Abstract
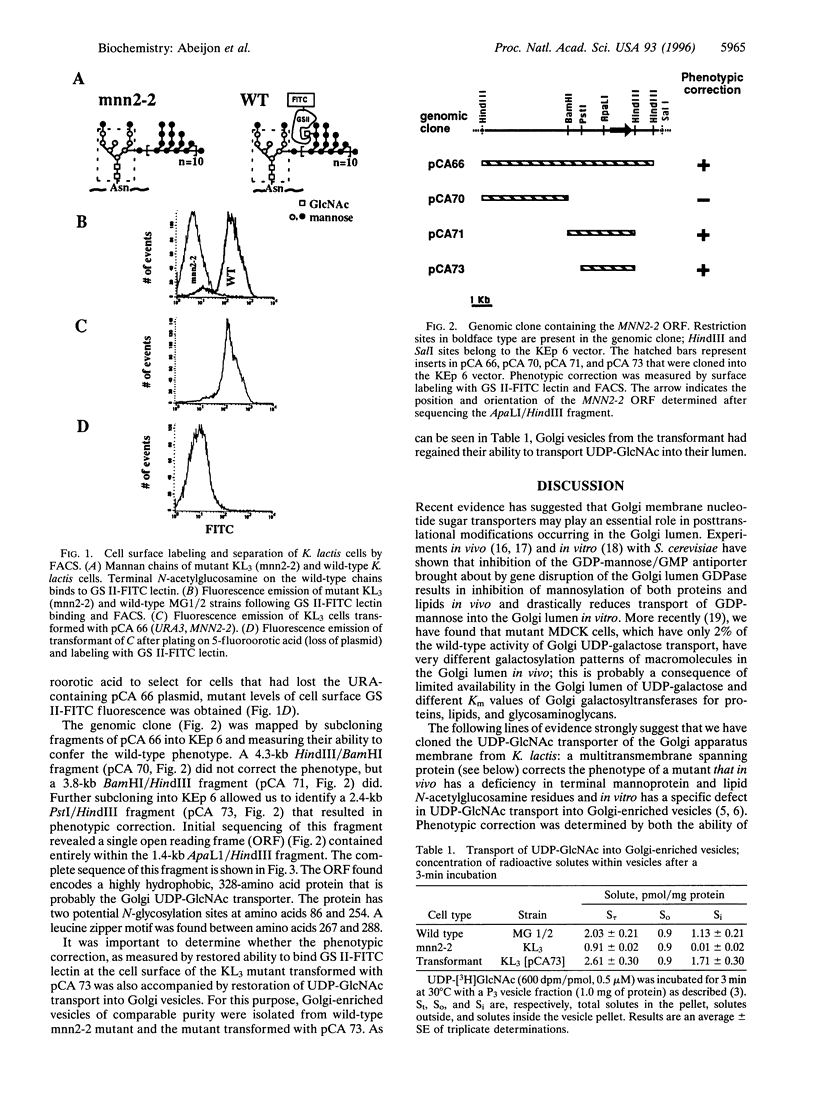
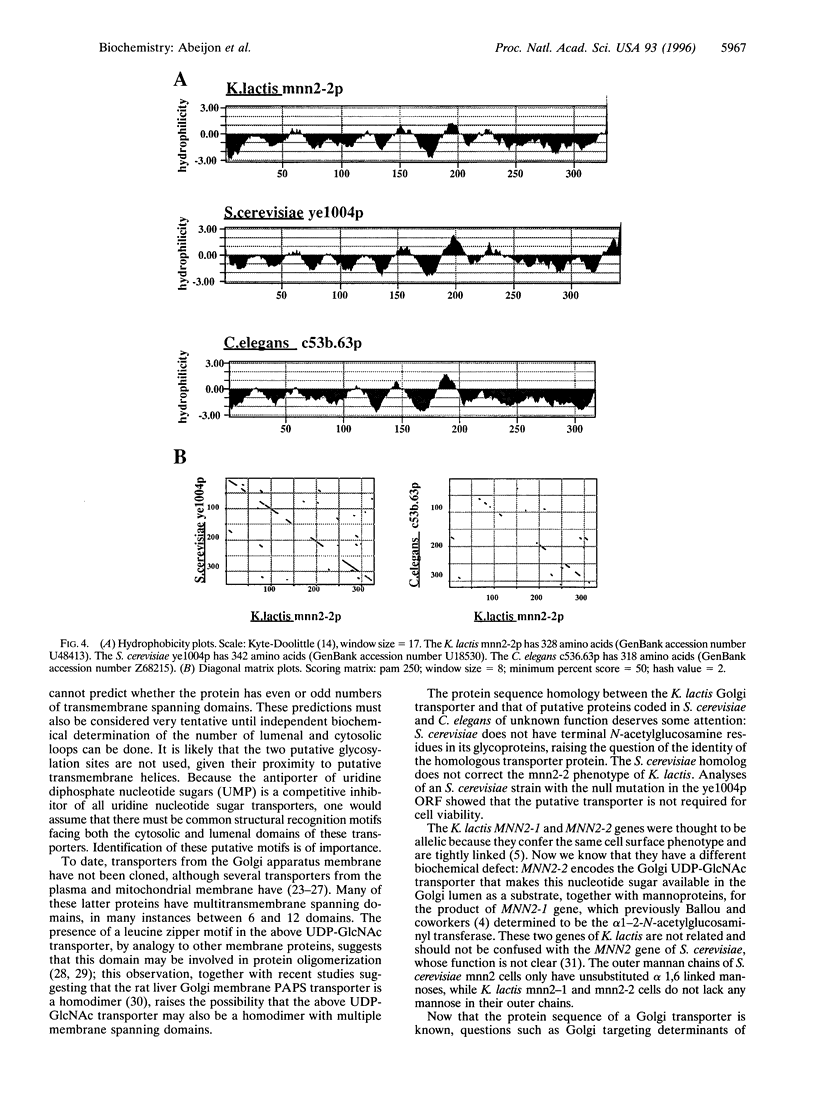
The mannan chains of Kluyveromyces lactis mannoproteins are similar to those of Saccharomyces cerevisiae except that they lack mannose phosphate and have terminal alpha1-->2-linked N-acetylglucosamine. The biosynthesis of these chains probably occurs in the lumen of the Golgi apparatus, by analogy to S. cerevisiae. The sugar donors, GDP-mannose and UDP-GlcNAc, must first be transported from the cytosol, their site of synthesis, via specific Golgi membrane transporters into the lumen where they are substrates in the biosynthesis of these mannoproteins. A mutant of K. lactis, mnn2-2, that lacks terminal N-acetylglucosamine in its mannan chains in vivo, has recently been characterized and shown to have a specific defect in transport of UDP-GlcNAc into the lumen of Golgi vesicles in vitro. We have now cloned the gene encoding the K. lactis Golgi membrane UDP-GlcNAc transporter by complementation of the mnn2-2 mutation. The mnn2-2 mutant was transformed with a genomic library from wild-type K. lactis in a pKD1-derived vector; transformants were isolated and phenotypic correction was monitored following cell surface labeling with fluorescein isothiocyanate conjugated to Griffonia simplicifolia II lectin, which binds terminal N-acetylglucosamine, and a fluorescent activated cell sorter. A 2.4-kb DNA fragment was found to restore the wild-type lectin binding phenotype. Upon loss of the plasmid containing this fragment, reversion to the mutant phenotype occurred. The above fragment contained an open reading frame for a multitransmembrane spanning protein of 328 amino acids. The protein contains a leucine zipper motif and has high homology to predicted proteins from S. cerevisiae and C. elegans. In an assay in vitro, Golgi vesicles isolated from the transformant had regained their ability to transport UDP-GlcNAc. Taken together, the above results strongly suggest that the cloned gene encodes the Golgi UDP-GlcNAc transporter of K. lactis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeijon C., Mandon E. C., Robbins P. W., Hirschberg C. B. A mutant yeast deficient in Golgi transport of uridine diphosphate N-acetylglucosamine. J Biol Chem. 1996 Apr 12;271(15):8851–8854. doi: 10.1074/jbc.271.15.8851. [DOI] [PubMed] [Google Scholar]
