Abstract
Ion transport across the cell membrane mediated by channels and carriers participate in the regulation of tumour cell survival, death and motility. Moreover, the altered regulation of channels and carriers is part of neoplastic transformation. Experimental modification of channel and transporter activity impacts tumour cell survival, proliferation, malignant progression, invasive behaviour or therapy resistance of tumour cells. A wide variety of distinct Ca2+ permeable channels, K+ channels, Na+ channels and anion channels have been implicated in tumour growth and metastasis. Further experimental information is, however, needed to define the specific role of individual channel isoforms critically important for malignancy. Compelling experimental evidence supports the assumption that the pharmacological inhibition of ion channels or their regulators may be attractive targets to counteract tumour growth, prevent metastasis and overcome therapy resistance of tumour cells. This short review discusses the role of Ca2+ permeable channels, K+ channels, Na+ channels and anion channels in tumour growth and metastasis and the therapeutic potential of respective inhibitors.
Keywords: Ca2+ channels, K+ channels, anion channels, cell proliferation, migration, apoptosis
1. Introduction
Ion transport across the cell membrane plays a crucial role in fundamental tumour cell functions [1–3], such as cell volume regulation [4,5], migration [5], cell cycle progression [5,6], cell proliferation [5,6] as well as cell death [4,5]. All those functions are critically important for tumour cell survival and metastasis [1]. Moreover, ion channels participate in the regulation of other cell functions again relevant for migration [7], and thus metastasis. Ion transport across the membrane of non-tumour cells may further be decisive for tumour cell survival. For instance, ion channels participate in the regulation of tumour vascularization [8] and ion channels are important for the proliferation and response of immune cells attacking tumour cells [9].
Tumour-relevant ion channels are upregulated by growth factors and hormones [10] to the extent that a given ion channel is critically important for the survival of a tumour cell, this ion channel may be considered a target for the treatment of the respective tumour [11]. Needless to say, however, that only those channels are clinically applicable for the suppression of tumour growth, which do not serve critically important functions in other cells, for example channels required for cardiac repolarization. Moreover, ion channels may be relevant for the proliferation and survival of cells other than tumour cells.
This short synopsis discusses the significance of Ca2+ channels, K+ channels, Na+ channels and Cl− channels in the cell membrane. For the involvement of other channels or transporters, such as mitochondrial channels [12], cell membrane water channels [13], Na+/H+ exchanger [14,15], Na+,K+,2Cl− cotransporters [16–19], KCl cotransporters [20], Na+,K+-ATPase [4,21–33], MDR [34], as well as several H+ transporters, such as vacuolar H+-ATPases, H+/Cl− symporters, monocarboxylate transporters, or Na+-dependent Cl−/HCO3- exchangers (for reviews, see [35–38]) in cell proliferation, cell death, tumour growth and migration, the reader is referred to the respective reviews or original papers. Moreover, the reader is encouraged to read the other contributions of this special issue on this exciting topic. In this review, the case is made that ion channels and transporters are indeed critically important for tumour growth and metastasis and are thus potential targets in the treatment of malignancy.
2. Ca2+ permeable cation channels
Cytosolic Ca2+ activity plays a decisive role in the regulation of cell proliferation [39–42]. Alterations of cytosolic Ca2+ activity are important for entering and accomplishing the S and M phase of the cell cycle [43,44]. Along those lines, Ca2+ signalling is altered in proliferating tumour cells (for review, see [45]).
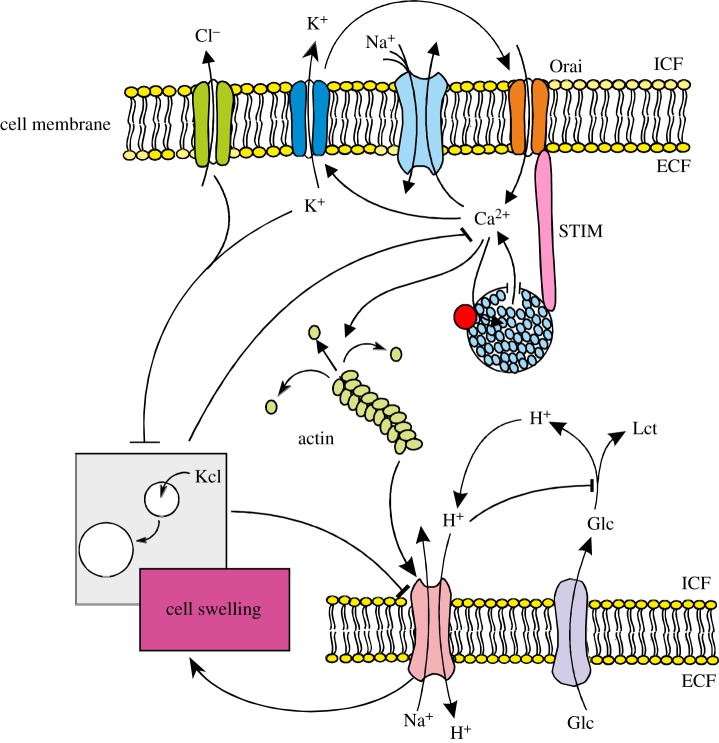
Growth factors stimulate store-operated Ca2+ entry (SOCE) or Ca2+ release-activated channels (CRACs) resulting in a CRAC current (ICRAC) [46,47], which in turn mediates Ca2+ entry and subsequent Ca2+ oscillations in proliferating cells. The Ca2+ oscillations govern a wide variety of cellular functions [39,40,42,48], including the depolymerization of actin filaments [49,50], which in turn leads to disinhibition of Na+/H+ exchanger and/or Na+,K+,2Cl− cotransporter resulting in an increase in cell volume [50]. Activation of ICRAC, Ca2+ oscillations, depolymerization of the actin filaments and cell volume increase are all prerequisites of cell proliferation [50]. It is intelligible that neither mitosis nor migration is possible without the timely depolymerization of actin filaments (figure 1).
Figure 1.
Tentative model of channels and Na+/H+ ion exchanger in cell proliferation. ICF, intracellular fluid; ECF, extracellular fluid; Glc, glucose; Lct, lactate; Orai/STIM = Ca2+ release-activated Ca2+ channel (adapted from Lang et al. [50]). (Online version in colour.)
The channel underlying SOCE or ICRAC is composed of the pore-forming units Orai1, 2 or 3 [51–54], which bind to their regulators STIM 1 or 2 [55–57]. Evidence points to a decisive role of Orai and STIM in the resistance to apoptosis, in proliferation and in migration of tumour cells [58–60]. Similarly, STIM1 silencing has been shown to inhibit the proliferation and to induce the arrest of the cell cycle at S and G2/M phase of cervical cancer cells [59].
Further channels contributing to altered Ca2+ signalling in tumour cells include Ca2+-permeable cation channels belonging to the canonical, melastatin and vanilloid families of transient receptor potential (TRP) channels (for review, see [61]). TRP channels are expressed in tumours [62]. Ca2+ entry through TRP channels may inhibit apoptosis [63,64], an effect partially attributed to the stimulation of NF-κB [64]. Moreover, TRPP2 channels may, by an increase in the endoplasmatic reticulum Ca2+ permeability, deplete Ca2+ stores thus blunting the intracellular Ca2+ release upon apoptotic stimuli [4,65].
In contrast to the oscillating increase in cytosolic Ca2+ activity in proliferating cells, a lasting increase in cytosolic Ca2+ activity owing to sustained Ca2+ entry through Ca2+-permeable cation channels may result in apoptosis [4,42,48,66–70] by affecting mitochondrial integrity [71,72] as well as stimulating proteinases, inducing cell shrinkage owing to the activation of Ca2+-sensitive K+ channels and triggering cell membrane scrambling [4]. Apoptosis-stimulating Ca2+ channels or unselective cation channels include NMDA receptors [73], purinergic receptors [74,75], as well as the TRP channels [76] TRPC1 [77], TRPC3 [78], TRPC6 [79–81], TRPM2 [82–84], TRPM8 [85,86], TRPML2 [87], TRPP5 [88], TRPV1 [71,89–91], TRPV2 [92,93] and TRPV4 [94].
