Abstract
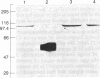
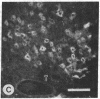
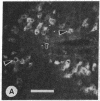
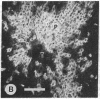
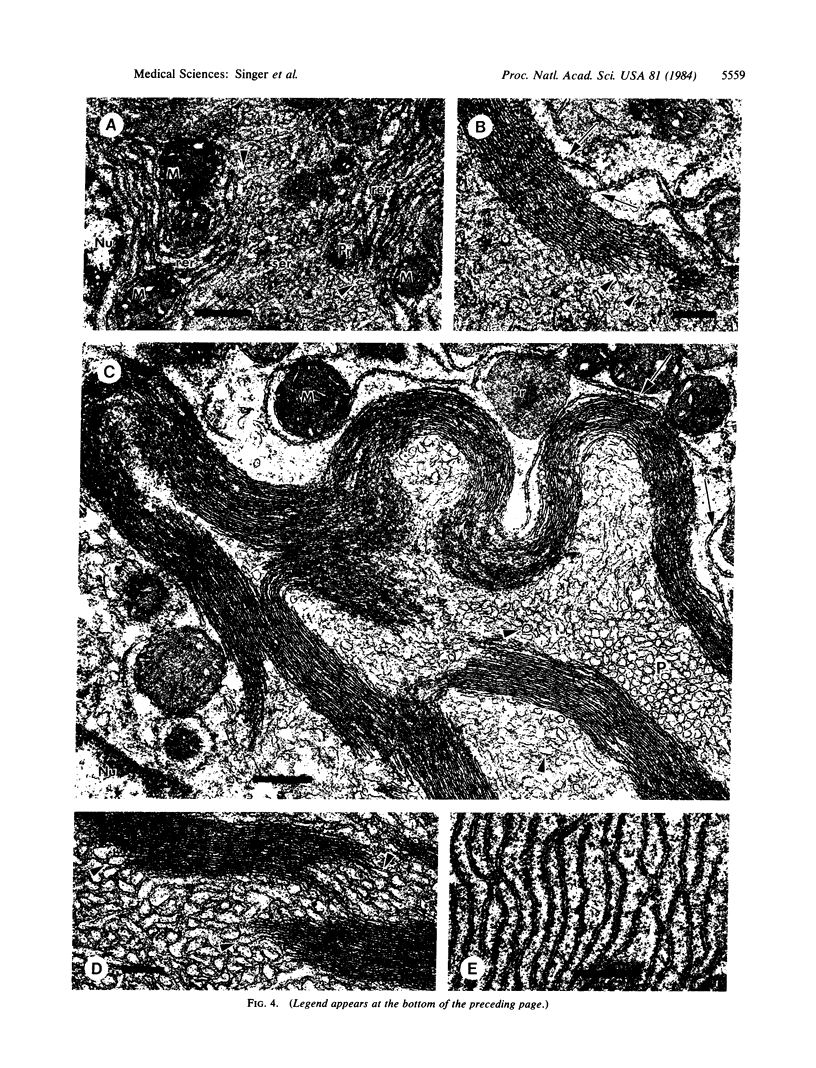
Mevinolin is a potent inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA reductase; EC 1.1.1.34), an enzyme that catalyzes the rate-limiting step in cholesterol biosynthesis. We have been studying the hepatic distribution of reductase with immunofluorescence microscopy and liver ultrastructure with electron microscopy in normal and drug-treated rats. In control animals, only about 20% of the hepatocytes were reductase positive. These cells were localized in the periportal lobular zones. The numbers of positive hepatocytes in animals given mevinolin or cholestyramine (or both) were directly proportional to the activities of the HMG-CoA reductase determined biochemically. This induction of HMG-CoA reductase immunofluorescence was centered periportally. Rats given 0.075% mevinolin alone had a homogeneous distribution of reductase staining in their hepatocyte cytoplasm, whereas a combination of 0.25% mevinolin and 3% cholestyramine caused a 150-fold increase in enzyme activity and induced prominent juxtanuclear immunofluorescent globules of HMG-CoA reductase in all hepatocytes. With electron microscopy, these bodies were composed of tightly packed stacks of smooth endoplasmic reticulum cysternae and aggregates of branched smooth endoplasmic reticulum tubules. Our data suggest that a subpopulation of periportal rat hepatocytes may be uniquely specialized for cholesterol synthesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts A. W., Chen J., Kuron G., Hunt V., Huff J., Hoffman C., Rothrock J., Lopez M., Joshua H., Harris E. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3957–3961. doi: 10.1073/pnas.77.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. G., Orci L., Brown M. S., Garcia-Segura L. M., Goldstein J. L. Ultrastructural analysis of crystalloid endoplasmic reticulum in UT-1 cells and its disappearance in response to cholesterol. J Cell Sci. 1983 Sep;63:1–20. doi: 10.1242/jcs.63.1.1. [DOI] [PubMed] [Google Scholar]
- Bilheimer D. W., Grundy S. M., Brown M. S., Goldstein J. L. Mevinolin and colestipol stimulate receptor-mediated clearance of low density lipoprotein from plasma in familial hypercholesterolemia heterozygotes. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4124–4128. doi: 10.1073/pnas.80.13.4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black V. H. The development of smooth-surfaced endoplasmic reticulum in adrenal cortical cells of fetal guinea pigs. Am J Anat. 1972 Nov;135(3):381–417. doi: 10.1002/aja.1001350307. [DOI] [PubMed] [Google Scholar]
- Bolender R. P., Weibel E. R. A morphometric study of the removal of phenobarbital-induced membranes from hepatocytes after cessation of threatment. J Cell Biol. 1973 Mar;56(3):746–761. doi: 10.1083/jcb.56.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin D. J., Luskey K. L., Anderson R. G., Faust J. R., Goldstein J. L., Brown M. S. Appearance of crystalloid endoplasmic reticulum in compactin-resistant Chinese hamster cells with a 500-fold increase in 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1185–1189. doi: 10.1073/pnas.79.