seizures prior to surgery (yrs, mean ± SD) | 7.0 ± 5.1 | 6.7 ± 5.2 | 7.8 ± 4.7 | 7.5 ± 6.0 | 13.5 | .31 |
Jonckheere-Terpstra test; logistic regression was also used to test this association for collapsed Engel scores (I vs. II, III, IV) and this was also non-significant (data not shown).
Table 3.
Presurgical MRI findings and seizure outcome in non-palliative cases (n=47*).
| n | Engel | P** | ||||
|---|---|---|---|---|---|---|
| I (%) | II (%) | III (%) | IV (%) | |||
| Total | 47 | 39 (83) | 4 (9) | 3 (6) | 1 (2) | |
| Presence of lesion(s) | .32 | |||||
| No lesion | 4 | 2 (50) | 1 (25) | 1 (25) | 0 | |
| Suspected/possible/non-specific | 1 | 1 (100) | 0 | 0 | 0 | |
| Definite lesion(s) | 42 | 36 (86) | 3 (7) | 2 (5) | 1 (2) | |
| Extent of definite lesion(s) | .84 | |||||
| Solitary/discrete | 3 | 3 (100) | 0 | 0 | 0 | |
| Diffuse/multilobar/hemispheric/multiple | 39 | 33 (85) | 3 (8) | 2 (5) | 1 (3) | |
| Laterality of definite lesion(s) | .68 | |||||
| Left | 25 | 21 (84) | 1 (4) | 2 (8) | 1 (4) | |
| Right | 15 | 13 (87) | 2 (13) | 0 | 0 | |
| Bilateral | 2 | 2 (100) | 0 | 0 | 0 | |
| Lesion type (vs. all other types) | ||||||
| Dysplasia | 7 | 7 (100) | 0 | 0 | 0 | .42 |
| Atrophic/cystic/gliotic | 32 | 28 (88) | 1 (3) | 2 (6) | 1 (3) | .83 |
| Hippocampal sclerosis | 3 | 3 (100) | 0 | 0 | 0 | .82 |
| Signal abnormality NOS | 3 | 1 (33) | 2 (67) | 0 | 0 | .25 |
All MRI findings refer to MRIs prior to any resective surgery. One patient with prior surgery at an outside institution did not have such an MRI available for review.
Mantel-Haenszel chi-square test
Table 4.
Presurgical EEG features, seizure semiology, and seizure outcome in non-palliative cases (n=48*).
| n | Engel | P* | |||||
|---|---|---|---|---|---|---|---|
| I (%) | II (%) | III (%) | IV (%) | ||||
| Total | 48 | 40 (83) | 4 (8) | 3 (6) | 1 (2) | ||
| EEG background symmetry | .48 | ||||||
| Symmetric | 6 | 6 (100) | 0 | 0 | 0 | ||
| Asymmetric | 42 | 34 (81) | 4 (10) | 3 (7) | 1 (2) | ||
| EEG background abnormality | .51 | ||||||
| Abnormal unilaterally | 34 | 28 (82) | 2 (6) | 3 (9) | 1 (3) | ||
| Abnormal bilaterally | 13 | 11 (85) | 2 (15) | 0 | 0 | ||
| Interictal epileptiform EEG activity | .17 | ||||||
| Unifocal | 14 | 12 | 2 | 0 | 0 | ||
| Multifocal, unilateral | 20 | 18 | 1 | 1 | 0 | ||
| Multifocal, bilateral | 14 | 11 | 1 | 1 | 1 | ||
| Bilateral synchrony | 12 | 8 | 2 | 1 | 1 | .07 | |
| Ictal EEG onset | .79 | ||||||
| Unilateral | 35 | 28 | 4 | 3 | 0 | ||
| Bilateral | 8 | 7 | 0 | 0 | 1 | ||
| Not captured | 5 | 5 | 0 | 0 | 0 | ||
| Seizure semiology (vs. all others) | |||||||
| Simple partial | 33 | 28 | 2 | 2 | 1 | 1.0 | |
| Complex partial | 39 | 33 | 3 | 3 | 0 | .42 | |
| Generalized | 15 | 13 | 1 | 0 | 1 | 1.0 | |
Mantel-Haenszel chi-square test
The relationships between etiology of epilepsy, prior surgical history, hemispherectomy technique and outcome are demonstrated in Table 5. There was no significant correlation detected between etiology and outcome (P = .67) with infarct (50%) and malformations of cortical development (MCD, 25%) comprising the majority. Within the MCD group, 2 patients had hemimegalencephaly, both achieving Engel I outcomes.
Table 5.
Seizure outcome for non-palliative cases (n=48), stratified by etiology, surgical history, and hemispherectomy technique.
| n | Engel | P* | ||||
|---|---|---|---|---|---|---|
| I (%) | II (%) | III (%) | IV (%) | |||
| Total | 48 | 40 (83) | 4 (8) | 3 (6) | 1 (2) | |
| Etiology | .67 | |||||
| Infarct | 24 | 22 (92) | 1 (8) | 1 (4) | ||
| MCD | 12 | 10 (83) | 1 (8) | 1 (8) | ||
| Rasmussen’s | 7 | 4 (57) | 2 (14) | 1 (14) | ||
| Sturge-Weber | 2 | 2 (100) | ||||
| Trauma | 1 | 1 (100) | ||||
| HHE syndrome | 1 | 1 (100) | ||||
| Tumor/radiation | 1 | 1 (100) | ||||
| Prior resective surgery | .005 | |||||
| None | 36 | 33 (92) | 1 (3) | 2 (6) | ||
| Non-hemispherectomy resection | 6 | 5 (83) | 1 (17) | |||
| Previous hemispherectomy | 6 | 2 (33) | 2 (33) | 1 (17) | 1 (17) | |
| Pair-wise comparisons | Previous hemispherectomy vs. None Previous hemispherectomy vs. Non-hemi resect/disconnect Non-hemi resection vs. None |
.006 .15 1.0 |
||||
| Hemispherectomy technique | .005 | |||||
| MLH | 42 | 37 (88) | 3 (7) | 2 (5) | ||
| Rasmussen’s functional | 2 | 2 (100) | ||||
| Anatomic | 4 | 1 (25) | 1 (25) | 1 (25) | 1 (25) | |
| Pair-wise comparisons | Anatomic vs. MLH Anatomic vs. Rasmussen’s functional Rasmussen’s functional vs. MLH |
.004 .33 1.0 |
||||
Exact Mantel-Haenszel test, exact test for pairwise comparisons
Twelve of the non-palliative cases had prior cranial surgery. Six of these were prior hemispherectomy procedures performed by other surgeons. The first three revision hemispherectomies were performed as anatomic hemispherectomies (Engel outcomes: II, III, IV), while the last 3 were done using the MLH technique with disconnections primarily of suspected connected tissue (Engel outcomes: I, I, II). Prior resective surgery was associated with worsened outcome (P = .005, Table 5). When pairwise comparisons were performed, this indicated that the difference was likely related to the previous hemispherectomy group (previous hemispherectomy vs. none, P = .006; non-hemispherectomy resection vs. none, P = 1.0, Table 5). However, a clear significant difference could not be shown between the non-hemispherectomy resection group and the previous hemispherectomy group (P = .15, Table 5).
