Abstract
Changes in telomere length are believed to reflect changes in physiological state and life expectancy in animals. However, much remains unknown about the determinants of telomere dynamics in wild populations, and specifically the influence of conditions during highly mobile life-history stages, for example migration. We tested whether telomere dynamics were associated with migratory behaviour and/or with stress during reproduction in free-living seabirds. We induced short-term stress during reproduction in chick-rearing, black-legged kittiwakes (Rissa tridactyla), tracked winter migration with geolocators and measured telomere length before and after winter migration. We found that time spent at wintering grounds correlated with reduced telomere loss, while stress during reproduction accelerated telomere shortening. Our results suggest that different life-history stages interact to influence telomere length, and that migratory patterns may be important determinants of variation in an individual's telomere dynamics.
Keywords: overwintering, migratory behaviour, breeding, seasonal effects, carry-over, telomeres
1. Introduction
Telomeres, the protective caps at the ends of linear chromosomes, are thought to be indicators of the individual state of an animal in terms of biological age [1]. In some animals, telomere length predicts survival better than chronological age [2,3]. Telomeres shorten with each cell cycle [4,5], but various intrinsic and extrinsic factors accelerate or reduce telomere shortening [5]. One suggested important driver of telomere dynamics is stress mediated by glucocorticoids [6,7]. In wild animals, telomere shortening has recently also been associated with a general cost of reproduction [8,9], especially under suboptimal ecological conditions [10]. However, reproduction represents only a limited part of the annual cycle in animals with complex life cycles, and other more poorly investigated life-history stages potentially explain a substantial amount of the residual variation in telomere dynamics.
Migration is thought to benefit an animal's fitness by optimizing its access to predictably fluctuating resources [11,12]. In addition, extending migration is suggested to allow the recovery from a stressful reproductive period [8]. However, it is difficult to quantify the conditions migratory animals face during life-history stages at which they are highly mobile. Seabirds, for example, disperse on the open ocean and may migrate long distances from their breeding colonies during the non-breeding season. Most research on the physiological state of individuals has therefore focused on the effects of conditions during periods of restricted movement, for example the reproductive period, although the migratory stage may constitute a larger part of the annual cycle. Owing to the suggested positive effects of migration on individuals, we suspected that variation in migration behaviour could explain variation in telomere dynamics of a migratory species.
We investigated the effects of migratory stage duration and stress encountered during the reproductive stage on telomere dynamics in the black-legged kittiwake (Rissa tridactyla). Most kittiwakes in the Atlantic Ocean migrate from their breeding colonies to areas east of Labrador/Newfoundland, which are likely to provide good feeding conditions during winter [13]. Based on the assumption that kittiwakes migrate to southern marine regions because conditions there are favourable, we hypothesized that time spent at these wintering grounds exhibits a positive effect on telomere length. By contrast, based on our previous observation that kittiwakes in our study colony maintain parental effort when facing stress [14], and since reproduction is costly [15], we predicted that experimentally induced stress during reproduction has a negative effect on telomere length.
2. Material and methods
We fitted 25 kittiwakes in July–August 2011 with geolocators at their breeding colony in Svalbard, Norway (79°00′ N, 12°09′ E), to track their winter migration, of which 20 were recovered in the following reproductive season (2012). For 16 individuals, we obtained both winter locations and repeated telomere samples (see below). Wintering time was calculated as the number of days individuals spent south of latitude 70° N. For details of geolocator deployment and their analyses see [16]. Telomeres were sampled in both the year of deployment and the year of recovery. Briefly, whole blood was collected from the brachial vein of individuals and temporarily stored in EDTA buffer. Precipitated red blood cells were then transferred into glycerol buffer and stored at −80°C until laboratory analysis. Whole telomere smears were measured with the telomere restriction fragment method following the Southern blot hybridization protocol [10,17]. Details are provided in the electronic supplementary material. Telomere data of two individuals were excluded owing to poor quality of the telomere smears. This was done blindly, i.e. without knowledge of the birds' identities. Inclusion of these data points did not change the major conclusions of our study. Telomere change was calculated as per cent change relative to initial telomere length, but total change (i.e. change in number of base pairs) produced similar results.
