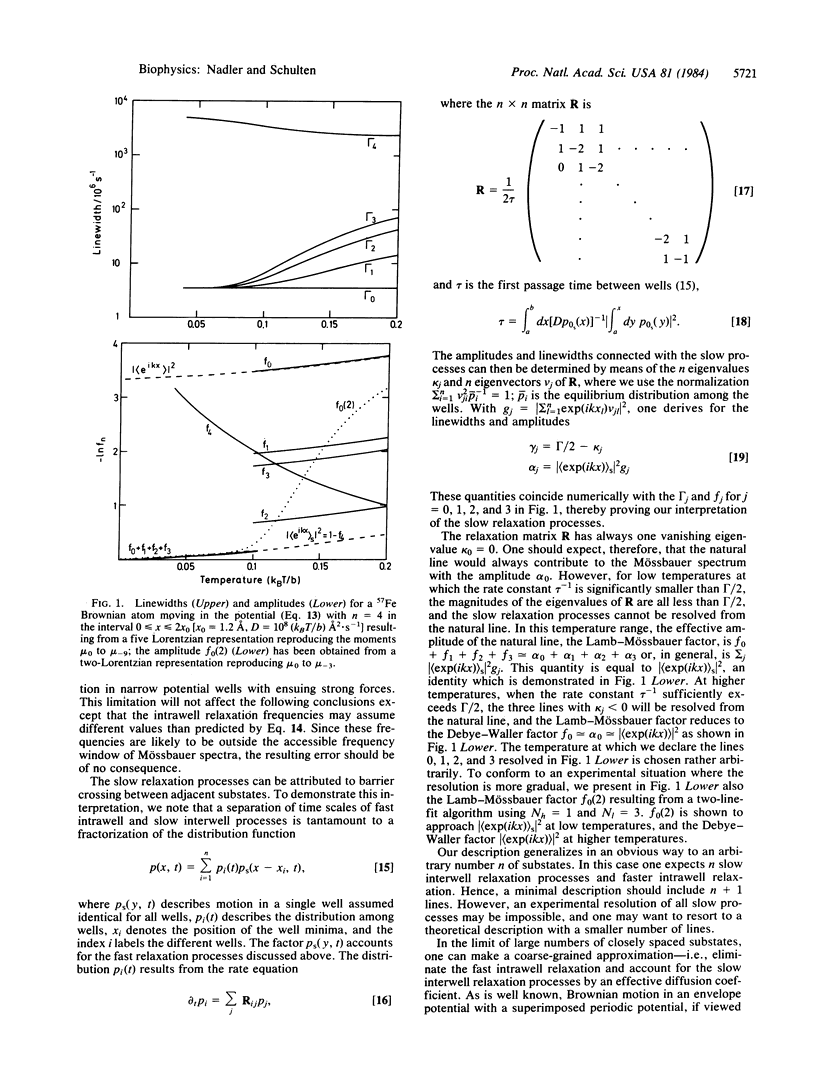
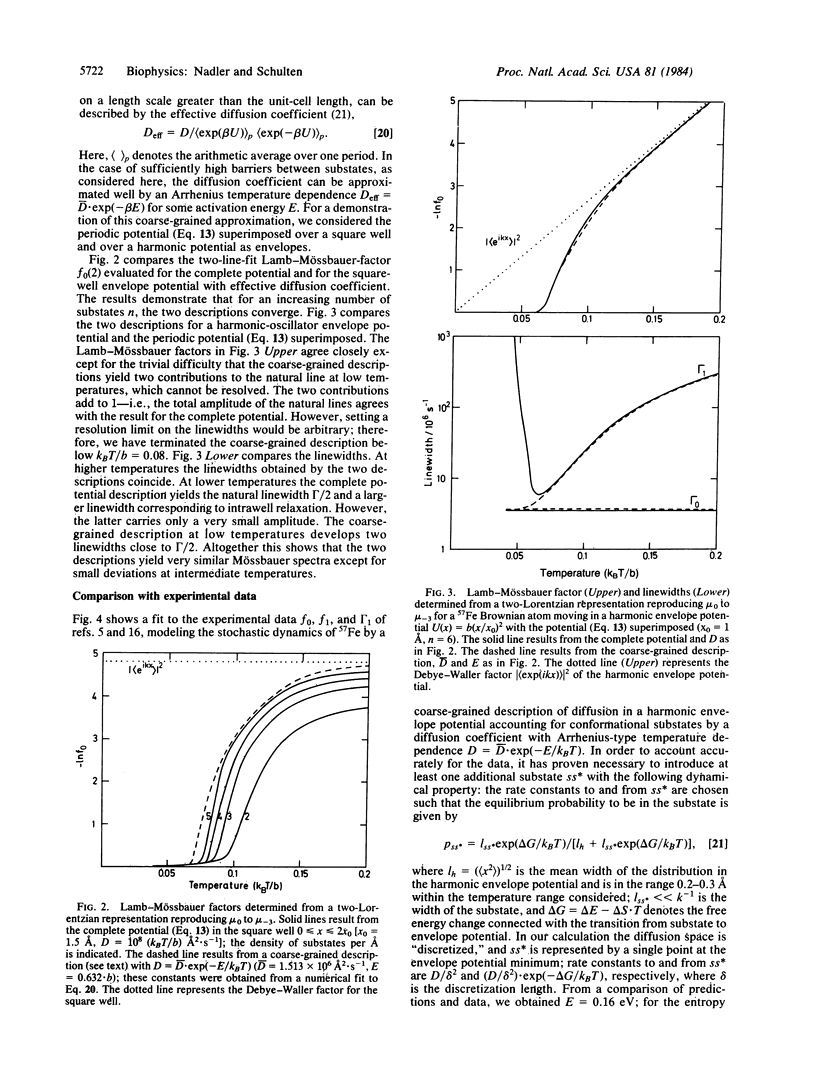
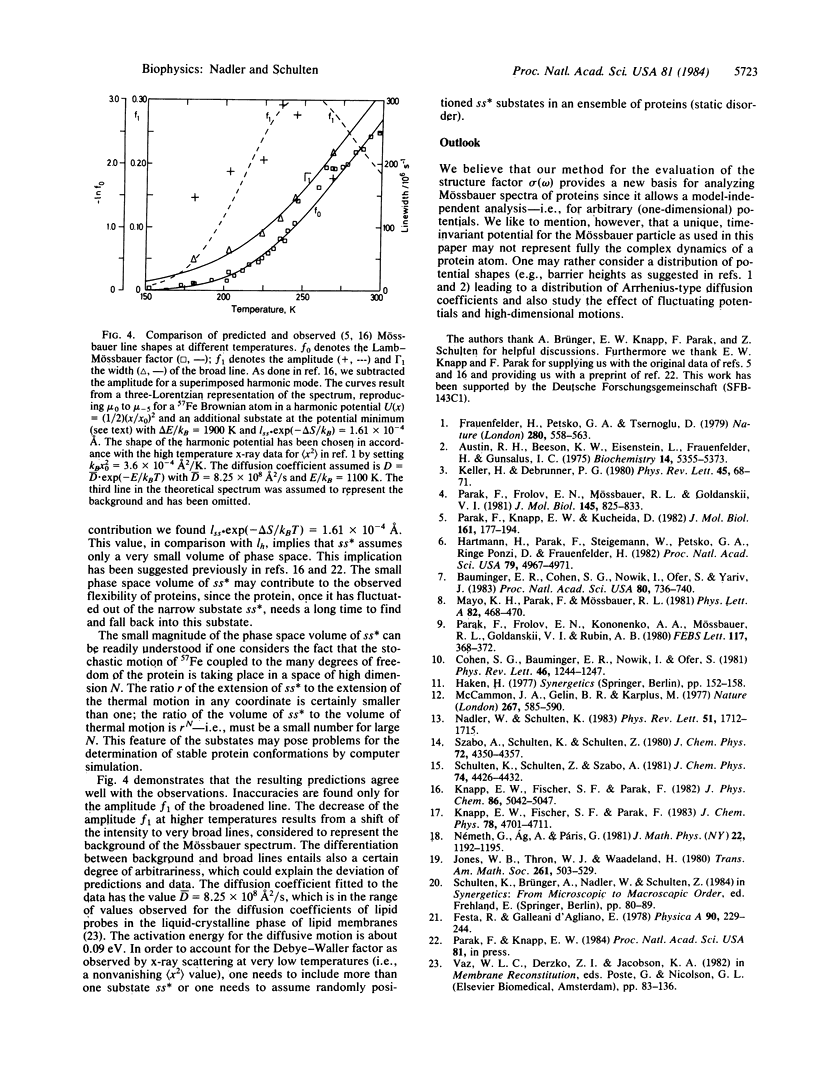
Abstract
Mössbauer spectra of 57Fe in proteins fluctuating between different conformational substates are evaluated by means of a two-sided Padée approximation, which can reproduce the low and high frequency dependence of the spectral line shape I(omega) to any desired accuracy. The dynamics of the atom is modeled as Brownian motion in a multiminimum potential and described by a Fokker-Planck equation. The Mössbauer spectrum is expanded in terms of Lorentzian contributions, which can be attributed separately to fluctuations between conformational substates (potential minima) and to relaxation within the substates. In the limit of closely spaced substates, the Mössbauer spectra can be accounted for by an effective diffusion coefficient with Arrhenius-type temperature dependence. We demonstrate that the observed temperature dependence of Mössbauer spectra