Hemispherectomy technique was a significant predictor of outcome with anatomic hemispherectomies showing worsened outcome (P = .005, Table 5). However, 3 of the 4 anatomic hemispherectomies performed were revision procedures on patients with previous hemispherectomies. A multivariate analysis was performed which showed that neither bilateral synchrony (P = .11) nor hemispherectomy technique (P = .22) were significant predictors when controlling for previous resective surgery. Previous resective surgery was the sole predictor of seizure outcome in the multivariate analysis.
Unfavorable seizure outcomes
Eight of the 48 patients targeted for seizure freedom had persistent seizures (4 Engel II, 3 Engel III, 1 Engel IV). Four of these patients had previous hemispherectomies (2 Engel II, 1 Engel III, 1 Engel IV). Two of the remaining 4 had MCD. The first was a 13-month-old female status post prior left temporal and parieto-occipital resections who achieved a Class IIC outcome on 2 antiepileptic medications (follow-up 5.0 years). The second patient was a 15-month-old boy with infantile spasms beginning at 4 months of age. He developed medically refractory complex partial right-sided seizures and a mild left hemiparesis. His brain MRI was normal but PET imaging showed diminished signal in the right frontal and temporal lobes. He underwent a right MLH. Histology showed focal cortical dysplasia (FCD 1A). Postoperatively he began to demonstrate left-sided seizures on EEG in addition to generalized events, although with markedly reduced frequency (follow-up 1.5 years, Engel IIIA). The 2 remaining patients had infarcts as the etiology of their epilepsy. The first patient was an 11-year-old female with a congenital large right porencephalic cyst, shunted hydrocephalus, cortical blindness, spastic diplegia and left hemiparesis. Seizure onset was at 16 months of age. Her preoperative video-EEG showed right-sided seizure onset but bilateral synchrony. She underwent a right MLH with a subsequent Engel IID outcome (nocturnal seizures only). Her postoperative MRI showed completed disconnections. A video-EEG study 6 years after surgery documented left-sided seizure onset. The second patient was a 12-year-old male who suffered an anoxic/hypotensive injury associated with a home birth with a left MCA infarct. His preoperative video-EEG showed clinical symptoms which preceded bihemispheric slowing leading to right greater than left bursts of 9–12 Hz activity. The activity would become more generalized over the right hemisphere and then engage the left. His right hemisphere appeared normal on MRI and PET. He underwent a left MLH which resulted in improved but not absolute seizure control (follow-up 6 months, Engel IIIA).
Cognitive evaluations and outcomes
Preoperatively, forty-six children (92%) underwent presurgical neuropsychological evaluations at a mean age of 8.3 ± 4.9 years). Of the 46 patients tested, 22 (48%) were able to complete more comprehensive neuropsychological evaluations. Three patients were too low functioning for calculation of a FSIQ, with an estimated level of cognitive functioning at approximately a 2-year age equivalency level. The mean preoperative FSIQ for the remaining 43 patients was 59.3 ± 11.7, which is in the mild range of mental retardation or intellectual disability (ID). However, there was significant variability in levels of functioning with a range from 36 (profound ID) to 83 (low average). Of those who completed a VABS (n=28), the mean composite score was 58.2 ± 17.1, which also is within the mild range of intellectual disability (range = 24 to 84). Twenty-four patients (52%) demonstrated generalized cognitive dysfunction; however, the subset of the patients (n = 22, 48%) who were relatively higher functioning demonstrated a cognitive profile suggestive of lateralized cognitive dysfunction. That is, the pattern of strengths and weaknesses observed on testing indicated greater impairment in cognitive abilities that are typically attributed to the dominant/usually left (i.e., language skills) or nondominant/usually right (i.e., visual-spatial or nonverbal skills) hemispheres. This pattern of lateralized dysfunction was generally consistent with the side of seizure focus and planned surgery: those with greater dysfunction lateralized to the right hemisphere underwent right hemispherectomies (10/10, 100%) and those with greater left-sided dysfunction underwent left hemispherectomies (11/12, 92%). Concordant cognitive lateralization did not correlate with seizure outcome (Spearman correlation, p = .47). It is important to note, however, that a significantly greater proportion of those with greater left-hemisphere dysfunction were also presumed to have undergone functional reorganization based on their neuropsychological profiles (90.9% vs. 0%, Fisher’s exact test p < .001). Functional reorganization is presumed to have taken place if an individual demonstrates intact or stronger functioning within cognitive domains that typically are lateralized to the hemisphere of known greater dysfunction.
Twenty-nine patients underwent a post-surgical evaluation. One patient was too low functioning for calculation of an FSIQ, with an estimated level of cognitive functioning at approximately a 2-year age equivalency level. The mean FSIQ for the remaining 28 patients was 62.7 ± 15.4, which again was within the mild range of ID. There was significant variability with a range from 42 (severe ID) to 97 (average). Of those who completed a VABS (n = 14), the mean composite score was 62.7 ± 17.7, which also is within the mild range of ID (range 27 to 91).
Twenty-seven patients completed both the pre- and post-surgical evaluations (Table 6). For this subset, mean FSIQ was 63.9 ± 9.9 before hemispherectomy, and 61.4 ± 14.1 after hemispherectomy. On an individual patient basis, 21 (78%) patients demonstrated relative stability of level of cognitive functioning (post-operative performance within 1 SD of pre-operative), 1 (4%) demonstrated a significant gain (> 1 SD), and 5 (19%) demonstrated a significant decline (> 1 SD) (For individual pre- and post-surgical changes, please see Figure, Supplemental Content 2). However, group level analysis revealed that the difference between the pre- and post-surgical FSIQ was not significant (p = .27). No preoperative variables were identified that correlated significantly with change in IQ after surgery (Table 6).
Table 6.
Preoperative and postoperative IQ in patients who underwent both pre- and postoperative testing (n=27), and the correlation between preoperative variables and change in FSIQ.
| Preoperative FSIQ | Postoperative FSIQ | P* |
|---|---|---|
| 63.9 ± 9.9 | 61.4 ± 14.1 | .27 |
| Preoperative variable vs. ΔFSIQ | ||
| Preoperative FSIQ | .82 | |
| Age at seizure onset | .40 | |
| Age at hemispherectomy | .10 | |
| Duration of seizures | .19 | |
| Side of hemispherectomy | .80 | |
FSIQ displayed as mean ± SD; paired samples t-test for group-level comparison of FSIQ, Spearman correlation for continuous variables, Wilcoxon rank-sum test for categorical variable (side of hemispherectomy)
Postoperative hydrocephalus
Seven of the 50 hemispherectomy patients had pre-existing hydrocephalus. Of the remaining 43, 11 (26%) developed hydrocephalus and underwent subsequent shunt placement. Several variables were analyzed as possible factors contributing to hydrocephalus. Variables analyzed included etiology of epilepsy, prior surgery, surgical technique, surgical time, use of Avitene (microfibrillar collagen hemostat, Davol, Warwick, RI) as a hemostatic agent, and cerebrospinal fluid profiles (cell counts and protein levels) sampled from external ventricular drains in the first 2 days following surgery (Table 7). The use of Avitene was the only significant predictor of future hydrocephalus, with an odds ratio of 5.8 (95% CI 1.2 – 28.4, P = .03). Hydrocephalus occurred in 56% (5/9) of the cases when Avitene was used, and 18% (6/34) of the cases without Avitene. Within the MLH cohort, hydrocephalus occurred in 5/7 (71%) cases with Avitene, and 4/31 (13%) without Avitene. Unfortunately, for the earlier cases in the series, including almost all of the non-MLH hemispherectomies, postoperative CSF sampling was not performed. This and the limited sample size prevented meaningful multivariate analysis.