In glioma cells, TRPC1 is required for cytokinesis in proliferation and migration [95,96]. Ca2+ entry through TRPM8 channels leads to the activation of Ca2+-sensitive K+ channels (KCa1.1), which participate in the machinery accomplishing migration [5,97]. The K+ channels can obviously be activated, however, even in the absence of external Ca2+ [5,97,98].
3. K+ channels
Ca2+ entry through Orai and similar channels requires polarization of the cell membrane and thus the activity of K+ channels, which are decisive for cell proliferation [5,99]. Along those lines, the pharmacological inhibition of K+ channels may compromise cell proliferation [100,101]. The cell membrane of tumour cells is, however, rather depolarized compared with excitable cells, epithelia or differentiated glial cells [102]. In glial cells, the depolarized state is associated with decreased Kir4.1 activity [5,103]. However, in ras oncogene-expressing fibroblasts repetitive activation of Ca2+-sensitive K+ channels resulted in short hyperpolarizing spikes owing to oscillations of cytosolic Ca2+ activity with the repetitive activation of Ca2+-sensitive K+ channels [47].
In glioma cells, membrane depolarization is apparently required for cell proliferation [5], as overexpression of Kir4.1 inhibited and blockade of Kir4.1 channels stimulated cell proliferation [103,104]. On the other hand, the inhibition of Kir channels has been shown to slow down cell proliferation [105,106].
A variety of tumour cells express Kv10.1 [107,108] and/or Kv11.1 channels [109]. Pharmacological inhibition of Kv10.1 interferes with the proliferation and migration of several myeloid leukaemia cell lines and expression of Kv10.1 in myeloid leukaemias was correlated with higher relapse rates and a significantly shorter overall survival [108]. Kv11.1 is similarly important for tumour cell proliferation and survival [106,109].
K+ channels participate in the machinery regulating cell cycle [110,111]. K+ channels may affect proliferation further by altering cell volume [5]. Along those lines, cell proliferation may be inhibited by the blockade of the voltage-gated K+ channels Kv1.3 and Kv1.5 [112,113] or ATP-sensitive K+ channels [111].
Ca2+-activated K+ channels, for example KCa1.1, participate in the regulation of migration [5,114]. Accordingly, KCa1.1 inhibition decreases migration [115].
K+ exit following the activation of K+ channels decreases cytosolic K+ concentration [4]. Cellular loss of K+ and organic osmolytes favours apoptosis [4]. The impact of K+ channels on apoptosis depends on the cell type and channel [5,116]. In glioma cells, the inhibition of outwardly rectifying K+ channels may trigger apoptosis [100,101]. Moreover, the inhibition of Ca2+-sensitive K+ channels may foster apoptosis [117–119]. On the other hand, sustained K+ channel activity may trigger apoptosis [116,120].
4. Na+ channels
A variety of carcinoma cells express voltage-gated Na+ channels [1,3,10]. Voltage-gated Na+ channels are particularly active in strongly metastatic cells where they appear to stimulate their functional expression thus establishing a positive feedback [10]. The Na+ current through voltage-gated Na+ channels enhances migration, invasion and metastasis in vivo [121]. It is tempting to speculate that the expression of voltage-gated Na+ channels accelerates depolarization with the subsequent more rapid and stronger activation of voltage-gated K+ channels thus increasing the frequency of Ca2+ oscillations. Beyond that, β subunits of the channels apparently mediate cellular adhesion and process extension [121]. Expression of the Nav1.5α subunit is correlated with poor prognosis in breast cancer [121]. Some evidence points to a decisive role of the hypoxia-sensitive persistent component of the voltage-gated Na+ channel current [1].
5. Anion channels
Activation of anion channels is followed by the exit of Cl−, organic osmolytes and HCO−3 [4]. In glioma cells, Na+,K+,2Cl− cotransporter activity [122] leads to intracellular Cl− accumulation up to concentrations of some 100 mM [123]. The high cytosolic Cl− activity and the sizable Cl− conductance result in depolarization of the glioma membrane potential [5,124]. The depolarization following the exit of anions drives K+ exit. Cellular loss of KCl and organic osmolytes lead to cell shrinkage [4]. A decrease in cell volume is observed immediately prior to the M phase, a phenomenon termed ‘premitotic condensation’ [123,125]. Cl− channel blockers prevent Cl− exit, and thus premitotic cellular condensation [5].
Moreover, the activation of Cl− channels and cell shrinkage are required to trigger Ca2+ oscillations [126], which are in turn required for the initiation of actin depolymerization (see above). It is tempting to speculate that premitotic condensation is triggering the Ca2+ oscillations with the subsequent depolymerization of the actin filamental cytoskeleton, thus setting the stage for mitosis. Osmotic cell swelling may slow down transition through the cell cycle and counteract cell proliferation [123,125]. During M-phase, both the Cl− conductance [125] and the expression levels of ClC-3 Cl− channels [123] are high. Pharmacological or genetic knockdown of ClC-3 decreases Cl− conductance, blunts premitotic condensation and delays the cell cycle [5,123,125].
Cl− channels important for cell proliferation, cell migration and metastasis further include anoctamin 1 (TMEM16A, Ano1), which is activated by the increase in cytosolic Ca2+ activity [127]. Ano1 expression is excessive in several gastrointestinal stromal tumours [127]. Notably, Ano1 apparently does not support cell proliferation in all cell types [127] and the isoform Ano6 triggers apoptosis rather than proliferation [127].
Cell volume changes have been suggested to modify cell proliferation by affecting cytoskeletal architecture [128], cell size checkpoints [112], cytosolic nutrient concentration [112], gene expression [129] and macromolecular crowding [112,128]. Macromolecular crowding may in turn be effective by modifying activity of kinases or further signalling molecules [50,112,125,130].
Not only increased but as well decreased cell volume inhibits cell proliferation [128]. Obviously, proliferating cells have to double their size, membrane and intracellular constituents in order to divide into two daughter cells of the same size as the parent cell.
Anion channels are further important for cell migration [5]. Cl− channel inhibitors [131–133] or the replacement of extracellular Cl− with impermeant anions [134] decrease migration. Genetic knockdown of ClC-3 similarly decreases the migration of glioma cells [134].
Cl− channels are further involved in apoptosis [5,135–138]. Cl− channel inhibitors counteract apoptotic cell shrinkage and activation of caspases [130]. Moreover, excessive hyperosmotic shock stimulates apoptosis [130]. Thus, enhanced Cl− channel activity may lead to death rather than proliferation of tumour cells. Similar to the impact of Ca2+ entry, the impact of Cl− channel activity may depend on the temporal pattern of the channel activity.
6. Ion channels as drug targets
Ion channels are ideal drug targets as the respective small molecules may be effective from the extracellular space and need not to enter the target cells. Thus, tumour cells are not able to protect themselves by expressing drug exporting carriers or pumps. It is indeed becoming increasingly clear that the inhibition of ion channels is effective in halting tumour growth and metastasis [3,5,139]. The use of channel inhibitors is, however, limited by side effects, if the target channels are required for decisive physiological functions, for example cardiac repolarization. Along those lines, the inhibition of Kv11.1 (HERG) channels may lead to long QT syndrome, severe cardiac arrhythmia and sudden cardiac death [140]. Nevertheless, several ion channel modulators are already in clinical use or currently tested in clinical trials [5,141]. Moreover, ion channels are considered as targets for vaccination against tumour-associated antigens [142]. The extracellular domains of the channels and transporters are accessible for antibodies [143]. Several clinical trials and mouse models highlight the feasibility of attacking tumours by targeting channels [144]. Those include the Cl− channel inhibitor tamoxifen [145] and chlorotoxin or TM-601 [146–148], substances accomplishing internalization of ClC-3 thus inhibiting migration of glioma cells [5,99]. Inhibitors of voltage-gated Na+ channels may be particularly valuable in the suppression of tumour metastasis [1,121]. In this respect, the voltage-gated Na+ channel blockers ranolazine and riluzole have been claimed as anti-metastatic agents [1]. Those substances are already in use for chronic treatment of cardiac angina and amyotrophic lateral sclerosis and could thus be considered relatively safe [1].