- Abeijon C., Orlean P., Robbins P. W., Hirschberg C. B. Topography of glycosylation in yeast: characterization of GDPmannose transport and lumenal guanosine diphosphatase activities in Golgi-like vesicles. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6935–6939. doi: 10.1073/pnas.86.18.6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeijon C., Yanagisawa K., Mandon E. C., Häusler A., Moremen K., Hirschberg C. B., Robbins P. W. Guanosine diphosphatase is required for protein and sphingolipid glycosylation in the Golgi lumen of Saccharomyces cerevisiae. J Cell Biol. 1993 Jul;122(2):307–323. doi: 10.1083/jcb.122.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Berninsone P., Miret J. J., Hirschberg C. B. The Golgi guanosine diphosphatase is required for transport of GDP-mannose into the lumen of Saccharomyces cerevisiae Golgi vesicles. J Biol Chem. 1994 Jan 7;269(1):207–211. [PubMed] [Google Scholar]
- Bernstein H. B., Tucker S. P., Kar S. R., McPherson S. A., McPherson D. T., Dubay J. W., Lebowitz J., Compans R. W., Hunter E. Oligomerization of the hydrophobic heptad repeat of gp41. J Virol. 1995 May;69(5):2745–2750. doi: 10.1128/jvi.69.5.2745-2750.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckland R., Wild F. Leucine zipper motif extends. Nature. 1989 Apr 13;338(6216):547–547. doi: 10.1038/338547a0. [DOI] [PubMed] [Google Scholar]
- Chen X. J., Wésolowski-Louvel M., Tanguy-Rougeau C., Bianchi M. M., Fabiani L., Saliola M., Falcone C., Frontali L., Fukuhara H. A gene-cloning system for Kluyveromyces lactis and isolation of a chromosomal gene required for killer toxin production. J Basic Microbiol. 1988;28(4):211–220. doi: 10.1002/jobm.3620280402. [DOI] [PubMed] [Google Scholar]
- Devlin C., Ballou C. E. Identification and characterization of a gene and protein required for glycosylation in the yeast Golgi. Mol Microbiol. 1990 Nov;4(11):1993–2001. doi: 10.1111/j.1365-2958.1990.tb02049.x. [DOI] [PubMed] [Google Scholar]
- Douglas R. H., Ballou C. E. Purification of an alpha-N-acetylglucosaminyltransferase from the yeast Kluyveromyces lactis and a study of mutants defective in this enzyme activity. Biochemistry. 1982 Mar 30;21(7):1561–1570. doi: 10.1021/bi00536a015. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Goud B., Salminen A., Walworth N. C., Novick P. J. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell. 1988 Jun 3;53(5):753–768. doi: 10.1016/0092-8674(88)90093-1. [DOI] [PubMed] [Google Scholar]