Table 7.
Perioperative variables and the development of post-hemispherectomy hydrocephalus (n=43*).
| n | No HCP (%) | HCP (%) | P** | |
|---|---|---|---|---|
| Total | 43 | 32 (74) | 11 (26) | |
| Avitene use | .03 | |||
| Yes | 9 | 4 (44) | 5 (56) | |
| No | 34 | 28 (64) | 6 (36) | |
| Prior resective surgery | .70 | |||
| Yes | 11 | 9 (82) | 2 (18) | |
| No | 32 | 23 (72) | 9 (28) | |
| Surgical Technique | 1.0 | |||
| MLH | 38 | 29 (76) | 9 (24) | |
| Rasmussen’s functional | 2 | 1 (50) | 1 (50) | |
| Anatomic | 3 | 2 (67) | 1 (33) | |
| Surgical Time (minutes, mean+SD) | 43 | 258 ± 79 | 263 ± 71 | .37 |
| CSF profile | ||||
| RBC POD #1 (per μL, mean ± SD) | 35 | 1.0 × 105 ± 1.5 × 105 | 2.7 × 105 ± 3.8 × 105 | .18 |
| RBC POD #2 (per μL, mean ± SD) | 34 | 1.1 × 105 ± 2.8 × 105 | 2.4 × 105 ± 4.1 × 105 | .71 |
| WBC POD #1 (per μL, mean ± SD) | 35 | 0.7 × 103 ± 1.1 × 103 | 2.0 × 103 ± 2.7 × 103 | .36 |
| WBC POD #2 (per μL, mean ± SD) | 34 | 1.8 × 103 ± 3.0 × 103 | 4.9 × 103 ± 9.5 × 103 | .44 |
| Protein POD #1 (mg/dL, mean ± SD) | 35 | 929 ± 610 | 1303 ± 1271 | .64 |
| Protein POD #2 (mg/dL, mean ± SD) | 34 | 795 ± 452 | 1042 ± 643 | .44 |
Seven patients had hydrocephalus prior to hemispherectomy surgery and were thus excluded.
Fisher’s Exact (Avitene, prior resective surgery, surgical technique) and Wilcoxon rank-sum tests (surgical time, CSF profile)
Complications
There were no (unanticipated) permanent neurological deficits or deaths. One patient early in the series developed a reversible ischemic neurological deficit involving the contralateral hemisphere (Case Illustration #1). One patient had a re-operation in the immediate post-operative period to address a missed disconnection (Case Illustration #2). Three patients (6%) developed wound infections in the postoperative period. Two of these patients had prior craniotomies from previous resections and were treated with antibiotics and bone flap removal with subsequent cranioplasty. The third infection was in a patient without prior surgery who underwent a modified lateral hemispherotomy (with an osteoplastic bone flap). This infection was successfully managed with drainage of a subgaleal abscess and antibiotics without removal of the bone flap.
The youngest patient (10 weeks old) in this series developed contralateral coronal craniosynostosis postoperatively. The details of this case have been previously reported22. The patient had undergone a posterior quadrantectomy that was converted to a hemispherectomy under the same anesthesia after intraoperative electrocorticography revealed persistent epileptiform activity. The craniosynostosis was postulated to be due to the substantial volume loss created by the posterior quadrantectomy. Due to this case, we have adopted disconnective procedures for posterior quadrantectomies23.
Case Illustration #1 – epidural drainage
Patients undergoing modified lateral hemispherotomy typically develop an epidural hematoma that is of no clinical significance. Early in this series, an epidural drain was attempted to prevent this occurrence, with unfavorable results. The patient was a 10-year-old female, born prematurely with Grade IV intraventricular hemorrhage and subsequent shunted hydrocephalus, left hemiparesis and intractable seizure onset during infancy. A comprehensive workup led to a right MLH. At the end of the procedure, in addition to the usual external ventricular catheter placed in the resection cavity, an epidural drain (10 French) was placed to bulb suction. She emerged from anesthesia with seizures, new right-sided weakness and increased tone. The epidural drain was removed from bulb suction and the seizures promptly ceased. An immediate MRI revealed increased T2 and fluid attenuated inversion recovery (FLAIR) signal in the contralateral thalamus with midline shift towards the resection cavity (Figure 2). She recovered to baseline status over the ensuing month with resolution of the thalamic T2/FLAIR hyperintensity on a 6 month follow-up MRI. She has remained seizure-free since the immediate postoperative period (follow-up period of 7 years). We have since avoided the use of negative pressure drains in the epidural space in any craniotomy involving a sizeable resection cavity.
Figure 2.
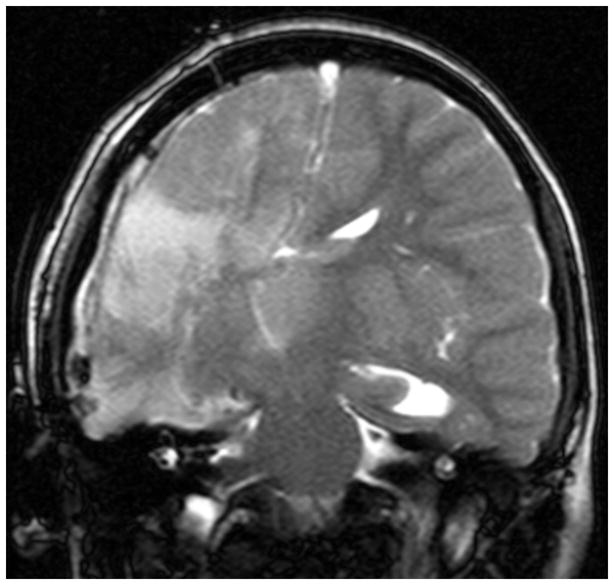
Immediate postoperative coronal T2-weighted MR image in a patient (Case Illustration #1) following a right modified lateral hemispherotomy with placement of an epidural negative pressure drain. Note the shifting of the 3rd ventricle towards the resection cavity and the hyperintensity within the left thalamus.