7. Conclusion
Several ion channels participate in the regulation of both cell proliferation and apoptosis. Stimulation of tumour cell proliferation may be paralleled by the following simplified scenario: the activation of Cl− channels leads to Cl− exit, depolarization of the cell membrane with subsequent increase in the driving force for K+ exit. The cellular loss of KCl leads to premitotic condensation, which in turn is required for triggering Ca2+ oscillations. The oscillations of cytosolic Ca2+ concentration lead to actin filament depolymerization, a prerequisite for entry of the cell into mitosis. The depolymerization of the cytoskeleton disinhibits the Na+/H+ exchanger Na+,K+,2Cl− cotransport, thus resulting in increase in cell volume and cytosolic alkalinization. The increase in cell volume is required for the generation of two daughter cells of the same size as the parent cell, the cytosolic alkalinization fosters the glycolytic flux, the main energy source of tumour cells. Needless to say that this simplified scheme ignores the majority of causal relationships in the complex machinery eventually doubling the cell number.
Besides their role in the regulation of cell proliferation, Ca2+ permeable cation channels, K+ channels and anion channels may participate in the triggering of suicidal cell death thus decreasing instead of increasing the cell number. Thus, Cl− channels, K+ channels and Ca2+ permeable channels participate in the machinery of both, cell proliferation and suicidal cell death. The eventual outcome depends on further properties of the cell. For instance, oscillating K+ channel activity in proliferating cells [47,149] differs from the sustained K+ channel activation in apoptotic cells [150]. The short-lived increases in cytosolic Ca2+ concentration triggered by oscillatory Ca2+ channel activity depolymerize the cytoskeleton [120,151–153] but are presumably too short to activate caspases [154] or to trigger cell membrane scrambling [155,156]. Moreover, the eventual outcome may depend on the extent of channel activation. The amplitude of TASK-3 K+ channel activity during apoptosis is one order of magnitude higher than the activity of the same channels in tumour cells [157,158]. Thus, the delicate machinery leading to cell proliferation may turn into triggering of cell death by subtle alterations of temporal organization and extent of channel activity.
References
- 1.Djamgoz MB, Onkal R. 2013. Persistent current blockers of voltage-gated sodium channels: a clinical opportunity for controlling metastatic disease. Recent Patents Anticancer Drug Discov. 8, 66–84. [DOI] [PubMed] [Google Scholar]
- 2.Arcangeli A, Becchetti A. 2006. Complex functional interaction between integrin receptors and ion channels. Trends Cell Biol. 16, 631–639. ( 10.1016/j.tcb.2006.10.003) [DOI] [PubMed] [Google Scholar]
- 3.Huber SM. 2013. Oncochannels. Cell Calcium 53, 241–255. ( 10.1016/j.ceca.2013.01.001) [DOI] [PubMed] [Google Scholar]
- 4.Lang F, Hoffmann EK. 2012. Role of ion transport in control of apoptotic cell death. Compr. Physiol. 2m 2037–2061. [DOI] [PubMed] [Google Scholar]
- 5.Turner KL, Sontheimer H. 2014. Cl− and K+ channels and their role in primary brain tumour biology. Phil. Trans. R. Soc. B 369, 20130095 ( 10.1098/rstb.2013.0095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becchetti A. 2011. Ion channels and transporters in cancer. 1. Ion channels and cell proliferation in cancer. Am. J. Physiol. Cell Physiol. 301, C255–C265. ( 10.1152/ajpcell.00047.2011) [DOI] [PubMed] [Google Scholar]
- 7.Kessler D, Gruen GC, Heider D, Morgner J, Reis H, Schmid KW, Jendrossek V. 2012. The action of small GTPases Rab11 and Rab25 in vesicle trafficking during cell migration. Cell Physiol. Biochem. 29, 647–656. ( 10.1159/000295249) [DOI] [PubMed] [Google Scholar]
- 8.Fiorio Pla A, Munaron L. 2014. Functional properties of ion channels and transporters in tumour vascularization. Phil. Trans. R. Soc. B 369, 20130103 ( 10.1098/rstb.2013.0103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panyi G, Beeton C, Felipe A. 2014. Ion channels and anti-cancer immunity. Phil. Trans. R. Soc. B 369, 20130106 ( 10.1098/rstb.2013.0106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraser SP, Ozerlat-Gunduz I, Brackenbury WJ, Fitzgerald EM, Campbell TM, Coombes RC, Djamgoz MBA. 2014. Regulation of voltage-gated sodium channel expression in cancer: hormones, growth factors and auto-regulation. Phil. Trans. R. Soc. B 369, 20130105 ( 10.1098/rstb.2013.0105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oosterwijk E, Gillies RJ. 2014. Targeting ion transport in cancer. Phil. Trans. R. Soc. B 369, 20130107 ( 10.1098/rstb.2013.0107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leanza L, Biasutto L, Manago A, Gulbins E, Zoratti M, Szabo I. 2013. Intracellular ion channels and cancer. Front. Physiol. 4, 227 ( 10.3389/fphys.2013.00227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tie L, Lu N, Pan XY, Pan Y, An Y, Gao JW, Lin YH, Yu HM, Li XJ. 2012. Hypoxia-induced up-regulation of aquaporin-1 protein in prostate cancer cells in a p38-dependent manner. Cell Physiol. Biochem. 29, 269–280. ( 10.1159/000337608) [DOI] [PubMed] [Google Scholar]
- 14.Reshkin SJ, Greco MR, Cardone RA. 2014. Role of pHi, and proton transporters in oncogene-driven neoplastic transformation. Phil. Trans. R. Soc. B 369, 20130100 ( 10.1098/rstb.2013.0100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen AP, Moreira JMA, Pedersen SF. 2014. Interactions of ion transporters and channels with cancer cell metabolism and the tumour microenvironment. Phil. Trans. R. Soc. B 369, 20130098 ( 10.1098/rstb.2013.0098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maeno E, Shimizu T, Okada Y. 2006. Normotonic cell shrinkage induces apoptosis under extracellular low Cl conditions in human lymphoid and epithelial cells. Acta Physiol. 187, 217–222. ( 10.1111/j.1748-1716.2006.01554.x) [DOI] [PubMed] [Google Scholar]
- 17.Maeno E, Takahashi N, Okada Y. 2006. Dysfunction of regulatory volume increase is a key component of apoptosis. FEBS Lett. 580, 6513–6517. ( 10.1016/j.febslet.2006.10.074) [DOI] [PubMed] [Google Scholar]
- 18.Nukui M, Shimizu T, Okada Y. 2006. Normotonic cell shrinkage induced by Na+ deprivation results in apoptotic cell death in human epithelial HeLa cells. J. Physiol. Sci. 56, 335–339. ( 10.2170/physiolsci.RP009606) [DOI] [PubMed] [Google Scholar]