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin D. J., Luskey K. L., Faust J. R., MacDonald R. J., Brown M. S., Goldstein J. L. Molecular cloning of 3-hydroxy-3-methylglutaryl coenzyme a reductase and evidence for regulation of its mRNA. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7704–7708. doi: 10.1073/pnas.79.24.7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke C. F., Edwards P. A., Lan S. F., Tanaka R. D., Fogelman A. M. Regulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase mRNA levels in rat liver. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3305–3308. doi: 10.1073/pnas.80.11.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards P. A., Lan S. F., Fogelman A. M. Alterations in the rates of synthesis and degradation of rat liver 3-hydroxy-3-methylglutaryl coenzyme A reductase produced by cholestyramine and mevinolin. J Biol Chem. 1983 Sep 10;258(17):10219–10222. [PubMed] [Google Scholar]
- Endo A., Kuroda M., Tsujita Y. ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinium. J Antibiot (Tokyo) 1976 Dec;29(12):1346–1348. doi: 10.7164/antibiotics.29.1346. [DOI] [PubMed] [Google Scholar]
- Endo A., Tsujita Y., Kuroda M., Tanzawa K. Effects of ML-236B on cholesterol metabolism in mice and rats: lack of hypocholesterolemic activity in normal animals. Biochim Biophys Acta. 1979 Nov 21;575(2):266–276. [PubMed] [Google Scholar]
- Fears R., Richards D. H., Ferres H. The effect of compactin, a potent inhibitor of 3-hydroxy-3-methylglutaryl coenzyme-A reductase activity, on cholesterogenesis and serum cholesterol levels in rats and chicks. Atherosclerosis. 1980 Apr;35(4):439–449. doi: 10.1016/0021-9150(80)90185-9. [DOI] [PubMed] [Google Scholar]
- Giloh H., Sedat J. W. Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gallate. Science. 1982 Sep 24;217(4566):1252–1255. doi: 10.1126/science.7112126. [DOI] [PubMed] [Google Scholar]
- Jones A. L., Fawcett D. W. Hypertrophy of the agranular endoplasmic reticulum in hamster liver induced by phenobarbital (with a review on the functions of this organelle in liver). J Histochem Cytochem. 1966 Mar;14(3):215–232. doi: 10.1177/14.3.215. [DOI] [PubMed] [Google Scholar]
- Jones A. L., Hradek G. T., Renston R. H., Wong K. Y., Karlaganis G., Paumgartner G. Autoradiographic evidence for hepatic lobular concentration gradient of bile acid derivative. Am J Physiol. 1980 Mar;238(3):G233–G237. doi: 10.1152/ajpgi.1980.238.3.G233. [DOI] [PubMed] [Google Scholar]
- Kovanen P. T., Bilheimer D. W., Goldstein J. L., Jaramillo J. J., Brown M. S. Regulatory role for hepatic low density lipoprotein receptors in vivo in the dog. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1194–1198. doi: 10.1073/pnas.78.2.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum L., Luskey K. L., Chin D. J., Ho Y. K., Goldstein J. L., Brown M. S. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase and its mRNA in rat liver as studied with a monoclonal antibody and a cDNA probe. J Biol Chem. 1983 Jul 10;258(13):8450–8455. [PubMed] [Google Scholar]
- Loud A. V. A quantitative stereological description of the ultrastructure of normal rat liver parenchymal cells. J Cell Biol. 1968 Apr;37(1):27–46. doi: 10.1083/jcb.37.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi H., Haba T., Tatami R., Miyamoto S., Sakai Y., Wakasugi T., Watanabe A., Koizumi J., Takeda R. Effect of an inhibitor of 3-hydroxy-3-methyglutaryl coenzyme A reductase on serum lipoproteins and ubiquinone-10-levels in patients with familial hypercholesterolemia. N Engl J Med. 1981 Aug 27;305(9):478–482. doi: 10.1056/NEJM198108273050902. [DOI] [PubMed] [Google Scholar]