Case Illustration #2 – use of navigation
The patient was an 11-year-old female, born prematurely at 25 weeks with a large left grade IV intraventricular hemorrhage and subsequent shunted hydrocephalus and right hemiparesis. She developed seizures at 2 years of age with frequent simple and complex partial seizures as well as frequent bouts of status epilepticus, refractory to medical therapy. Video-EEG monitoring, PET scan, and seizure semiology implicated the left hemisphere. Her MRI demonstrated left cerebral atrophy, corpus callosum dysgenesis, and slit ventricles of atypical morphology (Figure 3).
Figure 3.
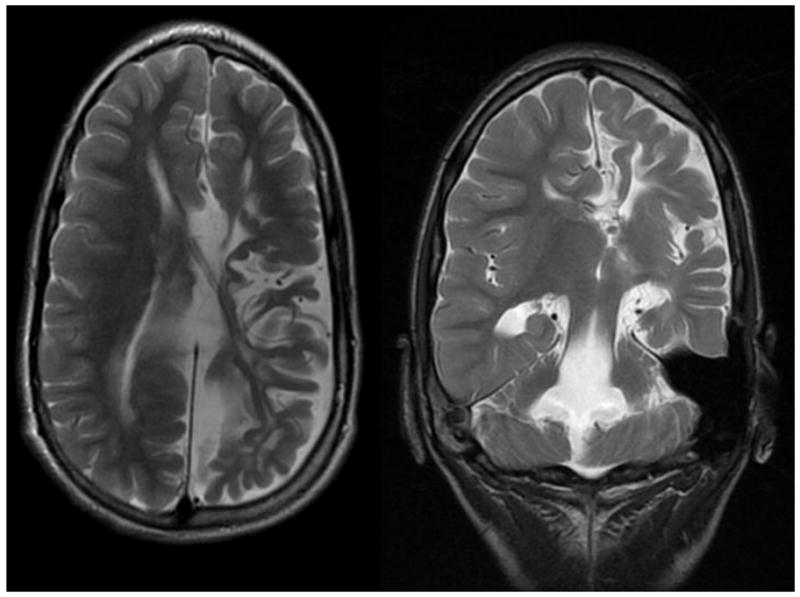
Preoperative axial (left) and coronal (right) T2-weighted MRI images in a patient with highly distorted ventricular anatomy undergoing a left modified lateral hemispherotomy (Case Illustration #2). Note the interhemispheric cyst and poorly defined left lateral ventricle.
Intraoperatively, the left frontal horn was indistinct and the callosal disconnection was created too laterally, leaving mesial frontal tissue connected. She had seizures in the immediate postoperative period and a reoperation was performed on postoperative day #5 to complete the callosal disconnection with the aid of frameless stereotactic navigation. She remains seizure-free following this procedure (2.5 year follow-up, Engel IA). Since this case, our practice has been to utilize navigation in patients with distorted or atypical ventricular anatomy.
DISCUSSION
Seizure outcome and relevant prognostic factors
Hemispherectomy surgery in the modern era is perhaps the most successful form of epilepsy surgery in terms of relief from seizure burden. The larger series published within the last decade reflect this, with seizure freedom rates ranging from 54–90%18, 24–32. Our results follow a similar pattern, with 83% of non-palliative cases (and 80% of all cases) resulting in seizure freedom (Engel I) and the majority of the remaining patients enjoying at least a significant reduction in their seizure burden.
Two patients were identified preoperatively that were felt to not have a chance at seizure freedom (but would still benefit from hemispherectomy surgery). These patients both had significant improvement (Engel IIIA, IIC). There is a precedent for palliative hemispherectomy surgery. Lupashko and colleagues reported on a child with a terminal condition (Alper’s disease) and refractory status epilepticus that underwent hemispherectomy for palliation to allow extubation and hospital discharge33. Ciliberto and colleagues reported on 7 patients with clearly defined bilateral seizure onset undergoing hemispherectomy surgery with three patients achieving seizure freedom (Engel I), and all patients with subjectively improved quality of life34. In an attempt to better identify factors affecting seizure outcome, we excluded the two palliative cases from that subset of analyses. We felt that this would allow us to best determine factors associated with unexpectedly poor seizure outcome.
Although there have been many publications reporting hemispherectomy outcome, in our review only six have identified statistically significant preoperative factors correlating with seizure outcome, and their conclusions differ24, 29, 35–38. This reflects the low statistical power afforded by the infrequency of this procedure at any single institution. Two of the 6 studies have implicated bilateral preoperative imaging abnormalities with adverse seizure outcome. In the largest series of hemispherectomy surgery published (n=186), Moosa and colleagues found that of all the preoperative variables assessed, only bilateral PET abnormalities had a significant correlation with seizure outcome29. In 2010, Boshuisen and colleagues35 reported the presence of contralateral MRI abnormalities as an adverse variable affecting outcome, a variable not found to be significant by Moosa et al. In our study we reviewed MRI findings with regard to the presence of abnormalities, the extent of lesions, laterality, and lesion type (Table 3). None of these variables approached significance. Of the remaining larger series reported (n>40), imaging is either not assessed as a potential predictive factor18, 24, 27, 28, 30, 31, 37, 39, 40, or not found to be significant38. These somewhat conflicting findings regarding the relevance of bilateral MRI abnormalities may be a result of low-powered studies. As it stands, the relevance of bilateral MRI abnormalities is unclear.
Two of the 6 studies demonstrated age at the time of surgery as a significant predictive factor, with younger patients achieving higher levels of seizure freedom24, 38. This variable was not significant in our study (Table 2) or in any other series, including the two largest published series that examined age as a potential factor27, 29. If youth is a predictor for improved outcome, it appears to not be a strong one.
Many hemispherectomy series conclude that epilepsy etiology is associated with seizure outcome25, 30, 31, 40, 41, but only the final 2 of the 6 studies demonstrate statistically significant evidence of such36, 37. Devlin and colleagues reported a series of 33 cases, concluding that developmental pathology was associated with significantly worse outcome compared to acquired or progressive pathology36. Kossoff and colleagues reached a similar conclusion, finding that malformations of cortical development, and hemimegalencephaly in particular, were associated with worsened outcomes37. No such statistically significant correlations were found between etiology and seizure outcome in our study (Table 5) or others examining the possibility24, 27, 29, 32, 38, 42, 43. It would make sense that malformations of cortical development would be more likely to be associated with bilateral pathology compared to other etiologies (infarct, Rasmussen’s, Sturge-Weber), and thus more likely to result in contralateral seizures following surgery. It has been postulated that Rasmussen’s encephalitis and cortical dysplasia can involve the deeper hemispheric structures such as the basal ganglia and thalamus that are left behind with some techniques, and that these affected structures may be a source of persistent seizures18. Hemimegalencephaly also poses greater surgical challenges that may lead to less reliable disconnections/resections. It is unclear if the failure of etiology to be a significant predictor of seizure outcome in most studies is a result of low-powered studies, or a true lack of effect.
In the present series, EEG findings did not correlate significantly with seizure outcome. Laterality of background abnormalities, interictal activity, and ictal onset were not significant. There was a trend towards significance with bilateral synchrony that was not significant in the multivariate analysis. Although two studies argue that preoperative EEG is predictive of outcome44, 45, both studies lack statistical power to bolster the assertion. Moosa and colleagues identified lateralized ictal EEG onset as a significant predictor in their univariate analysis, but it was not an independent predictor in the multivariate analysis29. Other studies investigating the possibility failed to show preoperative EEG parameters as significant predictors of seizure outcome32, 35, 38, 39, 42, 46.