- 19.Marklund L, Henriksson R, Grankvist K. 2001. Cisplatin-induced apoptosis of mesothelioma cells is affected by potassium ion flux modulator amphotericin B and bumetanide. Int. J. Cancer 93, 577–583. ( 10.1002/ijc.1363) [DOI] [PubMed] [Google Scholar]
- 20.Gagnon KB. 2012. High-grade glioma motility reduced by genetic knockdown of KCC3. Cell Physiol. Biochem. 30, 466–476. ( 10.1159/000339040) [DOI] [PubMed] [Google Scholar]
- 21.Bortner CD, Gomez-Angelats M, Cidlowski JA. 2001. Plasma membrane depolarization without repolarization is an early molecular event in anti-Fas-induced apoptosis. J. Biol. Chem. 276, 4304–4314. ( 10.1074/jbc.M005171200) [DOI] [PubMed] [Google Scholar]
- 22.Chueh SC, Guh JH, Chen J, Lai MK, Teng CM. 2001. Dual effects of ouabain on the regulation of proliferation and apoptosis in human prostatic smooth muscle cells. J. Urol. 166, 347–353. ( 10.1016/S0022-5347(05)66157-5) [DOI] [PubMed] [Google Scholar]
- 23.Esteves MB, Marques-Santos LF, Affonso-Mitidieri OR, Rumjanek VM. 2005. Ouabain exacerbates activation-induced cell death in human peripheral blood lymphocytes. An. Acad. Bras. Cienc. 77, 281–292. ( 10.1590/S0001-37652005000200008) [DOI] [PubMed] [Google Scholar]
- 24.Lang H, Schulte BA, Schmiedt RA. 2005. Ouabain induces apoptotic cell death in type I spiral ganglion neurons, but not type II neurons. J. Assoc. Res. Otolaryngol. 6, 63–74. ( 10.1007/s10162-004-5021-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McConkey DJ, Lin Y, Nutt LK, Ozel HZ, Newman RA. 2000. Cardiac glycosides stimulate Ca2+ increases and apoptosis in androgen-independent, metastatic human prostate adenocarcinoma cells. Cancer Res. 60, 3807–3812. [PubMed] [Google Scholar]
- 26.Nobel CSI, Aronson JK, van den Dobbelsteen DJ, Slater AFG. 2000. Inhibition of Na+/K+-ATPase may be one mechanism contributing to potassium efflux and cell shrinkage in CD95-induced apoptosis. Apoptosis 5, 153–163. ( 10.1023/A:1009684713784) [DOI] [PubMed] [Google Scholar]
- 27.Olej B, dos Santos NF, Leal L, Rumjanek VM. 1998. Ouabain induces apoptosis on PHA-activated lymphocytes. Biosci. Rep. 18, 1–7. ( 10.1023/A:1022259832207) [DOI] [PubMed] [Google Scholar]
- 28.Xiao AY, Wei L, Xia S, Rothman S, Yu SP. 2002. Ionic mechanism of ouabain-induced concurrent apoptosis and necrosis in individual cultured cortical neurons. J. Neurosci. 22, 1350–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu SP. 2003. Na+, K+-ATPase: the new face of an old player in pathogenesis and apoptotic/hybrid cell death. Biochem. Pharmacol. 66, 1601–1609. ( 10.1016/S0006-2952(03)00531-8) [DOI] [PubMed] [Google Scholar]
- 30.Sen N, Das BB, Ganguly A, Mukherjee T, Bandyopadhyay S, Majumder HK. 2004. Camptothecin-induced imbalance in intracellular cation homeostasis regulates programmed cell death in unicellular hemoflagellate Leishmania donovani. J. Biol. Chem. 279, 52 366–52 375. ( 10.1074/jbc.M406705200) [DOI] [PubMed] [Google Scholar]
- 31.Wang XQ, Xiao AY, Sheline C, Hyrc K, Yang A, Goldberg MP, Choi DW, Yu SP. 2003. Apoptotic insults impair Na+, K+-ATPase activity as a mechanism of neuronal death mediated by concurrent ATP deficiency and oxidant stress. J. Cell Sci. 116, 2099–2110. ( 10.1242/jcs.00420) [DOI] [PubMed] [Google Scholar]
- 32.Wang XQ, Xiao AY, Yang A, Larose L, Wei L, Yu SP. 2003. Block of Na+,K+-ATPase and induction of hybrid death by 4-aminopyridine in cultured cortical neurons. J. Pharmacol. Exp. Ther. 305, 502–506. ( 10.1124/jpet.102.045013) [DOI] [PubMed] [Google Scholar]
- 33.Panayiotidis MI, Bortner CD, Cidlowski JA. 2006. On the mechanism of ionic regulation of apoptosis: would the Na+/K+-ATPase please stand up? Acta Physiol. 187, 205–215. ( 10.1111/j.1748-1716.2006.01562.x) [DOI] [PubMed] [Google Scholar]
- 34.Hoffmann EK, Lambert IH. 2014. Ion channels and transporters in the development of drug resistance in cancer cells. Phil. Trans. R. Soc. B 369, 20130109 ( 10.1098/rstb.2013.0109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harguindey S, Arranz JL, Wahl ML, Orive G, Reshkin SJ. 2009. Proton transport inhibitors as potentially selective anticancer drugs. Anticancer Res. 29, 2127–2136. [PubMed] [Google Scholar]
- 36.Harguindey S, Orive G, Luis Pedraz J, Paradiso A, Reshkin SJ. 2005. The role of pH dynamics and the Na+/H+ antiporter in the etiopathogenesis and treatment of cancer. Two faces of the same coin—one single nature. Biochim. Biophys. Acta 1756, 1–24. ( 10.1016/j.bbcan.2005.06.004) [DOI] [PubMed] [Google Scholar]
- 37.Cardone RA, Casavola V, Reshkin SJ. 2005. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat. Rev. Cancer 5, 786–795. ( 10.1038/nrc1713) [DOI] [PubMed] [Google Scholar]
- 38.Parks SK, Chiche J, Pouyssegur J. 2011. pH control mechanisms of tumor survival and growth. J. Cell Physiol. 226, 299–308. ( 10.1002/jcp.22400) [DOI] [PubMed] [Google Scholar]
- 39.Berridge MJ, Bootman MD, Roderick HL. 2003. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4, 517–529. ( 10.1038/nrm1155) [DOI] [PubMed] [Google Scholar]
- 40.Berridge MJ, Bootman MD, Lipp P. 1998. Calcium: a life and death signal. Nature 395, 645–648. ( 10.1038/27094) [DOI] [PubMed] [Google Scholar]
- 41.Prevarskaya N, Ouadid-Ahidouch H, Skryma R, Shuba Y. 2014. Remodelling of Ca2+ transport in cancer: how it contributes to cancer hallmarks? Phil. Trans. R. Soc. B 369, 20130097 ( 10.1098/rstb.2013.0097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parekh AB, Penner R. 1997. Store depletion and calcium influx. Physiol. Rev. 77, 901–930. [DOI] [PubMed] [Google Scholar]
- 43.Steinhardt RA, Alderton J. 1988. Intracellular free calcium rise triggers nuclear envelope breakdown in the sea urchin embryo. Nature 332, 364–366. ( 10.1038/332364a0) [DOI] [PubMed] [Google Scholar]
- 44.Taylor JT, Zeng XB, Pottle JE, Lee K, Wang AR, Yi SG, Scruggs JA, Sikka SS, Li M. 2008. Calcium signaling and T-type calcium channels in cancer cell cycling. World J. Gastroenterol. 14, 4984–4991. ( 10.3748/wjg.14.4984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roderick HL, Cook SJ. 2008. Ca2+ signalling checkpoints in cancer: remodelling Ca2+ for cancer cell proliferation and survival. Nat. Rev. Cancer 8, 361–375. ( 10.1038/nrc2374) [DOI] [PubMed] [Google Scholar]
- 46.Qian D, Weiss A. 1997. T cell antigen receptor signal transduction. Curr. Opin. Cell Biol. 9, 205–212. ( 10.1016/S0955-0674(97)80064-6) [DOI] [PubMed] [Google Scholar]
- 47.Lang F, Friedrich F, Kahn E, Woll E, Hammerer M, Waldegger S, Maly K, Grunicke H. 1991. Bradykinin-induced oscillations of cell membrane potential in cells expressing the Ha-ras oncogene. J. Biol. Chem. 266, 4938–4942. [PubMed] [Google Scholar]
