Abstract
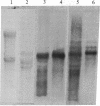
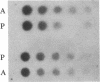
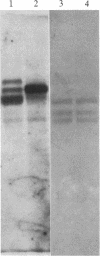
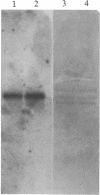
The parasitic protozoan Leishmania mexicana amazonensis has two developmental stages: a motile flagellated promastigote stage and a sessile intracellular amastigote stage. In our previous work, cells of the promastigote stage were found to synthesize more tubulin protein than those of the amastigote stage. Here, tubulin mRNAs in these leishmanias were analyzed. Based on dot blot hybridization between total leishmanial RNA and tubulin-specific cDNA probes derived from chicken brain, amastigotes and promastigotes were found to have approximately equal amounts of alpha- and beta-tubulin mRNAs. RNA blotting of leishmanial RNA, using chicken tubulin cDNA probes, showed that amastigotes and promastigotes both gave a single mRNA species of 2100 nucleotides for alpha-tubulin in roughly similar quantities. However, such analysis for beta-tubulin revealed mainly a single mRNA species of 3600 nucleotides for amastigotes and three species of 2800, 3600, and 4400 nucleotides for promastigotes, the smallest mRNA being the most predominant. Thus, regulation of gene expression appears to be different only for beta-tubulin between the two developmental stages of this protozoan.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum S. G., Wittner M., Nadler J. P., Horwitz S. B., Dennis J. E., Schiff P. B., Tanowitz H. B. Taxol, a microtubule stabilizing agent, blocks the replication of Trypanosoma cruzi. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4571–4575. doi: 10.1073/pnas.78.7.4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C., Garavito R. M., Armbruster B. Biochemical and structural analyses of microtubules in the pellicular membrane of Leishmania tropica. J Protozool. 1982 Nov;29(4):560–565. doi: 10.1111/j.1550-7408.1982.tb01335.x. [DOI] [PubMed] [Google Scholar]
- Chang K. P. Human cutaneous lieshmania in a mouse macrophage line: propagation and isolation of intracellular parasites. Science. 1980 Sep 12;209(4462):1240–1242. doi: 10.1126/science.7403880. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W. The tubulins: from DNA to RNA to protein and back again. Cell. 1983 Sep;34(2):330–332. doi: 10.1016/0092-8674(83)90366-5. [DOI] [PubMed] [Google Scholar]
- Cowan N. J., Dudley L. Tubulin isotypes and the multigene tubulin families. Int Rev Cytol. 1983;85:147–173. doi: 10.1016/s0074-7696(08)62372-4. [DOI] [PubMed] [Google Scholar]
- Cumming R., Williamson J. Differential labelling of trypanosome microtubules using tubulin subunit monoclonal antibodies. Cell Biol Int Rep. 1984 Jan;8(1):2–2. doi: 10.1016/0309-1651(84)90174-7. [DOI] [PubMed] [Google Scholar]
- Fong D., Chang K. P. Surface antigenic change during differentiation of a parasitic protozoan, Leishmania mexicana: Identification by monoclonal antibodies. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7366–7370. doi: 10.1073/pnas.79.23.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong D., Chang K. P. Tubulin biosynthesis in the developmental cycle of a parasitic protozoan, Leishmania mexicana: changes during differentiation of motile and nonmotile stages. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7624–7628. doi: 10.1073/pnas.78.12.7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo J. M., Anderton B. H. A subpopulation of trypanosome microtubules recognized by a monoclonal antibody to tubulin. EMBO J. 1983;2(4):479–483. doi: 10.1002/j.1460-2075.1983.tb01450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison E. H., Bowers W. E. Characterization of rat lymphocyte cell membranes by analytical isopycnic centrifugation. J Biol Chem. 1983 Jun 10;258(11):7134–7140. [PubMed] [Google Scholar]
- Hendricks L. D., Wood D. E., Hajduk M. E. Haemoflagellates: commercially available liquid media for rapid cultivation. Parasitology. 1978 Jun;76(3):309–316. doi: 10.1017/s0031182000048186. [DOI] [PubMed] [Google Scholar]