There is little evidence in our series that the chosen hemispherectomy technique affected seizure outcome. In the univariate analysis, technique was identified as a significant variable (P = .005) with worsened outcome with anatomic hemispherectomies. However, 3 of the 4 anatomic procedures were revision hemispherectomies and this technique was not a significant factor when controlling for prior resective surgery in the multivariate analysis. Comparing techniques is fraught with difficulty. Seizure outcome in this population is affected by patient selection, which cannot be controlled for when comparing results from various institutions. Within individual institutions, most series are similar to ours, insofar as a specific technique became preferred over time without a satisfactory volume of cases utilizing differing techniques to allow effective comparisons. One study has reported a fairly balanced distribution of cases between two hemispherectomy techniques with a demonstrably significant difference in outcome in their hands. Kwan and colleagues28 reported 21 cases of hemidecortication (HD) versus 20 cases of peri-insular hemispherotomy (PIH). They found significant differences in operative time (5 hrs HD vs. 7 hrs PIH, p<.001), and a significantly higher reoperation rate with HD (6/21) for persistent seizures with residual cortex removed at all reoperations. After initial surgery, 85% of PIH patients had Engel I or II outcomes versus 48% of the HD patients. The difference in efficacy in this study appeared to be related to persistent residual cortex left with the HD technique which may be a reflection of experience with the technique or simply that it is more difficult to adequately isolate a hemisphere with HD. Three other centers with adequate volume (>20 cases per technique) comparing different hemispherectomy methods performed at a single institution did not find significant differences in seizure outcome18, 24, 29.
We identified a history of prior resective surgery as a risk factor for persistent seizures after hemispherectomy (P = .005). Pairwise comparisons suggest that it was the patients with a history of prior hemispherectomy that were likely responsible for this effect. This was an expected finding. Vadera and colleagues recently published the largest series of revision hemispherectomy cases to date47. They reported >90% seizure reduction in 64% of patients, but only 19% achieved seizure freedom. In their study they identified generalized ictal onset and cortical dysplasia as variables associated with poor outcome. The patient with persistent seizures after hemispherectomy can pose a diagnostic challenge. High resolution MRI can demonstrate areas of residual connected tissue but it can be difficult to confirm those areas as the definitive source of persistent seizures. The success rate in this population probably relates more to patient selection rather than surgical technique. In our 6 revision hemispherectomy cases, all demonstrated areas of apparent residual connected ipsilateral cortex and the remainder of their workups were consistent with ipsilateral seizures. Despite this, only 4 patients achieved Engel I/II outcomes. Nevertheless, our findings are consistent with those of Vadera et al.; there exists a subset of patients with prior hemispherectomies and persistent epilepsy that will benefit from revision surgery.
Post-hemispherectomy hydrocephalus
Hydrocephalus is a known adverse outcome of hemispherectomy surgery. Incidence rates vary dramatically in individual series, ranging from 9% to 81%8, 18, 25, 28, 48–51. A recent multi-institutional review incorporated data on 690 hemispherectomy patients from 15 pediatric epilepsy centers (including ours)52. In that study, the overall incidence of hydrocephalus after hemispherectomy surgery was 23%. Prior cranial surgery and the anatomic hemispherectomy technique were identified as significant independent risk factors for developing hydrocephalus. In the present series, the overall incidence was 26%. When the MLH technique was initially adopted at our institution, Avitene was frequently used for hemostasis after removal of the opercular block of tissue. We noted in our early patients a high-rate of postoperative hydrocephalus, with 5 of the first 9 (56%) MLH patients developing hydrocephalus. After reviewing our technique at length we suspected the Avitene use was a factor. Since discontinuing its use, the hydrocephalus rate dropped significantly to 13% (in MLH cases), and statistical analysis identified Avitene as the only independent risk factor for developing hydrocephalus in this series. In the multi-institutional review52, the use of any hemostatic agent was a significant risk factor for developing hydrocephalus in the univariate analysis (but not the multivariate analysis). Avitene has been reported to induce granulomatous inflammatory responses post-craniotomy in both patients and animal models53, 54. It is possible that this inflammation poses a risk for hydrocephalus similar to that seen in patients with intraventricular infection or hemorrhage. The lead author (SML) has since discontinued usage of hemostatic agents in all surgeries involving ventricular exposure.
Neuropsychological outcome
Hemispherectomy did not appear to have a detrimental effect on overall cognitive function in this series. This reassuring finding is consistent with those of prior studies24, 27, 55–58. Despite the overall group-level stability, some individuals demonstrated significant changes in FSIQ. Other studies have identified differing preoperative factors significantly associated with pre-post cognitive improvement. These include lower presurgical intelligence24, non-hemimegalencephalic cortical dysplasia27, absence of contralateral MRI abnormalities35, older age at seizure onset59, shorter duration of seizures59, and postoperative seizure freedom24. In the current series, preoperative FSIQ, age at seizure onset, duration of seizures, age at hemispherectomy, and side of surgery failed to show significant correlation with change in FSIQ (Table 6).
Surgical technique
In the absence of clearly improved seizure outcome with one technique over another, the decision on which technique to use becomes a personal preference based on experience with differing techniques and perceptions regarding various advantages and disadvantages. Over the course of this series, the surgical technique has evolved significantly. The major change was early in the series when the MLH technique was adopted. There are two distinguishing features of the MLH technique. The first is the early sacrifice of the middle cerebral artery. This allows for excellent control of hemostasis during the remainder of the surgery, which in turn shortens the operative time. The second is the resection of the insular/basal ganglia/thalamic/opercular block of tissue lateral to the choroidal fissure. This provides excellent exposure of the ventricular system while assuring complete removal of the insular cortex. With this exposure, the callosal, frontal, and posterior disconnections can be made with direct lines of sight without brain retraction, reducing the possibility of a missed disconnection. The facile nature of the disconnections also leads to shorter operative times. With this technique we have found that surgery on infant brains does not pose any particular difficulties and we do not recommend postponing surgery due to concerns of blood loss or other perceived difficulties with infant surgery if hemispherectomy surgery appears inevitable.
Since adopting the MLH technique, we have made minor modifications. We now use a significantly smaller osteoplastic craniotomy, extending superiorly only to the superior temporal line (the midpupillary line was initially described as the superior extent18), as this provides adequate access to the relevant anatomy. The frontal disconnection is now created by making a trough extending from the Sylvian fissure to the inferior aspect of the rostrum of the corpus callosum with visualization of the ipsilateral anterior cerebral artery through the intact arachnoid. This minimizes any residual connected posterior mesial frontal lobe (Figure 1), a site implicated in hemispherectomy technical failures47.