- 48.Berridge MJ, Lipp P, Bootman MD. 2000. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1, 11–21. ( 10.1038/35036035) [DOI] [PubMed] [Google Scholar]
- 49.Dartsch PC, Ritter M, Haussinger D, Lang F. 1994. Cytoskeletal reorganization in NIH 3T3 fibroblasts expressing the ras oncogene. Eur. J. Cell Biol. 63, 316–325. [PubMed] [Google Scholar]
- 50.Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D. 1998. Functional significance of cell volume regulatory mechanisms. Physiol. Rev. 78, 247–306. [DOI] [PubMed] [Google Scholar]
- 51.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. 2006. Orai1 is an essential pore subunit of the CRAC channel. Nature 443, 230–233. ( 10.1038/nature05122) [DOI] [PubMed] [Google Scholar]
- 52.Putney JW., Jr 2007. New molecular players in capacitative Ca2+ entry. J. Cell Sci. 120, 1959–1965. ( 10.1242/jcs.03462) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vig M, et al. 2006. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 312, 1220–1223. ( 10.1126/science.1127883) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. 2006. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature 443, 226–229. ( 10.1038/nature05108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peinelt C, et al. 2006. Amplification of CRAC current by STIM1 and CRACM1 (Orai1). Nat. Cell Biol. 8, 771–773. ( 10.1038/ncb1435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Penna A, Demuro A, Yeromin AV, Zhang SL, Safrina O, Parker I, Cahalan MD. 2008. The CRAC channel consists of a tetramer formed by Stim-induced dimerization of Orai dimers. Nature 456, 116–120. ( 10.1038/nature07338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. 2005. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437, 902–905. ( 10.1038/nature04147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prevarskaya N, Skryma R, Shuba Y. 2011. Calcium in tumour metastasis: new roles for known actors. Nat. Rev. Cancer 11, 609–618. ( 10.1038/nrc3105) [DOI] [PubMed] [Google Scholar]
- 59.Chen YF, Chiu WT, Chen YT, Lin PY, Huang HJ, Chou CY, Chang HC, Tang MJ, Shen MR. 2011. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc. Natl Acad. Sci. USA 108, 15 225–15 230. ( 10.1073/pnas.1103315108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Flourakis M, et al. 2010. Orai1 contributes to the establishment of an apoptosis-resistant phenotype in prostate cancer cells. Cell Death Dis. 1, e75 ( 10.1038/cddis.2010.52) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chung LC, Tsui KH, Feng TH, Lee SL, Chang PL, Juang HH. 2011. Curcumin provides potential protection against the activation of hypoxia and prolyl 4-hydroxylase inhibitors on prostate-specific antigen expression in human prostate carcinoma cells. Mol. Nutr. Food Res. 55, 1666–1676. ( 10.1002/mnfr.201100328) [DOI] [PubMed] [Google Scholar]
- 62.Dhennin-Duthille I, Gautier M, Faouzi M, Guilbert A, Brevet M, Vaudry D, Ahidouch A, Sevestre H, Ouadid-Ahidouch H. 2011. High expression of transient receptor potential channels in human breast cancer epithelial cells and tissues: correlation with pathological parameters. Cell Physiol. Biochem. 28, 813–822. ( 10.1159/000335795) [DOI] [PubMed] [Google Scholar]
- 63.Selvaraj S, Watt JA, Singh BB. 2009. TRPC1 inhibits apoptotic cell degeneration induced by dopaminergic neurotoxin MPTP/MPP+. Cell Calcium 46, 209–218. ( 10.1016/j.ceca.2009.07.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thippegowda PB, Singh V, Sundivakkam PC, Xue J, Malik AB, Tiruppathi C. 2010. Ca2+ influx via TRPC channels induces NF-kappaB-dependent A20 expression to prevent thrombin-induced apoptosis in endothelial cells. Am. J. Physiol. Cell Physiol. 298, C656–C664. ( 10.1152/ajpcell.00456.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wegierski T, Steffl D, Kopp C, Tauber R, Buchholz B, Nitschke R, Kuehn EW, Walz G, Kottgen M. 2009. TRPP2 channels regulate apoptosis through the Ca2+ concentration in the endoplasmic reticulum. EMBO J. 28, 490–499. ( 10.1038/emboj.2008.307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fang KM, Chang WL, Wang SM, Su MJ, Wu ML. 2008. Arachidonic acid induces both Na+ and Ca2+ entry resulting in apoptosis. J. Neurochem. 104, 1177–1189. ( 10.1111/j.1471-4159.2007.05022.x) [DOI] [PubMed] [Google Scholar]
- 67.Svoboda N, Pruetting S, Grissmer S, Kerschbaum HH. 2009. cAMP-dependent chloride conductance evokes ammonia-induced blebbing in the microglial cell line, BV-2. Cell Physiol. Biochem. 24, 53–64. ( 10.1159/000227813) [DOI] [PubMed] [Google Scholar]
- 68.Green DR, Reed JC. 1998. Mitochondria and apoptosis. Science 281, 1309–1312. ( 10.1126/science.281.5381.1309) [DOI] [PubMed] [Google Scholar]
- 69.Liu XH, Kirschenbaum A, Yu K, Yao S, Levine AC. 2005. Cyclooxygenase-2 suppresses hypoxia-induced apoptosis via a combination of direct and indirect inhibition of p53 activity in a human prostate cancer cell line. J. Biol. Chem. 280, 3817–3823. ( 10.1074/jbc.M406577200) [DOI] [PubMed] [Google Scholar]
- 70.Spassova MA, Soboloff J, He LP, Hewavitharana T, Xu W, Venkatachalam K, van Rossum DB, Patterson RL, Gill DL. 2004. Calcium entry mediated by SOCs and TRP channels: variations and enigma. Biochim. Biophys. Acta 1742, 9–20. ( 10.1016/j.bbamcr.2004.09.001) [DOI] [PubMed] [Google Scholar]
- 71.Hu F, Sun WW, Zhao XT, Cui ZJ, Yang WX. 2008. TRPV1 mediates cell death in rat synovial fibroblasts through calcium entry-dependent ROS production and mitochondrial depolarization. Biochem. Biophys. Res. Commun. 369, 989–993. ( 10.1016/j.bbrc.2008.02.155) [DOI] [PubMed] [Google Scholar]
- 72.Santo-Domingo J, Demaurex N. 2010. Calcium uptake mechanisms of mitochondria. Biochim. Biophys. Acta 1797, 907–912. ( 10.1016/j.bbabio.2010.01.005) [DOI] [PubMed] [Google Scholar]
- 73.Villmann C, Becker CM. 2007. On the hypes and falls in neuroprotection: targeting the NMDA receptor. Neuroscientist 13, 594–615. ( 10.1177/1073858406296259) [DOI] [PubMed] [Google Scholar]
- 74.Diaz-Hernandez M, Diez-Zaera M, Sanchez-Nogueiro J, Gomez-Villafuertes R, Canals JM, Alberch J, Miras-Portugal MT, Lucas JJ. 2009. Altered P2X7-receptor level and function in mouse models of Huntington's disease and therapeutic efficacy of antagonist administration. FASEB J. 23, 1893–1906. ( 10.1096/fj.08-122275) [DOI] [PubMed] [Google Scholar]
- 75.Wang W, Xiao J, Adachi M, Liu Z, Zhou J. 2011. 4-aminopyridine induces apoptosis of human acute myeloid leukemia cells via increasing [Ca2+]i through P2X7 receptor pathway. Cell Physiol. Biochem. 28, 199–208. ( 10.1159/000331731) [DOI] [PubMed] [Google Scholar]