To simulate short-term food stress during the reproductive season, a 25 mm silastic tube filled with crystallized corticosterone was implanted for a 3 day period in nine individuals when chicks were about 10 days old. Small incisions were made at the end of the implants to facilitate a rapid and effective release of the hormone, and implants were then administered subcutaneously between the shoulders. Seven individuals received empty implants and served as a control group (for more details, see [14]). Corticosterone-treated individuals had a higher reproductive performance than controls and thus represent a group with high investment in reproduction when facing stress [14].
We fitted linear models containing the terms ‘wintering time’, ‘stress treatment’ and ‘sex’ and their interactions (see the electronic supplementary material). We used Akaike's information criterion corrected for finite sample sizes (AICc) for model selection [18]. Model fit was evaluated based on ΔAICc, and model-averaged estimates were calculated for the terms included in models with ΔAICc values of less than 2.
3. Results
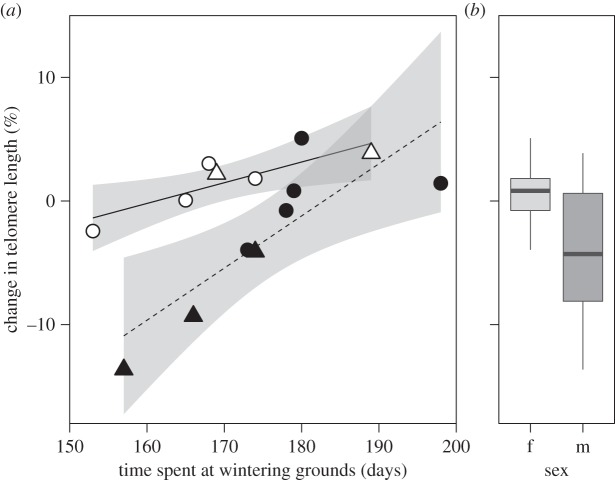
All models with ΔAICc values of less than 2 contained the terms ‘wintering time’ and ‘stress treatment’. Time spent at wintering grounds had a positive effect on telomere dynamics (figure 1a, model-averaged estimate: 3.56 ± 0.88 s.e.; see the electronic supplementary material). The slopes of this relationship were similar in both treatment groups (figure 1a), as indicated by high ΔAICc values for models including the interaction term ‘wintering time × stress treatment’ (see the electronic supplementary material). However, stress induced during reproduction resulted in an overall decrease in telomere length (figure 1a, model-averaged estimate: −4.38 ± 1.19 s.e.). Including information on sex of individuals (figure 1b) did not improve model fit, because the model including ‘sex’ was indistinguishable from the simpler model (ΔAICc = 0.70; see the electronic supplementary material).
Figure 1.
(a) Relationship between time spent at the wintering grounds (days south of 70° N) and change in telomere length (%) for control (open symbols, continuous line) and corticosterone-treated (filled symbols, dashed line). Males are indicated as triangles, females as circles. (b) Overall change of telomere length in both sexes. Box hinges correspond to the 25th and 75th percentiles, whiskers to values within 1.5× the interquartile range.
Initial telomere length, as an indicator of initial biological age, did not explain time spent at the wintering grounds (linear model: F1,14 = 1.705, p = 0.213).
4. Discussion
Our study provides novel evidence for a relationship between time spent at wintering grounds and telomere dynamics: birds that spent more time at the wintering grounds exhibited a reduced telomere shortening. This relationship was observed in both treatment groups, but birds that were exposed to a short-term stress during the reproductive season showed a larger telomere loss.
Theoretically, the relationship between telomeres and migration can be driven by chronological age, experience or individual quality of birds. The lack of an effect of initial telomere length on time spent at wintering grounds may indicate that age did not affect our results. However, until more detailed information is available on age-related and individual variation in telomere length and loss, we cannot exclude that age, experience and/or quality of individuals contributed to the observed pattern between migration and telomere dynamics.