Complications described in Case Illustrations #1 and #2 further altered our practices. All patients undergoing MLH surgery develop blood in the epidural space that is transient, asymptomatic, and does not cause midline shift. Nevertheless, as described in Case Illustration #1 we attempted to use a negative suction epidural drain in one of the earlier cases. This resulted in a contralateral hemispheric ischemic injury that was fortunately temporary. There are multiple reports regarding the dangers associated with negative pressure drains in both the subgaleal and epidural spaces as a cause of remote hemorrhage, bradycardia, and diminished consciousness60–63. We suspect these risks are exacerbated in the setting of a large intracranial potential space that will allow for migration of the midline structures towards the drain with associated distortion of the brain and its blood supply. Furthermore, the effectiveness of epidural drainage in preventing epidural and subgaleal collections is in doubt64. We have abandoned the use of epidural drainage in such patients. Case Illustration #2 highlights the utility of frameless stereotactic navigation in cases with distorted anatomic landmarks. It is now our practice to use navigation in all patients with prior resective surgery and in cases with markedly abnormal ventricular anatomy.
One potential disadvantage of the MLH technique is the incidence of postoperative hydrocephalus. The postoperative hydrocephalus rate for the technique is 24%. Since discontinuing the use of Avitene, the hydrocephalus rate with the MLH technique has been 13% (4/31 patients). This compares favorably to most larger series that report a hydrocephalus rate18, 38, 43, and to a recent multicenter study52, but is significantly higher than some peri-insular hemispherotomy series30, 40. It may be that the degree of brain resection involved or the greater exposure of the ventricular spaces increases the risk of hydrocephalus.
CONCLUSION
The modified lateral hemispherotomy is an effective method of achieving hemispherectomy, including revision hemispherectomy, with an acceptably low incidence of complications, and stable cognitive functioning postoperatively. Avitene use correlated with the development of hydrocephalus and is now avoided. Negative pressure epidural drainage is unnecessary and dangerous in the setting of a large resection cavity. Frameless stereotactic navigation is not routinely used with this technique but is suggested for patients with atypical ventricular anatomy or prior resective surgery. Revision hemispherectomy carries a lower rate of success but should be considered in select cases. Etiology of epilepsy did not correlate with seizure outcome. There is a lack of concordance between studies regarding prognostic factors for predicting seizure and neuropsychological outcomes following hemispherectomy. This is likely a byproduct of small sample sizes, and a meta-analysis or multi-institutional study is warranted.
Supplementary Material
Supplemental Content 1: Description of technique - modified lateral hemispherotomy
Supplemental Content 2: Figure – Pre- and post-surgical full scale IQ scores in the patients who underwent testing for both (n = 27).
Acknowledgments
We wish to thank Dr. Gary Mathern for his generosity in sharing his expertise and the modified lateral hemispherotomy technique. We also thank Daniel Eastwood for his data analysis, supported, in part, by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number 8UL1TR000055.
Footnotes
Disclosure: Statistical analysis for this project was supported, in part, by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number 8UL1TR000055. The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
References
- 1.McKenzie KG. The present status of a patient who had the right cerebral hemisphere removed. JAMA. 1938;111:168–183. [Google Scholar]
- 2.Krynauw RA. Infantile hemiplegia treated by removing one cerebral hemisphere. Journal of neurology, neurosurgery, and psychiatry. 1950 Nov;13(4):243–267. doi: 10.1136/jnnp.13.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oppenheimer DR, Griffith HB. Persistent intracranial bleeding as a complication of hemispherectomy. Journal of neurology, neurosurgery, and psychiatry. 1966 Jun;29(3):229–240. doi: 10.1136/jnnp.29.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falconer MA, Wilson PJ. Complications related to delayed hemorrhage after hemispherectomy. J Neurosurg. 1969 Apr;30(4):413–426. doi: 10.3171/jns.1969.30.4.0413. [DOI] [PubMed] [Google Scholar]
- 5.Wilson PJ. Cerebral hemispherectomy for infantile hemiplegia. A report of 50 cases. Brain. 1970;93(1):147–180. doi: 10.1093/brain/93.1.147. [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen T. Postoperative superficial hemosiderosis of the brain, its diagnosis, treatment and prevention. Transactions of the American Neurological Association. 1973;98:133–137. [PubMed] [Google Scholar]
- 7.Rasmussen T. Hemispherectomy for seizures revisited. The Canadian journal of neurological sciences. 1983 May;10(2):71–78. doi: 10.1017/s0317167100044668. [DOI] [PubMed] [Google Scholar]
- 8.Peacock WJ, Wehby-Grant MC, Shields WD, et al. Hemispherectomy for intractable seizures in children: a report of 58 cases. Childs Nerv Syst. 121996:376–384. doi: 10.1007/BF00395089. [DOI] [PubMed] [Google Scholar]
- 9.Brett E. Second thoughts on hemispherectomy in infantile hemiplegia. Developmental medicine and child neurology. 1969 Jun;11(3):374–376. doi: 10.1111/j.1469-8749.1969.tb01447.x. [DOI] [PubMed] [Google Scholar]
- 10.Wechsler D. Wechsler Intelligence Scale for Children. 4. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- 11.Wechsler D. Wechsler Intelligence Scale for Children. 3. San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- 12.Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 13.Elliot CD. The Differential Ability Scales. 2. San Antonio, TX: Harcourt Assessment, Inc; 2007. [Google Scholar]
- 14.Mullen EM. Mullen Scales of Early Learning, AGS Edition. Circle Pines, MN: American Guidance Service, Inc; 1995. [Google Scholar]
- 15.Roid GH. Stanford-Binet Intelligence Scales. 5. Itasca, IL: Riverside Publishing Corporation; 2003. [Google Scholar]