- 76.Abramowitz J, Birnbaumer L. 2009. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 23, 297–328. ( 10.1096/fj.08-119495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kusaba T, et al. 2010. Klotho is associated with VEGF receptor-2 and the transient receptor potential canonical-1 Ca2+ channel to maintain endothelial integrity. Proc. Natl Acad. Sci. USA 107, 19 308–19 313. ( 10.1073/pnas.0913844107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shan D, Marchase RB, Chatham JC. 2008. Overexpression of TRPC3 increases apoptosis but not necrosis in response to ischemia-reperfusion in adult mouse cardiomyocytes. Am. J. Physiol. Cell Physiol. 294, C833–C841. ( 10.1152/ajpcell.00313.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mukerji N, Damodaran TV, Winn MP. 2007. TRPC6 and FSGS: the latest TRP channelopathy. Biochim. Biophys. Acta 1772, 859–868. ( 10.1016/j.bbadis.2007.03.005) [DOI] [PubMed] [Google Scholar]
- 80.Sun YH, Li YQ, Feng SL, Li BX, Pan ZW, Xu CQ, Li TT, Yang BF. 2010. Calcium-sensing receptor activation contributed to apoptosis stimulates TRPC6 channel in rat neonatal ventricular myocytes. Biochem. Biophys. Res. Commun. 394, 955–961. ( 10.1016/j.bbrc.2010.03.096) [DOI] [PubMed] [Google Scholar]
- 81.Yu L, Lin Q, Liao H, Feng J, Dong X, Ye J. 2010. TGF-beta1 induces podocyte injury through Smad3-ERK-NF-kappaB pathway and Fyn-dependent TRPC6 phosphorylation. Cell Physiol. Biochem. 26, 869–878. ( 10.1159/000323996) [DOI] [PubMed] [Google Scholar]
- 82.Gao Y, Lei Z, Lu C, Roisen FJ, El Mallakh RS. 2010. Effect of ionic stress on apoptosis and the expression of TRPM2 in human olfactory neuroepithelial-derived progenitors. World J. Biol. Psychiatry 11, 972–984. ( 10.3109/15622975.2010.507784) [DOI] [PubMed] [Google Scholar]
- 83.Hecquet CM, Malik AB. 2009. Role of H2O2-activated TRPM2 calcium channel in oxidant-induced endothelial injury. J. Thromb. Haemost. 101, 619–625. [PMC free article] [PubMed] [Google Scholar]
- 84.Massullo P, Sumoza-Toledo A, Bhagat H, Partida-Sanchez S. 2006. TRPM channels, calcium and redox sensors during innate immune responses. Semin. Cell Dev. Biol. 17, 654–666. ( 10.1016/j.semcdb.2006.11.006) [DOI] [PubMed] [Google Scholar]
- 85.Li Q, Wang X, Yang Z, Wang B, Li S. 2009. Menthol induces cell death via the TRPM8 channel in the human bladder cancer cell line T24. Oncology 77, 335–341. ( 10.1159/000264627) [DOI] [PubMed] [Google Scholar]
- 86.Prevarskaya N, Zhang L, Barritt G. 2007. TRP channels in cancer. Biochim. Biophys. Acta 1772, 937–946. ( 10.1016/j.bbadis.2007.05.006) [DOI] [PubMed] [Google Scholar]
- 87.Lev S, Zeevi DA, Frumkin A, Offen-Glasner V, Bach G, Minke B. 2010. Constitutive activity of the human TRPML2 channel induces cell degeneration. J. Biol. Chem. 285, 2771–2782. ( 10.1074/jbc.M109.046508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xiao Y, et al. 2010. Overexpression of Trpp5 contributes to cell proliferation and apoptosis probably through involving calcium homeostasis. Mol. Cell Biochem. 339, 155–161. ( 10.1007/s11010-009-0379-8) [DOI] [PubMed] [Google Scholar]
- 89.Sappington RM, Sidorova T, Long DJ, Calkins DJ. 2009. TRPV1: contribution to retinal ganglion cell apoptosis and increased intracellular Ca2+ with exposure to hydrostatic pressure. Invest. Ophthalmol. Vis. Sci. 50, 717–728. ( 10.1167/iovs.08-2321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shirakawa H, Yamaoka T, Sanpei K, Sasaoka H, Nakagawa T, Kaneko S. 2008. TRPV1 stimulation triggers apoptotic cell death of rat cortical neurons. Biochem. Biophys. Res. Commun. 377, 1211–1215. ( 10.1016/j.bbrc.2008.10.152) [DOI] [PubMed] [Google Scholar]
- 91.Ziglioli F, Frattini A, Maestroni U, Dinale F, Ciufifeda M, Cortellini P. 2009. Vanilloid-mediated apoptosis in prostate cancer cells through a TRPV-1 dependent and a TRPV-1-independent mechanism. Acta Biomed. 80, 13–20. [PubMed] [Google Scholar]
- 92.Iwata Y, Katanosaka Y, Arai Y, Shigekawa M, Wakabayashi S. 2009. Dominant-negative inhibition of Ca2+ influx via TRPV2 ameliorates muscular dystrophy in animal models. Hum. Mol. Genet. 18, 824–834. [DOI] [PubMed] [Google Scholar]
- 93.Yamada T, Ueda T, Shibata Y, Ikegami Y, Saito M, Ishida Y, Ugawa S, Kohri K, Shimada S. 2010. TRPV2 activation induces apoptotic cell death in human T24 bladder cancer cells: a potential therapeutic target for bladder cancer. Urology 76, 507–509. ( 10.1016/j.urology.2010.03.029) [DOI] [PubMed] [Google Scholar]
- 94.Casas S, Novials A, Reimann F, Gomis R, Gribble FM. 2008. Calcium elevation in mouse pancreatic beta cells evoked by extracellular human islet amyloid polypeptide involves activation of the mechanosensitive ion channel TRPV4. Diabetologia 51, 2252–2262. ( 10.1007/s00125-008-1111-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cuddapah VA, Turner KL, Sontheimer H. 2013. Calcium entry via TRPC1 channels activates chloride currents in human glioma cells. Cell Calcium 53, 187–194. ( 10.1016/j.ceca.2012.11.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bomben VC, Sontheimer H. 2010. Disruption of transient receptor potential canonical channel 1 causes incomplete cytokinesis and slows the growth of human malignant gliomas. Glia 58, 1145–1156. ( 10.1002/glia.20994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wondergem R, Ecay TW, Mahieu F, Owsianik G, Nilius B. 2008. HGF/SF and menthol increase human glioblastoma cell calcium and migration. Biochem. Biophys. Res. Commun. 372, 210–215. ( 10.1016/j.bbrc.2008.05.032) [DOI] [PubMed] [Google Scholar]
- 98.Molenaar RJ. 2011. Ion channels in glioblastoma. ISRN Neurol. 2011, 590249 ( 10.5402/2011/590249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sontheimer H. 2008. An unexpected role for ion channels in brain tumor metastasis. Exp. Biol. Med. 233, 779–791. ( 10.3181/0711-MR-308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chin LS, Park CC, Zitnay KM, Sinha M, DiPatri AJ, Jr, Perillan P, Simard JM. 1997. 4-Aminopyridine causes apoptosis and blocks an outward rectifier K+ channel in malignant astrocytoma cell lines. J. Neurosci. Res. 48, 122–127. () [DOI] [PubMed] [Google Scholar]
- 101.Yang KB, Zhao SG, Liu YH, Hu EX, Liu BX. 2009. Tetraethylammonium inhibits glioma cells via increasing production of intracellular reactive oxygen species. Chemotherapy 55, 372–380. ( 10.1159/000235730) [DOI] [PubMed] [Google Scholar]