of proteins [Parak, F., Knapp, E.W. & Kucheida, D. (1982) J. Mol. Biol. 161, 177-194] can be accounted for by stochastic motion in a multiminimum potential.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Bauminger E. R., Cohen S. G., Nowik I., Ofer S., Yariv J. Dynamics of heme iron in crystals of metmyoglobin and deoxymyoglobin. Proc Natl Acad Sci U S A. 1983 Feb;80(3):736–740. doi: 10.1073/pnas.80.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frauenfelder H., Petsko G. A., Tsernoglou D. Temperature-dependent X-ray diffraction as a probe of protein structural dynamics. Nature. 1979 Aug 16;280(5723):558–563. doi: 10.1038/280558a0. [DOI] [PubMed] [Google Scholar]
- Hartmann H., Parak F., Steigemann W., Petsko G. A., Ponzi D. R., Frauenfelder H. Conformational substates in a protein: structure and dynamics of metmyoglobin at 80 K. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4967–4971. doi: 10.1073/pnas.79.16.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCammon J. A., Gelin B. R., Karplus M. Dynamics of folded proteins. Nature. 1977 Jun 16;267(5612):585–590. doi: 10.1038/267585a0. [DOI] [PubMed] [Google Scholar]
- Parak F., Frolov E. N., Kononenko A. A., Mössbauer R. L., Goldanskii V. I., Rubin A. B. Evidence for a correlation between the photoinduced electron transfer and dynamic properties of the chromatophore membranes from Rhodospirillum rubrum. FEBS Lett. 1980 Aug 11;117(1):368–372. doi: 10.1016/0014-5793(80)80982-3. [DOI] [PubMed] [Google Scholar]
- Parak F., Frolov E. N., Mössbauer R. L., Goldanskii V. I. Dynamics of metmyoglobin crystals investigated by nuclear gamma resonance absorption. J Mol Biol. 1981 Feb 5;145(4):825–833. doi: 10.1016/0022-2836(81)90317-x. [DOI] [PubMed] [Google Scholar]
- Parak F., Knapp E. W., Kucheida D. Protein dynamics. Mössbauer spectroscopy on deoxymyoglobin crystals. J Mol Biol. 1982 Oct 15;161(1):177–194. doi: 10.1016/0022-2836(82)90285-6. [DOI] [PubMed] [Google Scholar]