- 16.Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales. 2. Circle Pines, MN: AGS Publishing; 2005. [Google Scholar]
- 17.Tinuper P, Andermann F, Villemure JG, Rasmussen TB, Quesney LF. Functional hemispherectomy for treatment of epilepsy associated with hemiplegia: rationale, indications, results, and comparison with callosotomy. Annals of neurology. 1988 Jul;24(1):27–34. doi: 10.1002/ana.410240107. [DOI] [PubMed] [Google Scholar]
- 18.Cook SW, Nguyen ST, Hu B, et al. Cerebral hemispherectomy in pediatric patients with epilepsy: comparison of three techniques by pathological substrate in 115 patients. J Neurosurg Peds. 2004 Feb;100(2):125–141. doi: 10.3171/ped.2004.100.2.0125. [DOI] [PubMed] [Google Scholar]
- 19.Schramm J, Behrens E, Entzian W. Hemispherical deafferentation: an alternative to functional hemispherectomy. Neurosurgery. 1995 Mar;36(3):509–515. doi: 10.1227/00006123-199503000-00010. discussion 515–506. [DOI] [PubMed] [Google Scholar]
- 20.Schramm J, Kral T, Clusmann H. Transsylvian keyhole functional hemispherectomy. Neurosurgery. 2001 Oct;49(4):891–900. doi: 10.1097/00006123-200110000-00021. discussion 900–891. [DOI] [PubMed] [Google Scholar]
- 21.Villemure JG, Mascott CR. Peri-insular hemispherotomy: surgical principles and anatomy. Neurosurgery. 1995 Nov;37(5):975–981. doi: 10.1227/00006123-199511000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Jazayeri MA, Jensen JN, Lew SM. Craniosynostosis following hemispherectomy in a 2.5-month-old boy with intractable epilepsy. Journal of neurosurgery. 2011 Nov;8(5):450–454. doi: 10.3171/2011.8.PEDS11176. [DOI] [PubMed] [Google Scholar]
- 23.Daniel RT, Meagher-Villemure K, Farmer JP, Andermann F, Villemure JG. Posterior quadrantic epilepsy surgery: technical variants, surgical anatomy, and case series. Epilepsia. 2007 Aug;48(8):1429–1437. doi: 10.1111/j.1528-1167.2007.01095.x. [DOI] [PubMed] [Google Scholar]
- 24.Althausen A, Gleissner U, Hoppe C, et al. Long-term outcome of hemispheric surgery at different ages in 61 epilepsy patients. Journal of neurology, neurosurgery, and psychiatry. 2013 Dec 25; doi: 10.1136/jnnp-2012-303811. [DOI] [PubMed] [Google Scholar]
- 25.Basheer SN, Connolly MB, Lautzenhiser A, Sherman EM, Hendson G, Steinbok P. Hemispheric surgery in children with refractory epilepsy: seizure outcome, complications, and adaptive function. Epilepsia. 2007 Jan;48(1):133–140. doi: 10.1111/j.1528-1167.2006.00909.x. [DOI] [PubMed] [Google Scholar]
- 26.Caraballo R, Bartuluchi M, Cersosimo R, Soraru A, Pomata H. Hemispherectomy in pediatric patients with epilepsy: a study of 45 cases with special emphasis on epileptic syndromes. Childs Nerv Syst. 2011 Dec;27(12):2131–2136. doi: 10.1007/s00381-011-1596-5. [DOI] [PubMed] [Google Scholar]
- 27.Jonas R, Nguyen S, Hu B, et al. Cerebral hemispherectomy: hospital course, seizure, developmental, language, and motor outcomes. Neurology. 2004 May 25;62(10):1712–1721. doi: 10.1212/01.wnl.0000127109.14569.c3. [DOI] [PubMed] [Google Scholar]
- 28.Kwan A, Ng WH, Otsubo H, et al. Hemispherectomy for the control of intractable epilepsy in childhood: comparison of 2 surgical techniques in a single institution. Neurosurgery. 2010 Dec;67(2 Suppl Operative):429–436. doi: 10.1227/NEU.0b013e3181f743dc. [DOI] [PubMed] [Google Scholar]
- 29.Moosa AN, Gupta A, Jehi L, et al. Longitudinal seizure outcome and prognostic predictors after hemispherectomy in 170 children. Neurology. 2013 Jan 15;80(3):253–260. doi: 10.1212/WNL.0b013e31827dead9. [DOI] [PubMed] [Google Scholar]
- 30.Schramm J, Kuczaty S, Sassen R, Elger CE, von Lehe M. Pediatric functional hemispherectomy: outcome in 92 patients. Acta neurochirurgica. 2012 Nov;154(11):2017–2028. doi: 10.1007/s00701-012-1481-3. [DOI] [PubMed] [Google Scholar]
- 31.Shimizu H. Our experience with pediatric epilepsy surgery focusing on corpus callosotomy and hemispherotomy. Epilepsia. 2005;46( Suppl 1):30–31. doi: 10.1111/j.0013-9580.2005.461009.x. [DOI] [PubMed] [Google Scholar]
- 32.Terra-Bustamante VC, Inuzuka LM, Fernandes RM, et al. Outcome of hemispheric surgeries for refractory epilepsy in pediatric patients. Childs Nerv Syst. 2007 Mar;23(3):321–326. doi: 10.1007/s00381-006-0212-6. [DOI] [PubMed] [Google Scholar]
- 33.Lupashko S, Malik S, Donahue D, Hernandez A, Perry MS. Palliative functional hemispherectomy for treatment of refractory status epilepticus associated with Alpers’ disease. Childs Nerv Syst. 2011 Aug;27(8):1321–1323. doi: 10.1007/s00381-011-1495-9. [DOI] [PubMed] [Google Scholar]
- 34.Ciliberto MA, Limbrick D, Powers A, Titus JB, Munro R, Smyth MD. Palliative hemispherotomy in children with bilateral seizure onset. Journal of neurosurgery. 2012 Apr;9(4):381–388. doi: 10.3171/2011.12.PEDS11334. [DOI] [PubMed] [Google Scholar]
- 35.Boshuisen K, van Schooneveld MM, Leijten FS, et al. Contralateral MRI abnormalities affect seizure and cognitive outcome after hemispherectomy. Neurology. 2010 Nov 2;75(18):1623–1630. doi: 10.1212/WNL.0b013e3181fb4400. [DOI] [PubMed] [Google Scholar]
- 36.Devlin AM, Cross JH, Harkness W, et al. Clinical outcomes of hemispherectomy for epilepsy in childhood and adolescence. Brain. 2003 Mar;126(Pt 3):556–566. doi: 10.1093/brain/awg052. [DOI] [PubMed] [Google Scholar]
- 37.Kossoff EH, Vining EP, Pillas DJ, et al. Hemispherectomy for intractable unihemispheric epilepsy etiology vs outcome. Neurology. 2003 Oct 14;61(7):887–890. doi: 10.1212/01.wnl.0000090107.04681.5b. [DOI] [PubMed] [Google Scholar]
- 38.Ramantani G, Kadish NE, Brandt A, et al. Seizure control and developmental trajectories after hemispherotomy for refractory epilepsy in childhood and adolescence. Epilepsia. 2013 Jun;54(6):1046–1055. doi: 10.1111/epi.12140. [DOI] [PubMed] [Google Scholar]
- 39.Greiner HM, Park YD, Holland K, et al. Scalp EEG does not predict hemispherectomy outcome. Seizure. 2011 Dec;20(10):758–763. doi: 10.1016/j.seizure.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villemure JG, Daniel RT. Peri-insular hemispherotomy in paediatric epilepsy. Childs Nerv Syst. 2006 Aug;22(8):967–981. doi: 10.1007/s00381-006-0134-3. [DOI] [PubMed] [Google Scholar]
- 41.Villarejo-Ortega F, Garcia-Fernandez M, Fournier-Del Castillo C, et al. Seizure and developmental outcomes after hemispherectomy in children and adolescents with intractable epilepsy. Childs Nerv Syst. 2013 Mar;29(3):475–488. doi: 10.1007/s00381-012-1949-8. [DOI] [PubMed] [Google Scholar]