- 102.Blackiston DJ, McLaughlin KA, Levin M. 2009. Bioelectric controls of cell proliferation: ion channels, membrane voltage and the cell cycle. Cell Cycle 8, 3519–3528. ( 10.4161/cc.8.21.9888) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bordey A, Lyons SA, Hablitz JJ, Sontheimer H. 2001. Electrophysiological characteristics of reactive astrocytes in experimental cortical dysplasia. J. Neurophysiol. 85, 1719–1731. [DOI] [PubMed] [Google Scholar]
- 104.Higashimori H, Sontheimer H. 2007. Role of Kir4.1 channels in growth control of glia. Glia 55, 1668–1679. ( 10.1002/glia.20574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang CL, Tsai ML, Wu SN. 2012. Evidence for mitoxantrone-induced block of inwardly rectifying K+ channels expressed in the osteoclast precursor RAW 264.7 cells differentiated with lipopolysaccharide. Cell Physiol. Biochem. 30, 687–701. ( 10.1159/000341449) [DOI] [PubMed] [Google Scholar]
- 106.Banderali U, Belke D, Singh A, Jayanthan A, Giles WR, Narendran A. 2011. Curcumin blocks Kv11.1 (erg) potassium current and slows proliferation in the infant acute monocytic leukemia cell line THP-1. Cell Physiol. Biochem. 28, 1169–1180. ( 10.1159/000335850) [DOI] [PubMed] [Google Scholar]
- 107.Ufartes R, et al. 2013. Behavioural and functional characterization of Kv10.1 (Eag1) knockout mice. Hum. Mol. Genet. 22, 2247–2262. ( 10.1093/hmg/ddt076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Agarwal JR, Griesinger F, Stuhmer W, Pardo LA. 2010. The potassium channel Ether a go-go is a novel prognostic factor with functional relevance in acute myeloid leukemia. Mol. Cancer 9, 18 ( 10.1186/1476-4598-9-18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jehle J, Schweizer PA, Katus HA, Thomas D. 2011. Novel roles for hERG K+ channels in cell proliferation and apoptosis. Cell Death Dis. 2, e193 ( 10.1038/cddis.2011.77) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Urrego D, Tomczak AP, Zahed F, Stühmer W, Pardo LA. 2014. Potassium channels in cell cycle and cell proliferation. Phil. Trans. R. Soc. B 369, 20130094 ( 10.1098/rstb.2013.0094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huang L, Li B, Li W, Guo H, Zou F. 2009. ATP-sensitive potassium channels control glioma cells proliferation by regulating ERK activity. Carcinogenesis 30, 737–744. ( 10.1093/carcin/bgp034) [DOI] [PubMed] [Google Scholar]
- 112.Pardo LA. 2004. Voltage-gated potassium channels in cell proliferation. Physiology 19, 285–292. ( 10.1152/physiol.00011.2004) [DOI] [PubMed] [Google Scholar]
- 113.Yin LT, Fu YJ, Xu QL, Yang J, Liu ZL, Liang AH, Fan XJ, Xu CG. 2007. Potential biochemical therapy of glioma cancer. Biochem. Biophys. Res. Commun. 362, 225–229. ( 10.1016/j.bbrc.2007.07.167) [DOI] [PubMed] [Google Scholar]
- 114.Schwab A, Fabian A, Hanley PJ, Stock C. 2012. Role of ion channels and transporters in cell migration. Physiol. Rev. 92, 1865–1913. ( 10.1152/physrev.00018.2011) [DOI] [PubMed] [Google Scholar]
- 115.Kraft R, Krause P, Jung S, Basrai D, Liebmann L, Bolz J, Patt S. 2003. BK channel openers inhibit migration of human glioma cells. Pflugers Arch. 446, 248–255. ( 10.1007/s00424-003-1012-4) [DOI] [PubMed] [Google Scholar]
- 116.Lang F, Foller M, Lang KS, Lang PA, Ritter M, Gulbins E, Vereninov A, Huber SM. 2005. Ion channels in cell proliferation and apoptotic cell death. J. Membr. Biol. 205, 147–157. ( 10.1007/s00232-005-0780-5) [DOI] [PubMed] [Google Scholar]
- 117.Weaver AK, Liu X, Sontheimer H. 2004. Role for calcium-activated potassium channels (BK) in growth control of human malignant glioma cells. J. Neurosci. Res. 78, 224–234. ( 10.1002/jnr.20240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chang KH, Chen ML, Chen HC, Huang YW, Wu TY, Chen YJ. 1999. Enhancement of radiosensitivity in human glioblastoma U138MG cells by tetrandrine. Neoplasma 46, 196–200. [PubMed] [Google Scholar]
- 119.Khalid MH, Shibata S, Hiura T. 1999. Effects of clotrimazole on the growth, morphological characteristics, and cisplatin sensitivity of human glioblastoma cells in vitro. J. Neurosurg. 90, 918–927. ( 10.3171/jns.1999.90.5.0918) [DOI] [PubMed] [Google Scholar]
- 120.Lang F, Ritter M, Gamper N, Huber S, Fillon S, Tanneur V, Lepple-Wienhues A, Szabo I, Gulbins E. 2000. Cell volume in the regulation of cell proliferation and apoptotic cell death. Cell Physiol. Biochem. 10, 417–428. [DOI] [PubMed] [Google Scholar]
- 121.Brackenbury WJ. 2012. Voltage-gated sodium channels and metastatic disease. Channels 6, 352–361. ( 10.4161/chan.21910) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Haas BR, Sontheimer H. 2010. Inhibition of the sodium-potassium-chloride cotransporter isoform-1 reduces glioma invasion. Cancer Res. 70, 5597–5606. ( 10.1158/0008-5472.CAN-09-4666) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Habela CW, Olsen ML, Sontheimer H. 2008. ClC3 is a critical regulator of the cell cycle in normal and malignant glial cells. J. Neurosci. 28, 9205–9217. ( 10.1523/JNEUROSCI.1897-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ransom CB, O'Neal JT, Sontheimer H. 2001. Volume-activated chloride currents contribute to the resting conductance and invasive migration of human glioma cells. J. Neurosci. 21, 7674–7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Habela CW, Sontheimer H. 2007. Cytoplasmic volume condensation is an integral part of mitosis. Cell Cycle 6, 1613–1620. ( 10.4161/cc.6.13.4357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ritter M, Woll E, Waldegger S, Haussinger D, Lang HJ, Scholz W, Scholkens B, Lang F. 1993. Cell shrinkage stimulates bradykinin-induced cell membrane potential oscillations in NIH 3T3 fibroblasts expressing the ras-oncogene. Pflugers Arch. 423, 221–224. ( 10.1007/BF00374398) [DOI] [PubMed] [Google Scholar]
- 127.Wanitchakool P, Wolf L, Koehl GE, Sirianant L, Schreiber R, Kulkarni S, Duvvuri U, Kunzelmann K. 2014. Role of anoctamins in cancer and apoptosis. Phil. Trans. R. Soc. B 369, 20130096 ( 10.1098/rstb.2013.0096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rouzaire-Dubois B, Malo M, Milandri JB, Dubois JM. 2004. Cell size-proliferation relationship in rat glioma cells. Glia 45, 249–257. ( 10.1002/glia.10320) [DOI] [PubMed] [Google Scholar]
- 129.Burg MB, Kwon ED, Kultz D. 1996. Osmotic regulation of gene expression. FASEB J. 10, 1598–1606. [DOI] [PubMed] [Google Scholar]