Not surprisingly, experimentally induced stress during reproduction contributed to the shortening of telomeres. Glucocorticoid hormones may have a direct impact on telomere dynamics, e.g. by inhibiting the activity of telomerase, the enzyme that maintains telomeres [6]. Behavioural and physiological responses of kittiwakes in our study to elevated glucocorticoid levels may also help to explain this effect. As reported earlier, individuals in our study responded to experimentally elevated glucocorticoid levels with increased reproductive performance [14]. As reproductive investment competes with the maintenance of the soma [19], increased telomere shortening is likely to be a reflection of decreased self-investment. Our current empirical data, however, do not support a hypothesized [20] link between the adrenocortical and oxidative stress in the kittiwake (see the electronic supplementary material).
We suggest two possible explanations for why birds that spent more time south maintained their telomeres better. First, individuals could have benefited directly from positive effects of conditions they encountered at the wintering grounds. More time spent feeding in these areas, where food may be abundant [13], could provide birds with resources needed to run costly physiological maintenance machinery and/or to counteract oxidative damage, a factor that is believed to shorten telomeres [21]. According to this explanation, birds in our study that spent more time at the wintering grounds were able to fully recover from a costly bout of reproduction as reflected in their maintenance of telomeres. This is in line with previous studies that found migration facilitated recovery from reproduction [8]. As an alternative explanation, challenging conditions at the breeding grounds may have contributed to the shortening of telomeres. Spending time in the northern Arctic regions outside the peak of seasonal food productivity in this region could have resulted in food stress, which—as demonstrated in this study—can translate into telomere shortening. In addition, spending more time in cold Arctic conditions (temperatures at the colony are frequently below −10 and −20°C in late autumn and early spring, respectively) may increase the metabolic rates of birds. As a higher metabolism may increase oxidative stress if not counteracted by mitochondrial state or antioxidant status, this could have resulted in increased telomere shortening [21]. Additional migration data indicate that the timing of autumn departure rather than spring arrival was a more important determinant of telomere dynamics in kittiwakes (see the electronic supplementary material), which suggests that conditions during autumn at breeding or wintering grounds may play a more dominant role in governing the observed changes in telomere length. In summary, both favourable conditions at the wintering grounds and challenges at the breeding grounds may contribute to the positive effect of wintering time on telomere maintenance, and further investigations are needed to elucidate their specific importance.
In conclusion, our results suggest that conditions at the wintering grounds and the timing of migration, but also conditions encountered during reproduction, have a strong potential to impact biological ageing of animals. Changes in the environment that impose further constraints on any part of the annual cycle are, therefore, likely to affect individuals and populations. As migration behaviour in our study explained a major part of the variation in telomere dynamics, we encourage future research to make use of available technology to elucidate the influence of poorly studied life-history stages on the physiological state of animals.
Acknowledgements
We thank Vegard S. Bråthen, Dagfinn B. Skomsø, Jorg Welcker, Rebecca C. Young and Elin Noreen for assistance with the fieldwork, and Wojtek Moskal and the staff of the Sverdrup Research Station for logistic support.
Permission for all procedures was granted by the Norwegian Animal Research Authority, the Governor of Svalbard and University of Alaska IACUC.
Data accessibility
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.n4v8g.
Funding statement
Support was provided by the Research Council of Norway (no. 196181), the Svalbard Science Forum (Arctic Field Grants), the North Pacific Research Board (Graduate Student Research Award), the Fram Centre, and the French Polar Institute.