- 42.Limbrick DD, Narayan P, Powers AK, et al. Hemispherotomy: efficacy and analysis of seizure recurrence. Journal of neurosurgery. 2009 Oct;4(4):323–332. doi: 10.3171/2009.5.PEDS0942. [DOI] [PubMed] [Google Scholar]
- 43.Delalande O, Bulteau C, Dellatolas G, et al. Vertical parasagittal hemispherotomy: surgical procedures and clinical long-term outcomes in a population of 83 children. Neurosurgery. 2007 Feb;60(2 Suppl 1):ONS19–32. doi: 10.1227/01.NEU.0000249246.48299.12. discussion ONS32. [DOI] [PubMed] [Google Scholar]
- 44.Carmant L, Kramer U, Riviello JJ, et al. EEG prior to hemispherectomy: correlation with outcome and pathology. Electroencephalography and clinical neurophysiology. 1995 Apr;94(4):265–270. doi: 10.1016/0013-4694(95)98477-p. [DOI] [PubMed] [Google Scholar]
- 45.Smith SJ, Andermann F, Villemure JG, Rasmussen TB, Quesney LF. Functional hemispherectomy: EEG findings, spiking from isolated brain postoperatively, and prediction of outcome. Neurology. 1991 Nov;41(11):1790–1794. doi: 10.1212/wnl.41.11.1790. [DOI] [PubMed] [Google Scholar]
- 46.Doring S, Cross H, Boyd S, Harkness W, Neville B. The significance of bilateral EEG abnormalities before and after hemispherectomy in children with unilateral major hemisphere lesions. Epilepsy research. 1999 Mar;34(1):65–73. doi: 10.1016/s0920-1211(98)00101-6. [DOI] [PubMed] [Google Scholar]
- 47.Vadera S, Moosa AN, Jehi L, et al. Reoperative hemispherectomy for intractable epilepsy: a report of 36 patients. Neurosurgery. 2012 Aug;71(2):388–392. doi: 10.1227/NEU.0b013e31825979bb. discussion 392–383. [DOI] [PubMed] [Google Scholar]
- 48.Di Rocco C, Iannelli A. Hemimegalencephaly and intractable epilepsy: complications of hemispherectomy and their correlations with the surgical technique. A report on 15 cases. Pediatric neurosurgery. 2000 Oct;33(4):198–207. doi: 10.1159/000055953. [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez-Martinez JA, Gupta A, Kotagal P, et al. Hemispherectomy for catastrophic epilepsy in infants. Epilepsia. 2005 Sep;46(9):1518–1525. doi: 10.1111/j.1528-1167.2005.53704.x. [DOI] [PubMed] [Google Scholar]
- 50.Davies KG, Maxwell RE, French LA. Hemispherectomy for intractable seizures: long-term results in 17 patients followed for up to 38 years. J Neurosurg. 1993 May;78(5):733–740. doi: 10.3171/jns.1993.78.5.0733. [DOI] [PubMed] [Google Scholar]
- 51.Carson BS, Javedan SP, Freeman JM, et al. Hemispherectomy: a hemidecortication approach and review of 52 cases. J Neurosurg. 1996 Jun;84(6):903–911. doi: 10.3171/jns.1996.84.6.0903. [DOI] [PubMed] [Google Scholar]
- 52.Lew SM, Matthews AE, Hartman AL, Haranhalli N Workgroup obotP-HH. Posthemispherectomy hydrocephalus: results of a comprehensive, multiinstitutional review. Epilepsia. 2013 Feb;54(2):383–389. doi: 10.1111/epi.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Apel-Sarid L, Cochrane DD, Steinbok P, Byrne AT, Dunham C. Microfibrillar collagen hemostat-induced necrotizing granulomatous inflammation developing after craniotomy: a pediatric case series. Journal of neurosurgery. 2010 Oct;6(4):385–392. doi: 10.3171/2010.8.PEDS10248. [DOI] [PubMed] [Google Scholar]
- 54.Barbolt TA, Odin M, Leger M, Kangas L. Pre-clinical subdural tissue reaction and absorption study of absorbable hemostatic devices. Neurological research. 2001 Jul;23(5):537–542. doi: 10.1179/016164101101198794. [DOI] [PubMed] [Google Scholar]
- 55.Battaglia D, Chieffo D, Lettori D, Perrino F, Di Rocco C, Guzzetta F. Cognitive assessment in epilepsy surgery of children. Childs Nerv Syst. 2006 Aug;22(8):744–759. doi: 10.1007/s00381-006-0151-2. [DOI] [PubMed] [Google Scholar]
- 56.Pulsifer MB, Brandt J, Salorio CF, Vining EP, Carson BS, Freeman JM. The cognitive outcome of hemispherectomy in 71 children. Epilepsia. 2004 Mar;45(3):243–254. doi: 10.1111/j.0013-9580.2004.15303.x. [DOI] [PubMed] [Google Scholar]
- 57.Dunkley C, Kung J, Scott RC, et al. Epilepsy surgery in children under 3 years. Epilepsy research. Feb;93(2–3):96–106. doi: 10.1016/j.eplepsyres.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 58.Lettori D, Battaglia D, Sacco A, et al. Early hemispherectomy in catastrophic epilepsy: a neuro-cognitive and epileptic long-term follow-up. Seizure. 2008 Jan;17(1):49–63. doi: 10.1016/j.seizure.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 59.Thomas SG, Daniel RT, Chacko AG, Thomas M, Russell PS. Cognitive changes following surgery in intractable hemispheric and sub-hemispheric pediatric epilepsy. Childs Nerv Syst. 2009 Aug;26(8):1067–1073. doi: 10.1007/s00381-010-1102-5. [DOI] [PubMed] [Google Scholar]
- 60.Demetriades AK. Negative pressure suction from subgaleal drainage: bradycardia and decreased consciousness. Acta neurochirurgica. 2008 Oct;150(10):1111. doi: 10.1007/s00701-008-0028-0. [DOI] [PubMed] [Google Scholar]
- 61.Toshniwal GR, Bhagat H, Rath GP. Bradycardia following negative pressure suction of subgaleal drain during craniotomy closure. Acta neurochirurgica. 2007 Oct;149(10):1077–1079. doi: 10.1007/s00701-007-1246-6. discussion 1079. [DOI] [PubMed] [Google Scholar]
- 62.Karamchandani K, Chouhan RS, Bithal PK, Dash HH. Severe bradycardia and hypotension after connecting negative pressure to the subgaleal drain during craniotomy closure. British journal of anaesthesia. 2006 May;96(5):608–610. doi: 10.1093/bja/ael063. [DOI] [PubMed] [Google Scholar]
- 63.Yamanaka K, Iwai Y, Nakajima H, Kobayashi Y, Inoue T. Multiple remote brain hemorrhages after removal of a giant colloid cyst of the third ventricle--case report. Neurologia medico-chirurgica. 1998 Jan;38(1):24–27. doi: 10.2176/nmc.38.24. [DOI] [PubMed] [Google Scholar]
- 64.Guangming Z, Huancong Z, Wenjing Z, Guoqiang C, Xiaosong W. Should epidural drain be recommended after supratentorial craniotomy for epileptic patients? Surgical neurology. 2009 Aug;72(2):138–141. doi: 10.1016/j.surneu.2008.06.014. discussion 141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Content 1: Description of technique - modified lateral hemispherotomy
Supplemental Content 2: Figure – Pre- and post-surgical full scale IQ scores in the patients who underwent testing for both (n = 27).