- 130.Ernest NJ, Habela CW, Sontheimer H. 2008. Cytoplasmic condensation is both necessary and sufficient to induce apoptotic cell death. J. Cell Sci. 121, 290–297. ( 10.1242/jcs.017343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cuddapah VA, Turner KL, Seifert S, Sontheimer H. 2013. Bradykinin-induced chemotaxis of human gliomas requires the activation of KCa3.1 and ClC-3. J. Neurosci. 33, 1427–1440. ( 10.1523/JNEUROSCI.3980-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Catacuzzeno L, Aiello F, Fioretti B, Sforna L, Castigli E, Ruggieri P, Tata AM, Calogero A, Franciolini F. 2011. Serum-activated K and Cl currents underlay U87-MG glioblastoma cell migration. J. Cell Physiol. 226, 1926–1933. ( 10.1002/jcp.22523) [DOI] [PubMed] [Google Scholar]
- 133.Soroceanu L, Manning TJ, Jr, Sontheimer H. 1999. Modulation of glioma cell migration and invasion using Cl− and K+ ion channel blockers. J. Neurosci. 19, 5942–5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cuddapah VA, Sontheimer H. 2010. Molecular interaction and functional regulation of ClC-3 by Ca2+/calmodulin-dependent protein kinase II (CaMKII) in human malignant glioma. J. Biol. Chem. 285, 11 188–11 196. ( 10.1074/jbc.M109.097675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Szabo I, Lepple-Wienhues A, Kaba KN, Zoratti M, Gulbins E, Lang F. 1998. Tyrosine kinase-dependent activation of a chloride channel in CD95-induced apoptosis in T lymphocytes. Proc. Natl Acad. Sci. USA 95, 6169–6174. ( 10.1073/pnas.95.11.6169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shimizu T, Numata T, Okada Y. 2004. A role of reactive oxygen species in apoptotic activation of volume-sensitive Cl(-) channel. Proc. Natl Acad. Sci. USA 101, 6770–6773. ( 10.1073/pnas.0401604101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Okada Y, Maeno E. 2001. Apoptosis, cell volume regulation and volume-regulatory chloride channels. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 130, 377–383. ( 10.1016/S1095-6433(01)00424-X) [DOI] [PubMed] [Google Scholar]
- 138.Maeno E, Ishizaki Y, Kanaseki T, Hazama A, Okada Y. 2000. Normotonic cell shrinkage because of disordered volume regulation is an early prerequisite to apoptosis. Proc. Natl Acad. Sci. USA 97, 9487–9492. ( 10.1073/pnas.140216197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bortner CD, Cidlowski JA. 2014. Ion channels and apoptosis in cancer. Phil. Trans. R. Soc. B 369, 20130104 ( 10.1098/rstb.2013.0104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vandenberg JI, Perry MD, Perrin MJ, Mann SA, Ke Y, Hill AP. 2012. hERG K+ channels: structure, function, and clinical significance. Physiol. Rev. 92, 1393–1478. ( 10.1152/physrev.00036.2011) [DOI] [PubMed] [Google Scholar]
- 141.Wulff H, Castle NA. 2010. Therapeutic potential of KCa3.1 blockers: recent advances and promising trends. Expert Rev. Clin. Pharmacol. 3, 385–396. ( 10.1586/ecp.10.11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fuessel S, et al. 2006. Vaccination of hormone-refractory prostate cancer patients with peptide cocktail-loaded dendritic cells: results of a phase I clinical trial. Prostate 66, 811–821. ( 10.1002/pros.20404) [DOI] [PubMed] [Google Scholar]
- 143.Hartung F, Stuhmer W, Pardo LA. 2011. Tumor cell-selective apoptosis induction through targeting of K(V)10.1 via bifunctional TRAIL antibody. Mol. Cancer 10, 109 ( 10.1186/1476-4598-10-109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mamelak AN, Jacoby DB. 2007. Targeted delivery of antitumoral therapy to glioma and other malignancies with synthetic chlorotoxin (TM-601). Expert Opin. Drug Deliv. 4, 175–186. ( 10.1517/17425247.4.2.175) [DOI] [PubMed] [Google Scholar]
- 145.Zhang JJ, et al. 1994. Tamoxifen blocks chloride channels. A possible mechanism for cataract formation. J. Clin. Invest. 94, 1690–1697. ( 10.1172/JCI117514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mamelak AN, et al. 2006. Phase I single-dose study of intracavitary-administered iodine-131-TM-601 in adults with recurrent high-grade glioma. J. Clin. Oncol. 24, 3644–3650. ( 10.1200/JCO.2005.05.4569) [DOI] [PubMed] [Google Scholar]
- 147.Hockaday DC, Shen S, Fiveash J, Raubitschek A, Colcher D, Liu A, Alvarez V, Mamelak AN. 2005. Imaging glioma extent with 131I-TM-601. J. Nucl. Med. 46, 580–586. [PubMed] [Google Scholar]
- 148.Deshane J, Garner CC, Sontheimer H. 2003. Chlorotoxin inhibits glioma cell invasion via matrix metalloproteinase-2. J. Biol. Chem. 278, 4135–4144. ( 10.1074/jbc.M205662200) [DOI] [PubMed] [Google Scholar]
- 149.Pandiella A, Magni M, Lovisolo D, Meldolesi J. 1989. The effect of epidermal growth factor on membrane potential. Rapid hyperpolarization followed by persistent fluctuations. J. Biol. Chem. 264, 12 914–12 921. [PubMed] [Google Scholar]
- 150.Lang PA, Kaiser S, Myssina S, Wieder T, Lang F, Huber SM. 2003. Role of Ca2+-activated K+ channels in human erythrocyte apoptosis. Am. J. Physiol. Cell Physiol. 285, C1553–C1560. ( 10.1152/ajpcell.00186.2003) [DOI] [PubMed] [Google Scholar]
- 151.Dartsch PC, Ritter M, Gschwentner M, Lang HJ, Lang F. 1995. Effects of calcium channel blockers on NIH 3T3 fibroblasts expressing the Ha-ras oncogene. Eur. J. Cell Biol. 67, 372–378. [PubMed] [Google Scholar]
- 152.Ritter M, Woll E, Haller T, Dartsch PC, Zwierzina H, Lang F. 1997. Activation of Na+/H+-exchanger by transforming Ha-ras requires stimulated cellular calcium influx and is associated with rearrangement of the actin cytoskeleton. Eur. J. Cell Biol. 72, 222–228. [PubMed] [Google Scholar]
- 153.Lang F, Waldegger S, Woell E, Ritter M, Maly K, Grunicke H. 1992. Effects of inhibitors and ion substitutions on oscillations of cell membrane potential in cells expressing the RAS oncogene. Pflugers Arch. 421, 416–424. ( 10.1007/BF00370251) [DOI] [PubMed] [Google Scholar]
- 154.Whitfield JF, Bird RP, Chakravarthy BR, Isaacs RJ, Morley P. 1995. Calcium-cell cycle regulator, differentiator, killer, chemopreventor, and maybe, tumor promoter. J. Cell Biochem. Suppl. 22, 74–91. ( 10.1002/jcb.240590811) [DOI] [PubMed] [Google Scholar]
- 155.Dekkers DW, Comfurius P, Bevers EM, Zwaal RF. 2002. Comparison between Ca2+-induced scrambling of various fluorescently labelled lipid analogues in red blood cells. Biochem. J. 362, 741–747. ( 10.1042/0264-6021:3620741) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Woon LA, Holland JW, Kable EP, Roufogalis BD. 1999. Ca2+ sensitivity of phospholipid scrambling in human red cell ghosts. Cell Calcium 25, 313–320. ( 10.1054/ceca.1999.0029) [DOI] [PubMed] [Google Scholar]
- 157.Wang Z. 2004. Roles of K+ channels in regulating tumour cell proliferation and apoptosis. Pflugers Arch. 448, 274–286. ( 10.1007/s00424-004-1258-5) [DOI] [PubMed] [Google Scholar]
- 158.Patel AJ, Lazdunski M. 2004. The 2P-domain K+ channels: role in apoptosis and tumorigenesis. Pflugers Arch. 448, 261–273. ( 10.1007/s00424-004-1255-8) [DOI] [PubMed] [Google Scholar]