- HERMAN A., HALVORSON H. IDENTIFICATION OF THE STRUCTURAL GENE FOR BETA-GLUCOSIDASE IN SACCHAROMYCES LACTIS. J Bacteriol. 1963 Apr;85:895–900. doi: 10.1128/jb.85.4.895-900.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackenberg H., Klingenberg M. Molecular weight and hydrodynamic parameters of the adenosine 5'-diphosphate--adenosine 5'-triphosphate carrier in Triton X-100. Biochemistry. 1980 Feb 5;19(3):548–555. doi: 10.1021/bi00544a024. [DOI] [PubMed] [Google Scholar]
- Hebert D. N., Carruthers A. Uniporters and anion antiporters. Curr Opin Cell Biol. 1991 Aug;3(4):702–709. doi: 10.1016/0955-0674(91)90044-y. [DOI] [PubMed] [Google Scholar]
- Herscovics A., Orlean P. Glycoprotein biosynthesis in yeast. FASEB J. 1993 Apr 1;7(6):540–550. doi: 10.1096/fasebj.7.6.8472892. [DOI] [PubMed] [Google Scholar]
- Higgins C. F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Jones D. T., Taylor W. R., Thornton J. M. A model recognition approach to the prediction of all-helical membrane protein structure and topology. Biochemistry. 1994 Mar 15;33(10):3038–3049. doi: 10.1021/bi00176a037. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lin C. S., Hackenberg H., Klingenberg E. M. The uncoupling protein from brown adipose tissue mitochondria is a dimer. A hydrodynamic study. FEBS Lett. 1980 May 5;113(2):304–306. doi: 10.1016/0014-5793(80)80614-4. [DOI] [PubMed] [Google Scholar]
- Mandon E. C., Milla M. E., Kempner E., Hirschberg C. B. Purification of the Golgi adenosine 3'-phosphate 5'-phosphosulfate transporter, a homodimer within the membrane. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10707–10711. doi: 10.1073/pnas.91.22.10707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschke W. C., Ballou C. E. Characterization of a yeast mannan containing N-acetyl-D-glucosamine as an immunochemical determinant. Biochemistry. 1972 Sep 26;11(20):3807–3816. doi: 10.1021/bi00770a021. [DOI] [PubMed] [Google Scholar]
- Rost B., Sander C. Improved prediction of protein secondary structure by use of sequence profiles and neural networks. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7558–7562. doi: 10.1073/pnas.90.16.7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. L., Nakajima T., Ballou C. E. Biosynthesis of yeast mannan. Isolation of Kluyveromyces lactis mannan mutants and a study of the incorporation of N-acetyl-D-glucosamine into the polysaccharide side chains. J Biol Chem. 1975 May 10;250(9):3426–3435. [PubMed] [Google Scholar]
- Toma L., Pinhal M. A., Dietrich C. P., Nader H. B., Hirschberg C. B. Transport of UDP-galactose into the Golgi lumen regulates the biosynthesis of proteoglycans. J Biol Chem. 1996 Feb 16;271(7):3897–3901. doi: 10.1074/jbc.271.7.3897. [DOI] [PubMed] [Google Scholar]