References
- 1.Monaghan P, Haussmann MF. 2006. Do telomere dynamics link lifestyle and lifespan? Trends Ecol. Evol. 21, 47–53 (doi:10.1016/J.Tree.2005.11.007) [DOI] [PubMed] [Google Scholar]
- 2.Bize P, Criscuolo F, Metcalfe NB, Nasir L, Monaghan P. 2009. Telomere dynamics rather than age predict life expectancy in the wild. Proc. R. Soc. B 276, 1679–1683 (doi:10.1098/rspb.2008.1817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salomons HM, Mulder GA, van de Zande L, Haussmann MF, Linskens MHK, Verhulst S. 2009. Telomere shortening and survival in free-living corvids. Proc. R. Soc. B 276, 3157–3165 (doi:10.1098/rspb.2009.0517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackburn EH. 1991. Structure and function of telomeres. Nature 350, 569–573 (doi:10.1038/350569a0) [DOI] [PubMed] [Google Scholar]
- 5.Sahin E, DePinho RA. 2010. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature 464, 520–528 (doi:10.1038/Nature08982) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haussmann MF, Marchetto NM. 2010. Telomeres: linking stress and survival, ecology and evolution. Curr. Zool. 56, 714–727 [Google Scholar]
- 7.Tomiyama AJ, et al. 2012. Does cellular aging relate to patterns of allostasis?: an examination of basal and stress reactive HPA axis activity and telomere length. Physiol. Behav. 106, 40–45 (doi:10.1016/j.physbeh.2011.11.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plot V, Criscuolo F, Zahn S, Georges JY. 2012. Telomeres, age and reproduction in a long-lived reptile. PLoS ONE 7, e40855 (doi:10.1371/journal.pone.0040855) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauch C, Becker PH, Verhulst S. 2013. Telomere length reflects phenotypic quality and costs of reproduction in a long-lived seabird. Proc. R. Soc. B 280, 20122540 (doi:10.1098/rspb.2012.2540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young RC, Kitaysky AS, Haussmann MF, Descamps S, Orben RA, Elliott KH, Gaston AJ. 2013. Age, sex, and telomere dynamics in a long-lived seabird with male-biased parental care. PLoS ONE 8, e74931 (doi:10.1371/journal.pone.0074931) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fryxell JM, Sinclair ARE. 1988. Causes and consequences of migration by large herbivores. Trends Ecol. Evol. 3, 237–241 (doi:10.1016/0169-5347(88)90166-8) [DOI] [PubMed] [Google Scholar]
- 12.Dingle H. 1996. Migration: the biology of life on the move. Cary, NC: Oxford University Press [Google Scholar]
- 13.Frederiksen M, et al. 2012. Multicolony tracking reveals the winter distribution of a pelagic seabird on an ocean basin scale. Divers. Distrib. 18, 530–542 (doi:10.1111/j.1472-4642.2011.00864.x) [Google Scholar]
- 14.Schultner J, Kitaysky AS, Gabrielsen GW, Hatch SA, Bech C. 2013. Differential reproductive responses to stress reveal the role of life history strategies within a species. Proc. R. Soc. B 280, 20132090 (doi:10.1098/rspb.2013.2090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golet GH, Irons DB, Estes JA. 1998. Survival costs of chick rearing in black-legged kittiwakes. J. Anim. Ecol. 67, 827–841 (doi:10.1046/j.1365-2656.1998.00233.x) [Google Scholar]
- 16.Schultner J, Moe B, Chastel O, Tartu S, Bech C, Kitaysky AS. 2013. Corticosterone mediates carry-over effects between breeding and migration in the kittiwake Rissa tridactyla. Mar. Ecol. Progr. Ser. (doi:10.3354/meps10603) [Google Scholar]
- 17.Haussmann MF, Mauck RA. 2008. New strategies for telomere-based age estimation. Mol. Ecol. Resour. 8, 264–274 (doi:10.1111/j.1471-8286.2007.01973.x). [DOI] [PubMed] [Google Scholar]
- 18.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach. New York, NY: Springer [Google Scholar]
- 19.Stearns SC. 1977. The evolution of life history traits: a critique of the theory and a review of the data. Annu. Rev. Ecol. Syst. 8, 145–171 (doi:10.1146/annurev.es.08.110177.001045) [Google Scholar]
- 20.Costantini D, Marasco V, Moller AP. 2011. A meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates. J. Comp. Physiol. B 181, 447–456 (doi:10.1007/S00360-011-0566-2) [DOI] [PubMed] [Google Scholar]
- 21.von Zglinicki T. 2002. Oxidative stress shortens telomeres. Trends Biochem. Sci. 27, 339–344 (doi:10.1016/S0968-0004(02)02110-2) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.n4v8g.