- Hodgkinson V. H., Herman R., Semprevivo L. Leishmania donovani: correlation among assays of amastigote viability. Exp Parasitol. 1980 Dec;50(3):397–408. doi: 10.1016/0014-4894(80)90042-9. [DOI] [PubMed] [Google Scholar]
- Hunter K. W., Jr, Cook C. L., Hensen S. A. Temperature-induced in vitro transformation of Leishmania mexicana. I. Ultrastructural comparison of culture-transformed and intracellular amastigotes. Acta Trop. 1982 Jun;39(2):143–150. [PubMed] [Google Scholar]
- Kemphues K. J., Kaufman T. C., Raff R. A., Raff E. C. The testis-specific beta-tubulin subunit in Drosophila melanogaster has multiple functions in spermatogenesis. Cell. 1982 Dec;31(3 Pt 2):655–670. doi: 10.1016/0092-8674(82)90321-x. [DOI] [PubMed] [Google Scholar]
- Landfear S. M., McMahon-Pratt D., Wirth D. F. Tandem arrangement of tubulin genes in the protozoan parasite Leishmania enriettii. Mol Cell Biol. 1983 Jun;3(6):1070–1076. doi: 10.1128/mcb.3.6.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lopata M. A., Havercroft J. C., Chow L. T., Cleveland D. W. Four unique genes required for beta tubulin expression in vertebrates. Cell. 1983 Mar;32(3):713–724. doi: 10.1016/0092-8674(83)90057-0. [DOI] [PubMed] [Google Scholar]
- Lüttke A., Lurquin P. F., Bonotto S. Rapid method for the detection of small amounts of DNA in CsCl gradients with ethidium bromide. Anal Biochem. 1980 Oct;108(1):1–5. doi: 10.1016/0003-2697(80)90685-5. [DOI] [PubMed] [Google Scholar]
- Pateraki E., Portocala R., Labrousse H., Guesdon J. L. Antiactin and antitubulin antibodies in canine visceral leishmaniasis. Infect Immun. 1983 Nov;42(2):496–500. doi: 10.1128/iai.42.2.496-500.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rondinelli E., Soares M. C., de Castro J. F., de Castro F. T. Characterization of messenger RNA populations of Crithidia fasciculata. Arch Biochem Biophys. 1981 Jul;209(2):349–355. doi: 10.1016/0003-9861(81)90291-5. [DOI] [PubMed] [Google Scholar]
- Russell D. G., Miller D., Gull K. Tubulin heterogeneity in the trypanosome Crithidia fasciculata. Mol Cell Biol. 1984 Apr;4(4):779–790. doi: 10.1128/mcb.4.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seebeck T., Whittaker P. A., Imboden M. A., Hardman N., Braun R. Tubulin genes of Trypanosoma brucei: a tightly clustered family of alternating genes. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4634–4638. doi: 10.1073/pnas.80.15.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silflow C. D., Rosenbaum J. L. Multiple alpha- and beta-tubulin genes in Chlamydomonas and regulation of tubulin mRNA levels after deflagellation. Cell. 1981 Apr;24(1):81–88. doi: 10.1016/0092-8674(81)90503-1. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow L. S., Milhausen M., Rutter W. J., Agabian N. Tubulin genes are tandemly linked and clustered in the genome of trypanosoma brucei. Cell. 1983 Jan;32(1):35–43. doi: 10.1016/0092-8674(83)90494-4. [DOI] [PubMed] [Google Scholar]
- Wallach M., Cully D. F., Haas L. O., Trager W., Cross G. A. Histidine-rich protein genes and their transcripts in Plasmodium falciparum and P. lophurae. Mol Biochem Parasitol. 1984 May;12(1):85–94. doi: 10.1016/0166-6851(84)90046-x. [DOI] [PubMed] [Google Scholar]
- Wallach M. Efficient extraction and translation of Plasmodium falciparum messenger RNA. Mol Biochem Parasitol. 1982 Dec;6(6):335–342. doi: 10.1016/0166-6851(82)90023-8. [DOI] [PubMed] [Google Scholar]
- Wallach M., Fong D., Chang K. P. Post-transcriptional control of tubulin biosynthesis during leishmanial differentiation. Nature. 1982 Oct 14;299(5884):650–652. doi: 10.1038/299650a0. [DOI] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]
- Wirth D. F., Slater C. Isolation and characterization of an alpha-tubulin gene from Leishmania enriettii. Mol Biochem Parasitol. 1983 Sep;9(1):83–92. doi: 10.1016/0166-6851(83)90059-2. [DOI] [PubMed] [Google Scholar]