Abstract
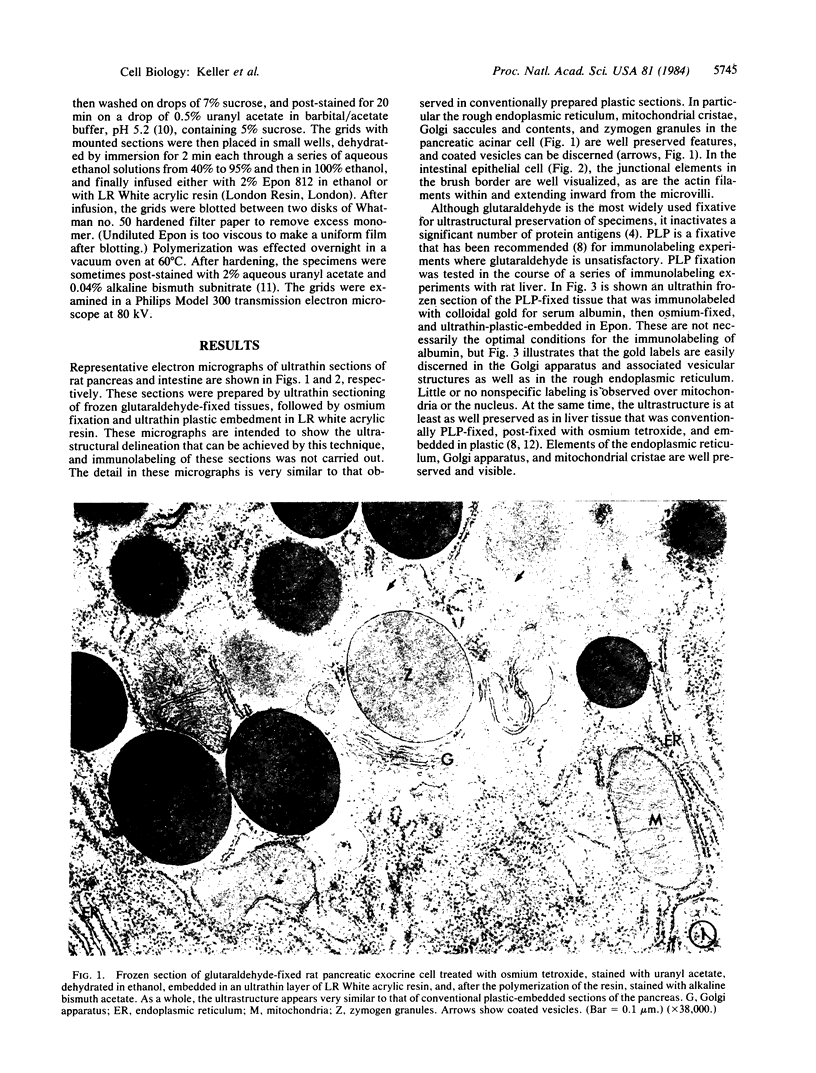
Ultrathin frozen sections are ideal substrates with which to carry out immunolabeling experiments in electron microscopy. However, the ultrastructural delineation in positively stained frozen sections has not been as detailed as in conventionally osmium-stained and plastic-embedded sections. We now describe a simple technique in which immunolabeled ultrathin frozen sections are subsequently treated with osmium tetroxide, dehydrated, and then embedded in plastic by impregnation with a monomer to the thickness of the section, followed by polymerization of the monomer. By this technique ultrastructural definition as good as that of conventional plastic sections is achieved, while the high density and specificity of immunolabeling characteristic of ultrathin frozen sections are retained.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dutton A. H., Tokuyasu K. T., Singer S. J. Iron-dextran antibody conjugates: General method for simultaneous staining of two components in high-resolution immunoelectron microscopy. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3392–3396. doi: 10.1073/pnas.76.7.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Dutton A. H., Tokuyasu K. T., Singer S. J. Immunoelectron microscope studies of membrane-microfilament interactions: distributions of alpha-actinin, tropomyosin, and vinculin in intestinal epithelial brush border and chicken gizzard smooth muscle cells. J Cell Biol. 1981 Dec;91(3 Pt 1):614–628. doi: 10.1083/jcb.91.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., van der Ley P. A., Scheffer R. C. Use of colloidal gold particles in double-labeling immunoelectron microscopy of ultrathin frozen tissue sections. J Cell Biol. 1981 Jun;89(3):653–665. doi: 10.1083/jcb.89.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Novikoff P. M., La Russo N. F., Novikoff A. B., Stockert R. J., Yam A., Le Sage G. D. Immunocytochemical localization of lysosomal beta-galactosidase in rat liver. J Cell Biol. 1983 Nov;97(5 Pt 1):1559–1565. doi: 10.1083/jcb.97.5.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T. A technique for ultracryotomy of cell suspensions and tissues. J Cell Biol. 1973 May;57(2):551–565. doi: 10.1083/jcb.57.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T., Dutton A. H., Geiger B., Singer S. J. Ultrastructure of chicken cardiac muscle as studied by double immunolabeling in electron microscopy. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7619–7623. doi: 10.1073/pnas.78.12.7619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T. Immunochemistry on ultrathin frozen sections. Histochem J. 1980 Jul;12(4):381–403. doi: 10.1007/BF01011956. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. Visualization of longitudinally-oriented intermediate filaments in frozen sections of chicken cardiac muscle by a new staining method. J Cell Biol. 1983 Aug;97(2):562–565. doi: 10.1083/jcb.97.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]