- Mabuchi H., Sakai T., Sakai Y., Yoshimura A., Watanabe A., Wakasugi T., Koizumi J., Takeda R. Reduction of serum cholesterol in heterozygous patients with familial hypercholesterolemia. Additive effects of compactin and cholestyramine. N Engl J Med. 1983 Mar 17;308(11):609–613. doi: 10.1056/NEJM198303173081101. [DOI] [PubMed] [Google Scholar]
- NOVIKOFF A. B. Cell heterogeneity within the hepatic lobule of the rat: staining reactions. J Histochem Cytochem. 1959 Jul;7(4):240–244. doi: 10.1177/7.4.240. [DOI] [PubMed] [Google Scholar]
- Ness G. C., Spindler C. D., Moffler M. H. Purification of 3-hydroxy-3-methylglutaryl coenzyme A reductase from rat liver. Arch Biochem Biophys. 1979 Oct 15;197(2):493–499. doi: 10.1016/0003-9861(79)90272-8. [DOI] [PubMed] [Google Scholar]
- Orci L., Brown M. S., Goldstein J. L., Garcia-Segura L. M., Anderson R. G. Increase in membrane cholesterol: a possible trigger for degradation of HMG CoA reductase and crystalloid endoplasmic reticulum in UT-1 cells. Cell. 1984 Apr;36(4):835–845. doi: 10.1016/0092-8674(84)90033-3. [DOI] [PubMed] [Google Scholar]
- Packard C. J., Shepherd J. The hepatobiliary axis and lipoprotein metabolism: effects of bile acid sequestrants and ileal bypass surgery. J Lipid Res. 1982 Nov;23(8):1081–1098. [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodwell V. W., Nordstrom J. L., Mitschelen J. J. Regulation of HMG-CoA reductase. Adv Lipid Res. 1976;14:1–74. doi: 10.1016/b978-0-12-024914-5.50008-5. [DOI] [PubMed] [Google Scholar]
- SHANK R. E., MORRISON G., CHENG C. H., KARL I., SCHWARTZ R. Cell heterogeneity within the hepatic lobule: quantitative histochemistry. J Histochem Cytochem. 1959 Jul;7(4):237–239. doi: 10.1177/7.4.237. [DOI] [PubMed] [Google Scholar]
- Sisson J. K., Fahrenbach W. H. Fine structure of steroidogenic cells of a primate cutaneous organ. Am J Anat. 1967 Sep;121(2):337–367. doi: 10.1002/aja.1001210211. [DOI] [PubMed] [Google Scholar]
- Tanaka R. D., Edwards P. A., Lan S. F., Knöppel E. M., Fogelman A. M. The effect of cholestyramine and Mevinolin on the diurnal cycle of rat hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase. J Lipid Res. 1982 Sep;23(7):1026–1031. [PubMed] [Google Scholar]
- Tobert J. A., Bell G. D., Birtwell J., James I., Kukovetz W. R., Pryor J. S., Buntinx A., Holmes I. B., Chao Y. S., Bolognese J. A. Cholesterol-lowering effect of mevinolin, an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme a reductase, in healthy volunteers. J Clin Invest. 1982 Apr;69(4):913–919. doi: 10.1172/JCI110530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobert J. A., Hitzenberger G., Kukovetz W. R., Holmes I. B., Jones K. H. Rapid and substantial lowering of human serum cholesterol by mevinolin (MK-803), an inhibitor of hydroxymethylglutaryl-coenzyme A reductase. Atherosclerosis. 1982 Jan;41(1):61–65. doi: 10.1016/0021-9150(82)90070-3. [DOI] [PubMed] [Google Scholar]
- Tsujita Y., Kuroda M., Tanzawa K., Kitano N., Endo A. Hypolipidemic effects in dogs of ML-236B, a competitive inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Atherosclerosis. 1979 Mar;32(3):307–313. doi: 10.1016/0021-9150(79)90174-6. [DOI] [PubMed] [Google Scholar]